Abstract
In the current operational facility, due to the absence of pellet pot facility, a novel technique is developed wherein the pellets with different chemistries prepared in the laboratory are fired in an induration machine by placing them inside the Inconel baskets. In the present work, an attempt is made to optimize the pellet chemistry by developing 2 different flux pellets, viz., low basicity (B2: CaO/SiO2 = 0.3) pellets and high basicity (B2 = 0.9) pellets, and further the effects of basicity on pellet properties, viz., cold compressive strength, porosity, swelling index, reducibility index, and softening and melting temperature, are studied. It has been found through the multiple operational trials that increasing the basicity of pellets from 0.3 to 0.9 results into improving the pellet strength, reducibility and softening and melting characteristics. Moreover, it is observed that the swelling index of pellets is reduced by adding 2% MgO to both low and high basicity pellets that could be attributed to the formation of high melting point slag in pellets. The operational trial has resulted into producing pellets that exhibit a combination of desired mechanical strength, improved swelling, and reducibility index and possess better softening–melting behavior among all the chemistries studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
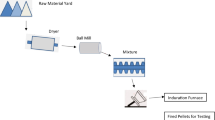
The iron-ore beneficiation process generates inevitably huge quantity of fines. These fines cannot be directly charged into the blast furnace as fines hinder the gas movement that undesirably causes adverse effects inside the blast furnace. Therefore, these fines are agglomerated into sinter and pellets and thereby are preferably charged into the blast furnace as a potential substitute to consume iron-ore fines generated during the beneficiation process. The sinter and pellets are the potential charge due to their shape and size that favors the gas movement and promotes desired permeability required for the stable blast furnace operation. The metallurgical characteristics of raw materials (sinter, pellets, and lump ore) are very different and hence they behave differently in the blast furnace under the same reducing conditions. The present work is focused on producing the pellets suitable for the blast furnace charge. The pelletizing process consists of a 2-step operation. The stage I consists of grinding iron ore in wet or dry ball mill, followed by mixing of raw materials in vertical or drum mixture, and subsequently forming the green pellets in drum or disc pelletizer with the addition of binder and moisture to achieve the necessary green ball strength. The stage II consists of firing of the fragile green balls either in the kiln or in straight grate to produce fired pellets with desired properties suitable for the blast furnace smelting process. The quality of fired pellets broadly depends on the type of ores, green pellet quality, type and amount of binders, fluxes used, and subsequent treatment employed during the firing process [1]. High-temperature metallurgical properties of fired pellets depend on their chemistry, induration conditions, raw material characteristics, and porosity. Therefore, pellet chemistry plays a vital role in controlling their behavior during its reduction in blast furnace [2]. Many studies are conducted to improve the high-temperature properties of pellets, like varying the size of fluxes and solid fuels, lowering of induration temperature, fluxing pellets with CaO and MgO bearing fluxes [3,4,5,6,7].
Fluxes such as limestone, dolomite, pyroxenite, magnesite, and quartzite are added to the blast furnace burden to control its slag chemistry [8,9,10]. However, the earlier studies have reported that addition of the carbonate fluxes like limestone and dolomite directly into the blast furnaces affects the blast furnace performance [11]. Also, with a rapid increase in the production rate of pellets in India, the increase of pellet proportion in blast furnace burden is inevitable. Therefore, the sinter basicity needs to be changed frequently if the pellets are being produced with low flux addition to control the blast furnace slag chemistry. However, looking into the sinter properties, there is a limitation to control sinter basicity and MgO content, which means addition of excess fluxes in sinter is limited. Therefore, addition of some part of fluxes into pellets becomes an essential countermeasure. Many studies have reported the effect of basicity and MgO on the properties of agglomerates, and the results show that adding a satisfactory amount of fluxes has improved the pellet properties [12,13,14,15,16]. In fluxed pellets, the bonding between the particles is achieved by the quantity of silicate melts formed during the induration that primarily depends on the amount of CaO and MgO content in the pellet. In fluxed pellets with a high amount of MgO, the formation of magnesio-ferrite and magnesio-silico ferrite takes place when MgO enters the magnetite lattice, while CaO reacts with silicate to form slag and with iron oxide to form calcium ferrite which allows crystal growth at substantially lower temperature. These slags help to bond the pellets to enhance the pellet strength [17,18,19]. An earlier work, reported by Sugiyama et al. and Lingtan et al., noted that increasing the MgO content in the form of magnesite/olivine increases the reducibility, and decreases the swelling because MgO increases the melting point of slag [20,21,22,23,24,25,26]. Dwarapudi et al. have reported the role of MgO and basicity on the pellet quality. It has been reported that by adding CaO in the form of limestone and by adding MgO in the different form (dolomite, pyroxenite, or magnesite), RDI is improved as basicity is increased, and by adding MgO, the swelling property is decreased because of the formation of low FeO slag that can resist reduction stress [17, 22, 23].
Most of the reported research were carried out either by varying the CaO or MgO content in pellet. However, there is limited information available about the influence of varying CaO and MgO content up to 3.7% and 2.4% respectively on the quality of pellets, which are important for maintaining the pellet quality. Moreover, the experimental trials are usually performed in laboratory, where the green pellets are first dried in an oven and the dried pellets are fired either in maffle furnace or rotary hearth furnace which are electrically heated either in absence or presence of air flow across the pellet bed, whereas the actual plant process are much different from the laboratory-based experimental trials. Few researchers have also reported the firing of green pellets in pot grate facility [27,28,29]. The pot grate is a pilot facility where the green pellets are fired according to a defined heating and cooling cycle (i.e., drying rate, heating rate, and cooling rate) which simulate the actual condition which occurs in a pellet induration machine. The advantage of pot grate over other heating method is that it helps in evaluating the effect of different ores, additives, and firing condition for fired pellet quality. It is important to note that there is no considerable literature reporting firing of laboratory prepared green pellets in induration furnace of pellet plant using Inconel baskets. This method is a novel way for firing the green pellets in absence of pellet pot facility to simulate the actual firing condition inside the induration machine in the plant. In the present work, an effort is made to develop pellet, by exploring the pellet chemistry at higher basicity and high CaO and MgO levels. Pellet properties were studied at two basicity (B2) levels, viz., 0.3 and 0.9, and varying the MgO content up to 2.0% and 2.4% by addition of limestone and olivine fluxes.
Table 1 shows the comparison between various available methods for firing of lab-prepared green pellets. Different pellet plants have different methods for firing of green pellets and most of the plants use muffle furnace. But the disadvantage of this method over pot grate is that the pellets inside muffle furnace are not subjected to the same condition as that in actual plant. To overcome this drawback, Inconel basket method is developed, in which pellets are fired inside the actual plant and thus the pellet quality is comparable to pellets produced in actual plant.
1.1 Experimental
1.1.1 Raw Materials
Material for the test work is collected directly from the grinding unit of the pellet plant to match the plant grinding condition. A representative sample post-grinding is collected, i.e., ground ore concentrate (GOC), and coning and quartering technique is conducted to get the composite sample for the test work. Blaine number of the composite sample measured was 3260 cm2/g. Along with GOC, bentonite (− 75 μm), limestone (− 10 mm), and olivine (− 5 mm) are used for preparing the green pellets. Limestone and olivine are ground separately in a laboratory ball mill to get the required fineness for pelletizing. GOC already has a carbon content of 1.1% as the plant has a dry co-grinding facility to grind coal and fluxes along with iron ore fines. Size analysis of GOC and other raw materials used is given in Table 2 and chemical analysis in Table 3.
1.1.2 Trial Plan
To study the effect of MgO content and basicity on pellet quality, pellets were prepared with two sets of basicity, i.e., 0.3 and 0.9 respectively, with varying MgO from 0.77 to 2.4%. Raw material mix proportion used for preparing green pellets with varying MgO contents along with basicity (CaO/MgO) and the chemical composition of green pellets are shown in Table 4. Ground ore concentrate as collected from plant is made as base 1 pellet batch whereas other pellet batches (i.e., base 2, trial 1, 2, and 3) were made by adding fluxes as per the plan to change the basicity and MgO contents.
1.1.3 Green Pellet Preparation
Green pellets were prepared from the mixture of ground ore concentrate, limestone, and olivine using a laboratory-scale pelletizing disc of 0.6 m in diameter and 0.2 m rim depth, rotational speed at 27 rpm and inclined at 45° horizontally. The green pellets were obtained at sizes of 10 to 12.5 mm in diameter by screening and the moisture of green pellets was about 8–9%. For all set of trials, bentonite dosage was fixed at 0.5% of dry ground ore concentrate. Bentonite acts as a binder. It is a water-soluble binder, which governs the green pellet strength. By varying the bentonite addition, green ball strength will also vary, which in turn will affect the fired pellet quality. Green pellets were tested for their drop strength, green compressive strength, and moisture content as shown in Table 5.
1.1.4 Pellet Firing Using Inconel Basket
In the absence of pellet pot grate facility, to simulate the firing condition of pellet plant indurating machine, the pellets are fired in pellet plant induration furnace using Inconel basket of size (100*100*100 mm) shown in Fig. 1. The steps for Inconel basket testing are shown in Fig. 2. The testing is done with an objective of evaluating new materials (like iron ore mix, different additives, binders, solid fuel etc.). The fired pellet obtained will be then tested for their chemical, physical, and high temperature properties. Based on the result, same changes can be then applied into the actual plant. Induration furnace consists of four zones, i.e., drying, preheating, firing, and cooling. Ambient air is supplied from cooling zone for creating oxidizing atmosphere inside the furnace. Hot air from firing zone is recirculated in drying and preheating zone to facilitate drying of moist pellets while the pallet car travels from drying to cooling zone. During this trial, five numbers of cubical Inconel baskets are filled with green pellets according to the experimental plan and are kept at the center of the pellet bed 100 mm above the hearth layer. Baskets are charged into the induration furnace, in a pallet car at the feeding station while the induration machine is running at a stable condition and the pallet car is marked for its tracking as shown in Fig. 3. Once the marked pallet car crosses the cooling zone of induration furnace, the machine is stopped in such a way that the marked car is placed in between the cooling zone and discharge bin hood. The baskets are removed from the car with the help of a shovel. The corresponding operating parameters of induration furnace during the trial are shown in Table 6.
1.1.5 Physical and Metallurgical Testing of Pellets
Fired pellets are tested for their cold compressive strength (CCS), porosity, swelling index, reducibility index (RI), and softening–melting characteristics. CCS and swelling index are tested as per IS 8625:1986 and IS 8624:1995 respectively. RI and porosity are tested as per TS000:2014 and ASTM D4404-10 respectively. Softening and melting test is conducted as per the procedure mentioned by Dwarapudi et al. [22]
2 Results and Discussions
2.1 Fact-Sage Studies
In this work, the ‘equilibrium’ module of the thermodynamic software, Fact-Sage (v6.2), is used to investigate the effect of flux type and dosage on melt formation and final phases in a pellet. Calculations are based on the principle of minimization of Gibbs free energy. Table 3 shows the composition of the pellet mixes used for thermodynamic simulations. The temperature of initial melt formation (TIM) and the amount of liquid melt formed during pelletizing are known to have a significant impact on the quality of pellets [30, 31] Hematite, silicate, and magnetite (in fluxed pellets) are the predominant phases in pellets and are formed as a result of several physicochemical reactions that take place during pelletizing. The relative amounts of these phases depend on various parameters such as the gangue content (CaO, MgO, SiO2, and Al2O3) of iron ore, the amount and type of flux added to the pellet mix, the amount of solid fuel used in the pellet mix, and the maximum temperature experienced by the pellets during induration. In Fact-Sage study the amount of melt phase formed within the pellet at peak induration temperature is determined and is correlated with the four-component basicity B4, i.e., (CaO + MgO)/(SiO2 + Al2O3). Figure 4 shows the relationship between the amount of melt at maximum temperature and the basicity of the pellet mix. It can be observed that the amount of melt increases with an increase in basicity B4 and this could be attributed to the increase in silicate phase with increase in pellet basicity [31]. And, with higher basicity and higher MgO level, the temperature of initial melt has also increased (Fig. 5). It is interesting to note that the amount of melt is higher even at higher MgO levels (> 2%) because MgO is known to increase the temperature of melt formation [30].
2.2 Microstructural Phase Quantification
Optical images of fired pellets (pellets A and B) with basicity 0.3 and MgO 1.4 and 2.4 MgO respectively are shown in Fig. 6. The phase quantification of fired pellets revealed that hematite, silicate melt, magnesioferrite, and unreacted olivine are the major phases, as shown in Table 7. It is observed that the porosity of the pellets increased with increase in MgO content. The increase in porosity is due to the formation of more high-temperature magnesio ferrite slag bonds. It was also inferred from microstructural quantification that magnesioferrite content in the pellet increased with increase in MgO content in fired pellets. The increased magnesioferrite may be attributed to the more availability of MgO to react with Fe2O3 [30].
2.3 Cold Compressive Strength
Cold compressive strength indicates the ability of pellets to withstand the load during their transportation, storage, and load of burden material in blast furnaces. Figure 7 shows that CCS of pellets with different chemistries are above the acceptable limit for the blast furnace. Pellet CCS shows a good correlation with the amount of melt formed in the pellets at peak firing temperature (1320 °C) and it is inversely proportional to the porosity of pellets as shown in Figs. 7 and 8. Pellet CCS increases with an increase of melt phase up to 10–12% and with further increase in melt phase it decreases. This could be attributed to formation of more slag bonds as compared to oxide–oxide recrystallization bonds as pellet matrix get weakens with the formation of excess melt and results in lower strength [30]. Figure 8 shows the pellet CCS and porosity as function of TIM. With increase in the TIM, the porosity is found to increase, and CCS decreases. This could be attributed to the delay in the melt formation as the pores inside the pellet are not fully filled with melt phase leading to high amount of open porosity leading to lower strength.
2.4 Swelling Index
Swelling of iron ore pellets takes place during their reduction from hematite to magnetite and wustite in the blast furnace. It can be mainly attributed to the increased volume requirements for the anisotropic growth of magnetite (111) planes parallel to the hematite (0001) planes [32]. Swelling is related to the strength of the slag phase to resist the stress developed in individual oxide particles during the reduction process. A high melting point slag would produce enough bonding strength to limit swelling and a low melting point slag enhances swelling. Figure 9 shows the swelling index of pellets with different chemistries. At basicity of 0.3 and 0.9, swelling index decreases with increases in MgO content and remains within the acceptable control limit. Low swelling index of pellet with an increase in MgO content can be compared with the earlier studies of Onoda et al. that have reported that the addition of MgO to pellets increases the melting point of the slag or silicate melt formed between the oxide particles [32], but higher porosity, on the other hand, increases the swelling due to the more open structure of pellets for the reducing gas as observed in trial 3.
2.5 Reducibility Index
Reducibility of the pellet is one of the important parameters along with the other properties. It is defined as the measure of the ability of pellet for transferring oxygen during indirect reduction in the blast furnace. By improving the reducibility of pellets, indirect reduction in blast furnace could be improved leading to better performance of blast furnace. Figure 10 shows the effect of basicity and MgO on the reducibility of pellets. Pellets with basicity 0.3 have shown an increase in reducibility index with the increase in MgO content. Pellets with basicity 0.9 have also exhibited high reducibility index but further increase in MgO content from 2 to 2.4% has lowered the reducibility index. This could be attributed to the higher TIM of trial 2 pellets than the trial 3 pellets, as showed in Fig. 5. During reduction process, the slag got soften and retards the reduction process by resisting the reducing gas flow within the pellet. Pellet with high MgO content, forms high melting point silicate melt between iron oxide grains which does not soften at reduction temperatures and keeps the pores open for reducing gas thereby enhancing reduction could be attributed to this improved reducibility of pellets [30].
2.6 Softening and Melting Characteristics
Softening and melting test is used to evaluate the performance of ferrous burden in the cohesive zone of a blast furnace. Softening and melting properties are affected by low melting point liquidus phase that is formed between the wustite and slag phase during reduction [30]. Figure 11 shows the softening and melting temperatures of pellets with different chemistries. Pellets with basicity 0.9 exhibited superior softening temperatures in the range of 1115 to 1121 °C whereas pellets with basicity 0.3 have shown relatively lower softening temperature in the range of 1072 to 1114 °C. This could be due to fact that MgO increases the melting point of slag. During reduction, MgO forms a solid solution with wustite called magnesio-wustite of higher melting point [33] that also helps in improving the softening–melting characteristics This could be because slag that formed at higher temperatures in the pellet also softens at higher temperatures during the reduction in the blast furnace. Inferior softening melting characteristics of trial 1 pellets could be attributed to very high silica content in the pellets, resulting from higher olivine addition for keeping the MgO at 2.4%. The excess silica, during reduction, reacts with Fe2+ to form a low melting point phase fayalite (Fe2SiO4) that melts at 1175 °C, leading to lower S-M temperatures [12].
3 Conclusion
This work has been undertaken to develop suitable chemistry regime to achieve good high-temperature metallurgical properties, by exploring the pellet chemistry at higher basicity and high MgO levels. Pellet properties like CCS, swelling index, and reducibility index are studied at two basicity (B2) levels, 0.3 and 0.9 CaO/SiO2, varying the MgO at 0.77% and 2.4%. Pellets are fired in the plant indurating machine in Inconel baskets. The following conclusions can be drawn from the experiment and test results obtained:
-
1.
CCS of both low and high basicity pellets are within the acceptable limit for the blast furnace. High fluxed pellets with 0.9 basicity have better strength as compared to pellets with 0.3 basicity due to more melt phase formation.
-
2.
Porosity of the high basicity pellets did not drop, despite higher melt phase, due to the presence of MgO which is known to increase the temperature of melt formation, TIM. High basicity pellets with high MgO exhibited high TIM and high porosity.
-
3.
MgO in pellet considerably reduced the swelling tendency of pellets due to the formation of high melting point slag that gives enough bond strength to withstand the reduction stresses.
-
4.
MgO addition considerably improved the reducibility of the pellets. The formation of less amount of liquid slag due to the presence of MgO could be attributed to this improved reducibility of pellets.
-
5.
High basicity and high MgO pellets exhibited high softening temperatures compared to low basicity pellet as presence of MgO results in the formation of high melting point slag and magnesio-wustite phase during reduction.
-
6.
This operational practice of using Inconel basket is now established and it has become a part of standard operating procedure for testing of any new material in pellet plant before its usage in actual plant.
References
Meyer K (1980) Pelletizing of Iron Ores. Springer-Verlag, Berlin, Heidelberg, Verlag Stahleisen mBH, Dusseldorf
Panigraphy SC, Jena BC, Rigaud M (1990) Characterization of bonding and crystalline phases in fluxed pellets using peat moss and bentonite as binders. Metall Trans B 21:463–474
Adrian IP, Borje B (1977) Experiences with dolomite fluxed pellets by Lkab. Proceedings of Ironmaking. New York: AIME, 366−383
Chizhikova VM, Vainshtein RM (2003) Composition of iron-ore pellets with different types of additives. Metallurgist 47(9):349–352
Bleifuss RL, Goetzman HE, Lopez RD, Beckman CA (1986) Development of Minnesota taconite fluxed pellets as an improved blast-furnace material, Proceedings of AIME 47th Annual Mining Symposium. New York: AIME, 1−31
Osamu T, Takeshi S, Mamoru O, Isao F (1980) Effect of MgO-component on various metallurgical properties of self-fluxed pellets. J Iron and Steel Ins Japan 66(13):1840–1849
Pal J, Ghoari S, Ammasi A, Hota SK, Koranne VM, Venugopalan T (2016) Improving reducibility of iron ore pellets by optimization of physical parameters. J Min Metall Sect B-Metall
Yu-zhu Z, Ming-shan C, Li X, Wen T (2005) The influence of MgO and basicity on the viscosity of BF slag. J Mater Metall 4(4):253–256
EL-Geassy AA, Nasr MI, Khedr MH, Abdel-Halim KS (2004) Reduction behaviour of iron ore fluxed pellets under load at 1 023−1 273 K . ISIJ International 44(3): 462−469
Guan X, Hou H, Tao OU (2001) The way of heightening MgO content of sintering and the effect of BF. Sinter Pellet 26(2): 1−5
Anil K (2005) Biswas, 1st edn. Principles of blast furnace ironmaking, SBA Publications, Kolkata, India
Nutter SM, Li K (1991) Effect of composition and structure on fluxed pellet reduction kinetics. Transact Iron and Steel Soc AIME 12:71–83
Xin J, Gang-sheng WU (2006) Effect of MgO on sintering process and metallurgical properties of sinter. Iron and Steel 41(3):8−12
Agrawal A, Gavel DJ, Shaik MB, Dwarapudi S Paul I (2021) Optimum pellet basicity desirable for blast furnace operation. J Inst Eng India Ser D 102(1):87–93. https://doi.org/10.1007/s40033-021-00258-1
Yong-bin Y, Gui-xiang H, Tao J, Zhu-chong H (2007) Application of organic binder as substitutes for bentonite in pellet preparation. Journal of Central South University: Science and Technology 38(5):851–857
Feng X, Zhang Y, Li Z (2004) The effect of basicity on sintering strength in the condition of low-Si. Sinter Pellet29(2): 9−12
Dwarapudi S, Ghosh TK, Shankar A, Tathavadkar V, Bhattacharjee D, Venugopal R (2011) Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. Int J Miner Process 99:43–53
Yang Z, Liu Z, Chu M, Gao L, Feng C, Tang J (2021) Effect of basicity on metallurgical properties of fluxed pellets with high MgO content. ISIJ Int 61(5):1431–1438
Wang R, Zhang J, Liu Z, Li Y, Chenyang Xu (2021) Effect of CaO and MgO additives on the compressive strength of pellets: exploration on the decisive stage during induration. Powder Technol 390:496–503
Sugiyama T, Shirouchi S, Tsuchi O, Onoda M, Fujta I (1983) High temperature reduction and softening properties of pellets with magnesite. Trans ISIJ 23:153–160
Lingtan K, Yang L, Lu W-K (1983) The role of magnesia in iron ore pellets. Scand J Metall 4:166–176
Dwarapudi S, Ghosh TK, Shankar A, Tathavadkar V, Bhattacharjee D, Venugopal R (2010) Effect of pyroxenite flux on the quality and microstructure of hematite pellets. Int J of Miner Process 96:45–53
Dwarapudi S, Ghosh TK, Tathavadkar V, Denys MB, Bhattacharjee D, Venugopal R (2012) Effect of MgO in the form of magnesite on the quality and microstructure of hematite pellets. Int J Miner Process 112–113:55–62
Mandal AK, Sinha OP (2015) Characterization of fluxed iron ore pellets as compared to feed material for blast furnace. J Progress Res Chem 2(1)
Umadevi T, Kumar A, Karthik P, Srinidhi R, Manjini S (2018) Characterisation studies on swelling behaviour of iron ore pellets. Ironmaking Steelmaking 45:157–165
Meyer M, Lagoeiro LE, Graça LM, Silva CJ (2016) Phase and microstructural characterization of iron ore pellet and their relation with cold crushing strength test. Min Process Extract Metall, Rev 37:295–304
Tommasi L, Tiburzio S (2015) Danieli New Pot Grate Testing Facilities: a case study – Ahmsa Pellet Plant p. 106–116. In: 45º Ironmaking / 16º Iron Ore / 3º Agglomeration, Rio de Janeiro
Wendling SS, Nascimento RC (2017) Comparative study of pellets fired in pot grate and grate kiln pilot furnaces. Miner Metall Process 34(2):84–90
Praes GE, de Arruda JD, Lemos LR, Tavares RP (2019) Assessment of iron ore pellets production using two charcoals with different content of materials volatile replacing partially anthracite fines. J Mater Res Technol 8(1):1150–1160
Onoda M, Tsuchiya O, Sugiyama T, Fujita I (1980) Quality improvements of lime fluxed pellets. Proc. of ISS-AIME 40th Iron Making Conf., Toronto, Ontario Canada, pp. 286–298
Dwarapudi S, Sekhar C, Paul I, Modi K, Pal AR, Chakraborty U, Das BK (2017) Effect of fluxing agents on the quality and microstructure of hematite pellets. Int J Metallurgic Eng 6(1): 18–30
Frazer FW, Westenberger H, Boss KH, Thumm W (1975) The relation between basicity and swelling on reduction of iron ore pellets. Int J Miner Process 2:353–365
Narita K, Maekawa M, Shigaki I (1977) On the permeability resistance of pellets containing MgO in the softening and melting zone of blast furnace. Trans ISIJ 18:712–720
Acknowledgements
We would like to acknowledge the entire pellet plant operation, mechanical and scientific services team of Tata Steel Jamshedpur for allowing us to conduct operational trials. Last but not the least, we would like to thank Tata Steel Limited for allowing us to carry out the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hota, S.K., Agrawal, A., Sekhar, C. et al. Optimizing the Metallurgical Properties of Pellets Through Operational Trial Using Inconel Basket. Mining, Metallurgy & Exploration 39, 1641–1649 (2022). https://doi.org/10.1007/s42461-022-00619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00619-8