Abstract
In recent decades cyanide is the most widely used for the extraction of gold and silver, for being economical and efficient, however, other alternatives have been considered because of its toxicity to the environment, for this reason in this work we study a new leaching agent that seeks to be a viable alternative to cyanide, which is commercially called DEZO and is considered ecological due to the low quantity of the main complexing agent which is cyanate, and other components such as sodium oxide, nitrogen, ammonium, calcium, iron, which is used for gold and silver extractions. For the development of the study a gold and silver ore provided by the mining company "Las Chispas", located in Arizpe, Sonora, Mexico, was used. The ore contains 15.50 g/T of Au and 1550 g/T of Ag. Leaching was carried out at moderate pressures using sodium cyanide and DEZO as lixiviants for Au and Ag extraction. XRD and SEM–EDS analyses confirm the presence of quartz, fluorite and argentite species. Pressure leaching was performed using NaCN, with conditions of T = 70 °C and P = 0.62 MPa, NaCN [300 mg/L], -270 mesh, 20% solids, time 1 h and 600 rpm, obtaining 98.3% extraction of Au and only 8.8% of Ag. Next, pressure leaching was performed using the DEZO eco-friendly lixiviant, with conditions of T = 70 °C and P = 0.62 MPa, NaCNO [300 mg/L], -270 mesh, 20% solids, time 1 h and 600 rpm, obtaining 93.9% Au extraction and only 7.7% Ag. Subsequently, the adjustment of the shrinking core model was performed by varying the temperature in the pressure leaching, the activation energy (Ea) using both leaching reagents (NaCN and DEZO) was less than 20 kJ/mol, which defines that the gold and silver leaching are controlled by diffusion through the product layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the beginning of the extraction of precious metals from their ores, cyanide has been the main leaching agent, having more than a century of experience in this industry, due to its high extractions and low cost. The leaching of gold and silver with cyanide in the presence of oxygen is reported as follows [1,2,3,4,5,6]:
However, although the cyanidation process has been the most widely used, environmental pollution has progressively inhibited cyanide leaching [7], due to its toxic nature that represents a significant threat to health if exposed to ecological entities. Moreover, in recent years cyanidation has been banned through its legislation, in countries such as Costa Rica, in some states of the United States and provinces of Argentina [8].
During the last few years, research on alternative lixiviants to cyanide has increased significantly [8], searching for a lixiviant that is effective, environmentally friendly and economical [1,2,3, 5,6,7].
Over the years, numerous investigations have been carried out as an alternative to the use of cyanide, including thiosulfates [3, 9,10,11,12,13,14,15], thiourea [3, 15,16,17,18,19,20], thiocyanate [1, 21,22,23,24], polysulfides [1, 25,26,27] and halides [1,2,3, 8, 27,28,29,30,31], which have made great contributions to the gold and silver industry.
DEZO reagent is a commercial product that is mainly composed of sodium cyanate. Its advantages are that it is environmentally friendly and works in a very similar way to cyanide, so it can be used directly for gold production without changing the original cyanide gold extraction technology and related equipment, which is considered a viable option to replace the use of cyanide [32]. The main reactions to using DEZO are as follows:
DEZO reagent is a stable product at ambient pressure and temperature, any production waste and water waste is only necessary to measure the pH and if necessary, neutralize. Table 1 shows some chemical and physical properties of DEZO reagent according to its safety data sheet [33].
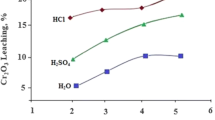
The following figure shows information to compare the use of cyanide vs. DEZO, which may prove to be a promising leachant.
In the present study we worked with a gold and silver ore from "Las Chispas" mine located in Arizpe, Sonora, Mexico. Leaching was performed using moderate oxygen pressures and sodium cyanide (NaCN) vs. the environmentally friendly commercial reagent DEZO (NaCNO) in order to compare their extractions for Au and Ag and to adjust the kinetic model of decreasing nucleus to determine the controlling stage of each of the leaching agents in the process.The shrinking core model can be presented in three controlling stages, which are presented in Table 2 [34].
Such a net result of an unreacted core shrinking as leaching proceeds, with a diffusion layer surrounding it. The original radius is r_0 and the radius of the shrinking nucleus is r [34].
The rate constant for any reaction must be expressed in the form of the Arrhenius equation,
When the activation energy is calculated, the controlling stage of the process can be determined. It is known that for a chemical reaction controlling stage, the value of Ea is higher than 40 kJ/mol, while for mass transport (diffusion) controlled reactions the activation energy is between 5 and 20 kJ/mol [5].
2 Materials and Methods
2.1 Characterization of the Mineral
First, homogenization and particle size reduction were carried out using the coning and quartering technique and the RO-TAP® equipment, ring pulverizer (TM/G 1500) and the Coulter equipment (LS 100Q) to obtain the particle sizes proposed in the study. The equipment used is shown in Fig. 1.
Atomic absorption spectrophotometer (AAS Perkin Elmer AAnalist 400) and induction coupled plasma (Optima 8300 ICP-OES Perkin Elmer) equipment were used for the determination of some elements present in the sample. The fire assay technique is used for the determination of gold and silver precious metals. Tables 3 and 4 show the results of the AAS, ICP and fire assay analyses.
Analyses were also performed on scanning electron microscopy-energy dispersive microscopy (SEM–EDS, JSM- 5410LV) and X-ray diffraction (XRD, BRUKER, Mod. D8Advance) equipment, in order to determine the elements and/or mineralogical species that make up the sample.
3 Experimental Methods
After characterizing the ore, conventional leaching was performed using sodium cyanide and DEZO to determine gold and silver extractions and reagent consumption. For both leachings the same parameters were used, particle size 88 μm, [leaching reagent] = 500 mg/L, ambient temperature and pressure, pH = 10.5–11 and time 72 h.
The next step was to carry out leaching at moderate pressures with sodium cyanide and DEZO using the titanium autoclave (Parr 1L, Mod.4523) with pressure and temperature controllers, shown in Fig. 2.
The conditions used for both sodium cyanide and DEZO leaching reagents were [lixiviant] = 300 mg/L, particle sizes of 88 μm and 53 μm, temperatures of 25, 50 and 70 °C, oxygen pressures of 0.34 and 0.62 MPa, agitation of 600 rpm, pH = 10.5–11 and time of 1 h.
Leaching was also carried out for both leaching reagents at a pressure of 0.62 MPa, particle size of 53 μm, agitation of 600 rpm and varying temperatures of 25, 50 and 70 °C at different residence times to carry out the kinetic study and determine the controlling stage of each process.
4 Results and Discussions
4.1 Characterization of the Mineral
After duly preparing the ore for analysis, it was determined that the particle sizes to be used in the study were 88 μm (#170 mesh) and 53 μm (#270 mesh), which were corroborated by the Coulter equipment (LS 100Q), indicating that 80% of each of the samples analyzed corresponded to those particle sizes.
The prepared ore was characterized by different techniques, in order to know its species and determine the gold and silver grades. The samples were made in duplicate by the fire assay technique, placing a blank for each sample analyzed. The blank corresponds to the amount of fluxes for the fusion mixture, therefore a blank is added to avoid erroneous results. These results were also corroborated by the combination method in Atomic Absorption Spectroscopy and by the ICP-OES technique, it is important to mention that all the analysis by these analytical techniques was of the main sample that we call it (head sample). Tables 3 and 4 show the results of the analyses by AAS, ICP-OES and fire assay.
The fire assay technique was used to determine the ore grades, which indicate that the gold and silver values are relevant for this study. The SEM–EDS micrographs show clear images of the presence of silver surrounded by silicates, shown in Fig. 3, while Fig. 4 and Table 5 show in detail the presence of gold and several silver species present in the sample. On the other hand, the analysis of the mineral by XRD shows fluorite and silicates such as quartz and orthoclase as the main species, which are shown in Fig. 5 and Table 6.
According to those obtained in the characterization, it indicates that the mineral has a high silver and gold grade, which according to the SEM–EDS point micrographs, are surrounded (not occluded) by some silicates such as quartz and orthoclase, and it was also observed that gold and silver are found within the species of kustelite (Ag, Au) and argentite (Ag2S). While for the diffractogram acquired by XRD shows that the mineral has species such as silicates and fluorite.
4.2 Leaching
As a next step to the mineral characterization, leaching tests were performed comparing the two leaching reagents NaCN and NaCNO, using for both leaches the same parameters, particle size 88 μm, [leaching reagent] = 500 mg/L, pH = 10.5–11, ambient temperature and pressure, time 72 h. Gold extractions comparing sodium cyanide and DEZO are shown in Fig. 6 and silver extractions comparing both leachants are shown in Fig. 7.
The leaching tests under atmospheric conditions were carried out in a reactor for 72 h, where solution was replenished at each sampling in order for the leaching reaction to continue and to ensure that the cyanide and DEZO reagents were not completely consumed. The results were satisfactory, obtaining with cyanide extractions of 83.5% Au and 80% Ag, consuming 0.6 g/L NaCN and 0.3 g/L lime, while using DEZO extractions of 85.8% Au and 79.3% Ag were obtained, with a NaCNO consumption of 1.35 g/L and a lime consumption of 0.4 g/L. In terms of reagent consumption, it was shown that DEZO is consumed more rapidly, consuming more than twice as much reagent as sodium cyanide for each test performed. Although it can be said that DEZO, in addition to being environmentally friendly, another advantage is that it can be used under normal conditions equal to those of cyanide, which would facilitate its handling in established processes [33].
4.3 Pressure Leaching
Pressure leaching was then carried out using the Parr reactor with a capacity of 1 L, where again the two leaching reagents of NaCN and NaCNO were compared, using for both leaches the same parameters, [NaCN] = 300 mg/L, [NaCNO] = 300 mg/L, pH = 10. 5 -11, particle size of 88 μm and 53 μm, temperatures of 25, 50 and 70 °C, oxygen pressures of 0.34 and 0.62 MPa, agitation of 600 rpm, pH = 10.5–11 and time of 1 h. Figures 8 and 9 show the results of each of the extractions for gold and silver.
The main advantage of pressure leaching is to reduce the test time, since the addition of oxygen increases the mass transfer inside the autoclave generating an ideal oxidizing environment, which accelerates the dissolution of gold and silver, providing good extractions in a minimum time [5]. Another advantage is the facility to handle different pressures and temperatures inside the autoclave, which allowed performing the leaching tests where it was observed that for both lixiviants the gold extractions were increasing as the particle size is smaller and the temperature increases, because the more the particle size decreases, the greater the contact area between the particle and the leaching solution, however, when heat is applied to the process, two opposite factors occur that can affect the dissolution of the metals. As the temperature increases, the rate of dissolution accelerates. However, as temperatures increase, the amount of oxygen in the solution decreases, due to the fact that the solubility of gases decreases with increasing temperature [35]. As for the tests performed by adding oxygen pressure and maximum temperatures of 70 °C, satisfactory results were obtained, where the gold extractions were 98.3% of Au using cyanide and 93.9% of Au using DEZO.
In the silver extractions, the same behavior could be observed in terms of the parameters of pressure, temperature and particle size, where by increasing pressure, temperature and decreasing the particle size an increase in the extractions was obtained, however, they were not satisfactory, since using cyanide only 8.8% of Ag was obtained and with DEZO 7.5% of Ag was obtained. It can be said that these results were due to the concentration of the lixiviant that was used, since it was not enough to finish the test and where it was also demonstrated that both lixiviants are more related to gold, which was extracted first, and that is the reason for the low silver extractions.
Once the leaching experiments were performed with NaCN and DEZO reagents, at the following conditions: leachant concentration 300 mg/L, Mesh #270, 70 °C and PO2 = 0.62 MPa, the solid residues were characterized using atomic absorption spectroscopy (AAS), fire assay technique, where the results obtained are shown in Tables 7 and 8, inductively coupled plasma (ICP-OES), these results are shown in Tables 9 and 10. They were also analyzed using X-ray diffraction (XRD) and scanning electron microscopy-energy dispersive microscopy (SEM–EDS), the analyses are shown in Tables 11 and 12 and Figs. 10 and 11.
The characterization of the residues shows the large amount of silver that remained unreacted due to the low concentration initially used in the pressure leaching, which will be a parameter that will be taken into account for the continuation of this work. It was also observed the low presence of gold in the residues, which indicates that both cyanide and DEZO are more akin to gold.
4.4 Kinetic Study
The fundamental kinetic study the variables with the best results of Au and Ag extractions were taken (PO2 = 0. 62 MPa, particle size 53 μm), the temperature was varied (25, 50 and 70 °C) and the experimental data were adjusted to the shrinking core model (SCM) with the objective of determining the controlling stage of each process and for this it was necessary to calculate the reaction rate and activation energy (Ea), which defines that, for controlled chemical reactions, the value of Ea > 40 kJ/mol, while for the controlling stage by diffusion through the product layer the Ea < 20 kJ/mol [5]. Graphs and calculations of the reaction rate (k) and activation energy (Ea) were obtained for both leaching reagents, which are shown in Fig. 12 and 16 and Tables 13 and 14.
In studies related to kinetic models, Qihao Gui et al. studied the kinetics of a novel synthetic green leachant for gold where they found that the leaching kinetics was mainly controlled by internal diffusion [36]. On the other hand, the researchers Huiqun Niu et al., performed pressure leaching using trichloroisocinauric acid (ATCC) as an alternative lixiviant to cyanide in gold extractions, being an effective reagent and of low toxicity to the environment, obtaining 94.39% Au, as for the kinetic study it was determined that the process was controlled by the diffusion model through the layers of solid products [37]. In the present work, the kinetic study was carried out for pressure leaching using sodium cyanide and DEZO, where the variables of the study were adjusted and the activation energies were calculated, obtaining for gold extractions an Ea = 4. 2413 kJ/mol using sodium cyanide and Ea = 11.7394 kJ/mol using DEZO, as for silver extractions using sodium cyanide the Ea = 10.1370 kJ/mol and using DEZO the Ea = 9. 9866 kJ/mol, where each activation energy value is less than 20 kJ/mol [5], which indicates that the stage that mainly controls the pressure leaching process for both leaching agents (sodium cyanide and DEZO) is given by the diffusion through the product layer, which is due to the predominance of the gas phase and the mass transport that exists in the formation of leaching products.
With the results obtained up to the realization of these tests, it can be said that DEZO presents a similar behavior to sodium cyanide and at the moment of using the working temperatures, pressures did not present any complication, however, the handling of the reagent was also taken care of and to use it within the normal parameters in which cyanide works as temperature not higher than 80 °C, pH above 10.5, in order to be able to carry out a good comparison between them. With this it was also demonstrated that its behavior microscopically was the same as cyanide, since both are dominated by the diffusion between the layer of products generated in the process. On the other hand, a considerable disadvantage observed was the high consumption of DEZO in each of the tests, which can generate high expenses in the use of this reagent. It can also be said that DEZO competes in cost with sodium cyanide.
5 Conclusions
According to the characterization, the ore grade was found to be 15.5 g/ton gold and 1550 g/ton silver. XDR and SEM–EDS analysis found quartz and orthoclase silicates as main species, as well as fluorite and several silver species such as kustelite and argentite.
When performing conventional leaching using sodium cyanide and DEZO, very similar gold and silver extractions were obtained, obtaining 83.5% Au and 80% Ag extractions with cyanide, while using DEZO, 85.8% Au and 79.3% Ag extractions were obtained. The consumption of DEZO reagent was twice as high compared to the consumption of sodium cyanide, which could be a disadvantage for this alternative lixiviant. However, the use of DEZO lixiviant is equal to that of cyanide which would facilitate the substitution of cyanide in any established process.
The pressure leaching using sodium cyanide obtained very favorable gold extractions, reaching extractions of up to 98.3%, in the silver extractions the extractions were low, only reaching an extraction of 8.8% Ag. In the leaching using the DEZO reagent, favorable extractions were also achieved for gold up to 93.9%, while for silver extractions were also discouraging, reaching only 7.5% Ag. It was observed that when using either of the two lixiviants, Au and Ag extractions increased as the temperature increased and the particle size decreased. Also, it was observed that the consumption of DEZO reagent was double compared to the consumption of sodium cyanide, which could be its strongest disadvantage.
The kinetic study of Au and Ag extractions using sodium cyanide and DEZO reagents showed that for both leaching agents the stage that controls the pressure leaching processes was diffusion through the product layer due to the predominance of the gas phase and the mass transport that exists in the formation of the leaching products.
References
Liu Z-W, Guo X-y, Tian Q-H, Zhang L (2022) A systematic review of gold extraction: Fundamentals, advancements, and challenges toward alternative lixiviantes. J Hazard Mater 440:129778. https://doi.org/10.1016/j.jhazmat.2022.129778
Sousa R, Regufe MJ, Fiúza A, Machado M, Futuro A (2022) A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extract Ind Soc 9:101018. https://doi.org/10.1016/j.exis.2021.101018
Birich A, Stopic S, Friedrich B (2019) Kinetic Investigation and Dissolution Behavior of Cyanide Alternative Gold Leaching Reagents. Sci Rep 9:7191. https://doi.org/10.1038/s41598-019-43383-4
Fleming CA (1992) Hydrometallurgy of precious metals recovery. Hydrometallurgy 30(1):127–162. https://doi.org/10.1016/0304-386X(92)90081-A
Marsden JO, House CI (2006) The Chemistry of Gold Extraction, 2nd edn. Society for Mining, Metallurgy, and Exploration, Littleton
Zhang Y, Cui M, Wang J, Liu X, Lyu X (2022) A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviantes. Miner Eng 176:107336. https://doi.org/10.1016/j.mineng.2021.107336
Guo X-Y, Liu Z-W, Tian Q-H, Li D, Zhang L (2022) Gold extraction from Carlin-type concentrate by a novel environmentally friendly lixiviant. Hydrometallurgy 211:105884. https://doi.org/10.1016/j.hydromet.2022.105884
Seisko S, Lampinen M, Aromaa J, Laari A, Koiranen T, Lundström M (2018) Kinetics and mechanisms of gold dissolution by ferric chloride leaching. Miner Eng 115:131–141. https://doi.org/10.1016/j.mineng.2017.10.017
Jeon S, Tabelin CB, Park I, Nagata Y, Ito M, Hiroyoshi N (2020) Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 191:105214. https://doi.org/10.1016/j.hydromet.2019.105214
Dong Z, Jiang T, Bin Xu, Zhang B, Liu G, Li Q, Yang Y (2021) A systematic and comparative study of copper, nickel and cobalt-ammonia catalyzed thiosulfate processes for eco-friendly and efficient gold extraction from an oxide gold concentrate. Sep Purif Technol 272:118929. https://doi.org/10.1016/j.seppur.2021.118929
Jiajia Wu, Ahn J, Lee J (2022) Characterization of gold deportment and thiosulfate extraction for a copper-gold concentrate treated by pressure oxidation. Hydrometallurgy 207:105771. https://doi.org/10.1016/j.hydromet.2021.105771
Yang Ou, Yang Y, Li Ke, Gao W, Wang L, Li Q, Jiang T (2023) Eco-friendly and low-energy innovative scheme of self-generated thiosulfate by atmospheric oxidation for green gold extraction. J Clean Prod 387:135818. https://doi.org/10.1016/j.jclepro.2022.135818
Yang Ou, Yang Y, Wang L, Li Ke, Gao W, Zhang Y, Li Q, Jiang T (2023) A clean and efficient innovative technology for refractory sulfide gold ore: In situ gold extraction via self-generated thiosulfate. J Clean Prod 419:138280. https://doi.org/10.1016/j.jclepro.2023.138280
Hou L, Valdivieso AL, Chen P, Zhang G, Qi Zhang Yu, Chen SS, Jia F (2023) An electrochemical study of the dissolution behavior of gold in a novel glycine-thiosulfate system. Miner Eng 202:108273. https://doi.org/10.1016/j.mineng.2023.108273
Lin Y, Xianzhi Hu, Zi F, Chen Y, Chen S, Li X, Zhao Li, Li Y (2023) Synergistical thiourea and thiosulfate leaching gold from a gold concentrate calcine with copper-ammonia catalysis. Sep Purif Technol 327:124928. https://doi.org/10.1016/j.seppur.2023.124928
Guo X-y, Zhang L, Tian Q-H, Qin H (2020) Stepwise extraction of gold and silver from refractory gold concentrate calcine by thiourea. Hydrometallurgy 194:105330. https://doi.org/10.1016/j.hydromet.2020.105330
Gamiño-Arroyo Z, Pareau D, Buch A, Gomez-Castro FI, Sánchez-Cadena LE, Stambouli M, El Bekri J, Avila-Rodriguez M (2021) Design of Multistage Extraction System for Simultaneous Separation of Silver and Gold from Thiourea Solutions. Chem Eng Process 164:108391. https://doi.org/10.1016/j.cep.2021.108391
Zhang L, Guo X-y, Tian Q-H, Li D, Zhong S-P, Qin H (2022) Improved thiourea leaching of gold with additives from calcine by mechanical activation and its mechanism. Miner Eng 178:107403. https://doi.org/10.1016/j.mineng.2022.107403
Ke Li A, Zhang Yan, Li Qian, Liu Xiaoliang, Yang Yongbin, Jiang Tao (2023) Role of foreign ions in the thiourea leaching of gold. Min Eng 202:108265. https://doi.org/10.1016/j.mineng.2023.108265
Sasaki K, Suyama I, Aoki Y, Konadu KT, Cindy CC, Miki H, Hirajima T (2023) Significance of Fe contents on the surface of the gold ores in gold leaching by thiourea and ethylene thiourea. Miner Eng 191:107957. https://doi.org/10.1016/j.mineng.2022.107957
Hao Wu, Feng Y, Huang W, Li H, Liao S (2019) The role of glycine in the ammonium thiocyanate leaching of gold. Hydrometallurgy 185:111–116. https://doi.org/10.1016/j.hydromet.2019.01.019
Azizitorghabeh A, Wang J, Ramsay JA, Ghahreman A (2021) A review of thiocyanate gold leaching – Chemistry, thermodynamics, kinetics and processing. Miner Eng 160:106689. https://doi.org/10.1016/j.mineng.2020.106689
Azizitorghabeh A, Mahandra H, Ramsay J, Ghahreman A (2021) Gold Leaching from an Oxide Ore Using Thiocyanate as a Lixiviant: Process Optimization and Kinetics. ACS Omega 6:17183–17193. https://doi.org/10.1021/acsomega.1c00525
Rezaee M, Abdollahi H, Saneie R, Mohammadzadeh A, Rezaei A, Darvanjooghi MHK, Brar SK, Magdouli S (2022) A cleaner approach for high-efficiency regeneration of base and precious metals from waste printed circuit boards through stepwise oxido-acidic and thiocyanate leaching. Chemosphere 298:134283. https://doi.org/10.1016/j.chemosphere.2022.134283
Wen Q, Yufeng Wu, Wang X, Zhuang Z, Yan Yu (2017) Researches on preparation and properties of sodium polysulphide as gold leaching agent. Hydrometallurgy 171:77–85. https://doi.org/10.1016/j.hydromet.2017.04.008
Zhang L, Guo X-y, Tian Q-H, Zhong S-P, Qin H (2022) Extraction of gold from typical Carlin gold concentrate by pressure oxidation pretreatment - Sodium jarosite decomposition and polysulfide leaching. Hydrometallurgy 208:105743. https://doi.org/10.1016/j.hydromet.2021.105743
Li J, Kou J, Sun C, Zhang Na, Zhang H (2023) A review of environmentally friendly gold lixiviants: Fundamentals, applications, and commonalities. Miner Eng 197:108074. https://doi.org/10.1016/j.mineng.2023.108074
Wang Q, Liu T, Chen Y, Chen S, Qin X, Nie Y, Zi F (2020) Eco-friendly and economical extraction of gold from a refractory gold ore in iodide solution using persulfate as the oxidant. Hydrometallurgy 198:105502. https://doi.org/10.1016/j.hydromet.2020.105502
Wang Q, Xianzhi Hu, Zi F, Qin X, Nie Y, Zhang Y (2029) Extraction of gold from refractory gold ore using bromate and ferric chloride solution. Miner Eng 136:89–98. https://doi.org/10.1016/j.mineng.2019.02.037
Ahtiainen R, Lundström M (2019) Cyanide-free gold leaching in exceptionally mild chloride solutions. J Clean Prod 234:9–17. https://doi.org/10.1016/j.jclepro.2019.06.197
Liu Xiang, Wang Yongliang, Xiao Li, Ma Licheng, Han Peiwei, Ye Shufeng (2022) Eco-friendly and rapid extraction of gold by in-situ catalytic oxidation with N-bromosuccinimide. Heliyon 8:09706. https://doi.org/10.1016/j.heliyon.2022.e09706
Consejo Integrador de la construcción, la industria y el Desarrollo, INCIDE A.C. (2019) http://consejoincide.com/2019/12/17/lixiviantechemie/#:~:text=El%20reactivo%20de%20extracci%C3%B3n%20de,preparaci%C3%B3n%20del%20mineral%20de%20oro
Jinan Dezo Chemical CO LTD (2018) Hoja de seguridad DEZO. www.dezo.hk
Sohn HY, Milton E Wadsworth (1986) Cinética de los procesos de la metalurgia extractiva, México: Editorial Trillas p 167-194
Américo S, Ramos H (2019) Lixiviación de plata a temperaturas altas en mineral complejo de pirita. Rev Soc Quím Perú 85(1):97–108. https://doi.org/10.37761/rsqp.v85i1.240
Gui Qihao, Likang Fu, Yuting Hu, Di Haokai, Liang Ming, Wang Shixing, Zhang Libo (2023) Gold extraction using alternatives to cyanide: Ultrasonic reinforcement and its leaching kinetics. Min Eng 191:107939. https://doi.org/10.1016/j.mineng.2022.107939
Niu Huiqun, Yang Hongying, Tong Linlin (2023) Research on gold leaching of carbonaceous pressure-oxidized gold ore via a highly effective, green and low toxic agent trichloroisocyanuric acid. J Cleaner Prod 419:138062. https://doi.org/10.1016/j.jclepro.2023.138062
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bracamontes-Landavazo, M.A., Valenzuela-García, J.L., Parga-Torres, J.R. et al. Kinetic Study for the Extraction of Gold and Silver from an Ore Comparing Lixiviants Sodium Cyanide and DEZO using Moderate Pressures. Mining, Metallurgy & Exploration (2024). https://doi.org/10.1007/s42461-024-01036-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42461-024-01036-9