Abstract
Application of biochar technology in the remediation of organic contaminated soils has drawn growing interest in recent years. In this study, sorption and degradation of two typical neonicotinoid insecticides, imidacloprid (IMI) and clothianidin (CLO) in Chinese typical paddy soil and red soil amended with six kinds of biochars were investigated. The results showed that surface area (SA), pH, total organic carbon and dissolved organic carbon (DOC) of the two soils all increased after biochar amendment, while H/C decreased. With biochar pyrolyzing temperature (PT) increasing from 300 °C to 700 °C, the sorption of the two insecticides on biochar–soil mixtures increased by more than 4.3-fold, due to the increasing SA and decreasing H/C. The acidic pH of the two tested soils also favored the enhanced sorption of the insecticides by removing the ash on biochar. The amendment of low-PT (300 °C) biochar promoted the biodegradation of IMI and CLO by 11.3–41.9% via providing more DOC and available N for microorganisms, while inhibiting the chemical degradation. Oppositely, the high-PT (500–700 °C) biochars inhibited the biodegradation of the insecticides by decreasing their bioavailability and promoted the chemical degradation by providing mineral active groups, and generating ·OH and other free radicals. In addition, soil type also affected the effects of biochar remediation. The highest 60-day degradation extent was achieved for CLO (90.5%) and IMI (81.4%) in paddy soil by adding biochar derived from pig manure at 700 °C PT. In summary, the effect of biochar on the fate of organic contaminants in soil is a comprehensive result involving several processes and a systematic study considering the type and property of biochar and soil is needed to optimize biochar technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides provide guarantee for stable and low-cost supplies of grains and vegetables to the human society, however, intemperate application of pesticides has caused extensive pollution in the environment and adverse effects to non-target organisms (Hallmann et al. 2014). How to alleviate the adverse effects has drawn much attention. The fate of pesticides is a comprehensive result depending on different environmental processes, including sorption/desorption, transport (leaching, runoff, volatilization and plant uptake), and degradation (biodegradation, photodegradation and chemical degradation) (Ren et al. 2017). Among these processes, sorption is a basic process that begins immediately upon pesticides entering into the soil, which changes pesticide combination state and bioavailability, and hence affects the magnitude of other processes, such as transport, bioaccumulation and ecotoxicological impacts on organisms (Yavari et al. 2015). Accordingly, there are two ways to reduce the adverse effects (e.g., toxicity and bioaccumulation potentials) of pesticides, i.e., reducing their bioavailability via enhanced sorption or accelerating degradation (Sohi 2010; Ren et al. 2016a).
Neonicotinoids are a class of increasingly used insecticides in the world (Jeschke et al. 2011; Zhang et al. 2016), and the adverse effects of neonicotinoids to non-target organisms, e.g., insectivorous birds, pollinators, and aquatic invertebrates have attracted increasing concerning in recent years (Kessler et al. 2015; Rundlof et al. 2015). Due to their high water solubility, the potential of neonicotinoids to leach into surface runoff or groundwater is one of the major concerns regarding the risk of large-scale application of neonicotinoids in farmlands (Botias et al. 2015). Imidacloprid (IMI) and clothianidin (CLO) are two commonly detected neonicotinoids in surface runoff or groundwater from agricultural areas as a result of their wide applications (Anderson et al. 2015). Hence, development of effective approaches to mitigate the ecological risks of these neonicotinoids in farmland soils is clearly needed.
Biochar has attracted increasing interest as a novel soil amendment in recent 15 years. It has been shown that biochar is a promising adsorbent for various kinds of pollutants, due to the great surface area (SA), high carbon content and aromatic intensity of biochar (Qiang et al. 2019; Lun et al. 2020). Many studies have been conducted on pesticide sorption by biochar (Zhang et al. 2013; Trigo et al. 2014). The sorption process has been proposed to occur via hydrophobic partitioning, pore-filling, n/π–π electron donor–acceptor (EDA) interactions, H-bonding, electrostatic attraction and cationic bridging (Lattao et al. 2014; Sigmund et al. 2020). For polar pesticides, the specific interactions like H-bonding and n/π–π EDA, electrostatic attraction and cationic bridging may contribute much besides hydrophobic partitioning, and hence the optimum biochar for the sorption of polar pesticides [usually biochar derived from the medium pyrolysis temperature (PT)] may be different from that for hydrophobic organic compounds (usually high-PT biochar) (Ren et al. 2016a; Zhang et al. 2018a). Biochar amendment can increase the total soil sorption capacity for pesticides, which was mainly ascribed to the increased SA and total organic carbon (TOC) content (Trigo et al. 2014). The enhanced sorption by biochar amendment in soil leads to reduced leaching and physical and biological availabilities of pesticides (Chai et al. 2012; Khorram et al. 2016), which greatly mitigate the risk of pesticides to the environment and ecosystem. Only a few studies have reported the sorption of neonicotinoids in biochar-amended soil (Jin et al. 2016; Mandal et al. 2017), and it was found that biochar amendment increased the sorption of IMI and thiacloprid (THI) in soils (Jin et al. 2016; Zhang et al. 2018b). The sorption mechanisms and the sequential effects of the sorption on the fate of neonicotinoids in soil are still yet to be explored.
Moreover, biochar amendment has been found to be able to improve soil micro-ecosystem in ways of both total density and diversity due to the porous structure and the dissolved organic matter (DOM) and trace nutrient elements released from biochar (Ren et al. 2016a). The prosperity of microorganisms in turn favors the biodegradation of pesticides. Tong et al. (2014) reported that a rape-straw-derived biochar could improve the microbial dechlorination of pentachlorophenol in contaminated soils. However, enhanced biodegradation of pesticides in biochar-amended soils does not usually occur due to that the enhanced sorption by biochar amendment lowers the bioavailability of pesticides (Zhang et al. 2013; Oleszczuk et al. 2014).
More recently, biochar was reported to be able to enhance chemical degradation of pesticides, which endows biochar an extra feature to be a promising remediation agent for pesticide contamination (Zhang et al. 2018b). Mechanism studies using biochar suspensions revealed that biochar is able to catalyze hydrolysis of carbaryl, atrazine and THI via the combined effects of elevated pH, dissolved transition metal ions, and metal oxide minerals on biochar surface (Zhang et al. 2013, 2018b). Furthermore, environmentally persistent free radicals (EPFRs) in biochars have attracted considerable attention, and it was reported that EPFRs can accelerate the oxidation of organic pollutants by activating small free radicals such as superoxide radical anion and hydroxyl radical (·OH), which can breakdown organic chemicals (Fang et al. 2014; Yang et al. 2016).
In summary, biochar amendment can influence the fate of a pesticide via several processes. Which process dominates depends on biochar characteristics, soil properties and pesticide structures and needs a comprehensive study for a specific case. Moreover, upon entering the soil, the structure and properties of biochar change due to the interactions with soil constitutes (Sun et al. 2013; Ren et al. 2018a). Soil constitutes and soil-dissolved substances could compete for binding sites or block pores of biochar, making biochar surface less available for pesticides (Ren et al. 2016a). The effects of soil type on biochar and consequent effects on pesticide fates have seldom been studied especially for acid soil.
Hence, a comprehensive study on the influences of biochar amendment on the fate of IMI and CLO in paddy soil (PS) and red soil (RS) was conducted to obtain more complete knowledge of the effect of the interactions between biochars and soils on the fate of polar pesticides. The aims of this paper were to (1) investigate the changes in soil physico-chemical properties due to the amendment of various biochars, (2) evaluate the sorption of IMI and CLO and the mechanism in PS and RS with or without biochars, and (3) evaluate the effect and mechanism of biochar amendment to the degradation of IMI and CLO in the two soils. The results gained in this paper will give deep insight into the effect of biochar amendment on the fate of polar pesticides in soil, which is important information for the design of biochar remediation technology for pesticide contamination.
2 Materials and methods
2.1 Chemicals
IMI (96%) and CLO (97%) were purchased from Beijing J & K Technology (Beijing, China). The structures and selected properties of the two pesticides are shown in Table S1 in the Supplementary Information (SI). The stock solutions of IMI and CLO were prepared in acetonitrile and stored at 4 °C.
2.2 Biochar and soil preparation and characterization
Biochars used in this study were derived by charring pig manure and maize straw at 300, 500 and 700 °C for 4 h under oxygen-limited conditions, respectively. The detailed preparation procedure and physico-chemical properties of the biochars were all given in our previous paper (Zhang et al. 2018a). For convenience, this information could also be referred in Text S1 and Table S2. The produced biochars are recorded as Mn (maize straw) and Pn (pig manure), with n = 3, 5, 7 indicating PTs (300, 500 and 700 °C).
The two soils, PS and RS used in this paper are Chinese typical soil types and were collected at 0–20 cm depth from farmlands, respectively. After passing through a 2 mm sieve, fresh PS and RS samples were separated into two parts: one for physico-chemical properties tests and sorption experiments, and another for degradation experiments. The physico-chemical properties of PS and RS samples are shown in Table S3. These two soils were both acidic (pH 3.8 and 4.4) with relatively less organic matter content (< 0.67%).
Biochars were separately added into PS and RS at the quality fraction of 2% (w/w) with sufficient mixing. An element analyzer (ElementarVarioEL, Germany) was employed to analyze the bulk elemental composition (C, H and N) of biochars, soils and their mixtures. Ash content of the samples was measured by the residual weight after heating the samples at 750 °C for 6 h, and the oxygen (O) content was calculated by mass difference (Keiluweit et al. 2010). An SA analyzer was used to analyze the SA and pore volume of biochars, soils and their mixtures (Quantachrome NOVA 2200e, USA). The pH, electric conductivity (EC), available N, total soluble N (TSN) and dissolved organic carbon (DOC) were determined according to Jones et al.’s methods (Jones et al. 2011). For \({\text{NH}}_{4}^{+}\)-N and \({\text{NO}}_{3}^{-}\)-N determinations, KCl extraction–indophenol blue method and dual-wavelength ultraviolet spectrophotometry method were used, respectively (Wang et al. 2017; Yokoyama et al. 2017).
2.3 Sorption experiments
The sorption isotherms of IMI and CLO on PS and RS with or without biochar amendment were determined by the batch sorption experiments in triplicates. An aliquot of 0.10 g biochar was weighted into a 40.0 mL vial, and an aliquot of 2.0 g soil with or without biochar was weighed into a 12.0 mL vial. Then, 10.0 mL or 40.0 mL of a background solution containing 5.0 mM CaCl2 and 200 mg/L HgCl2 were added in the vials. Screw-lined caps were used to seal the vials, and the suspension was pre-equilibrated by being shaken at 200 rpm and 25 ± 1 °C for 2.0 h. Then, designated amounts of 5000 mg/L IMI or CLO stock solutions were spiked into the vials. Seven initial solution concentrations ranging from 0.5 to 24.0 mg/L were used. The vials were placed on a shaking table oscillated with 200 rpm at 25±1 °C for 48 h. After equilibration, the vials were centrifuged at 3500 rpm for 10 min, and then the supernatant was filtered through 0.45 μm PTFE syringe filters and stored at 4 °C for further analysis using the high-performance liquid chromatography (HPLC). Before being analyzed by HPLC, the external standards were also filtered to correct the possible solute loss due to filtration (less than 3%). The analysis method is described in Text S2.
2.4 Degradation experiment
Degradations of IMI and CLO in sterile and unsterile soil slurries with or without biochar were examined in triplicates. An aliquot of 10.0 g (calculated as dry weight) soils with or without biochars were weighed into 20.0 mL vials, and sterilized deionized water was added to adjust water content with 60% of the soil maximum water-holding capacity. As for abiotic degradation experiment, soil samples were sterilized by autoclaving (121 °C for 30 min) three times continuously (Han et al. 2017) and sterilized deionized water containing 10 mg/L HgCl2 was used to further inhibit microorganism growth (Ren et al. 2016a). IMI and CLO in sterilized deionized water were, respectively, spiked into the vials to obtain an initial concentration of 4.95 ± 0.15 mg/kg. After being shaken for 1 min, the treated samples were put into a culture incubator. The experiment lasted for 60 days in the dark at 25 ± 1 °C and the vials were weighed every 10 days to monitor water loss and sterilized deionized water was supplemented if needed. Samples were sacrificed on 0, 2, 5, 10, 20, 40 and 60 days, and then freeze-dried and stored at − 20 °C until pretreatment for HPLC analysis. The preparation and analysis methods are described in Text S2. The above procedure gave recoveries > 90% for IMI and CLO in different soil slurries with or without biochar.
To investigate catalytic mechanisms of biochar to the chemical degradation of IMI and CLO, the degradation experiments in biochar leachates/suspensions were designed: (1) degradation in deionized water at neutral and alkaline pH (6.5 and 11.0), according to the measured pH values of biochar suspensions; (2) degradation in suspensions of 0.1 g biochar or biochar ash in 40 mL of deionized water with the original solution pH and the solution pH adjusted to the neutral level (6.5 ± 0.2), biochar ash were prepared at 300, 500 and 700 °C under aerobic conditions; (3) degradation in suspensions of 0.1 g HCl/HF-deashed biochars obtained according to the method of Zhang et al. (2013); (4) degradation in biochar leachate with the original solution pH and the solution pH adjusted to neutral (6.5 ± 0.2) (Zhang et al. 2018b). IMI or CLO stock solutions were spiked into each vial, respectively, to reach a concentration of 5.0 mg/L. These vials were capped and shaken at 200 rpm for 10 d (25 ± 1 °C in the dark). Concentrations of IMI and CLO on biochar particles and in liquid phase were then measured (Text S2).
2.5 Data analyses
Sorption isotherm data were analyzed using the Freundlich model: \(\text{ln }{C}_{\text{s}}=\text{ln }{K}_{\text{f}}+n\text{ ln}{C}_{\text{e}}\), where Cs and Ce are the equilibrium concentrations of the sorbate in solid-phase (mg/kg) and liquid-phase (mg/L), respectively. Kf ((mg/kg)(mg/L) − n) is the Freundlich sorption affinity, and n is the isotherm nonlinearity factor. To compare the sorption capacities of biochar, soil and their mixture samples, the sorption distribution coefficient (Kd) of IMI and CLO was calculated at Ce = 0.05 mg/L using \({K}_{\text{d}}={C}_{\text{s}}/{C}_{\text{e}}\). Degradation parameters of IMI and CLO in the soils and biochar–soil mixtures were computed using the first-order kinetic model: \({C}_{\text{t}}={C}_{0}{\text{e}}^{-k t}\), where C0 is the initial concentration of IMI and CLO (mg/kg), Ct is the concentration of IMI and CLO (mg/kg) at time t (d), and k is the degradation rate constant (d−1) obtained as the slope of the logarithmic form of the above model. The half-life (t1/2) was computed from the rate constant (t1/2 = 0.693/k).
3 Results and discussion
3.1 Characterization of biochar–soil mixtures
Results of the characterization of biochar–soil mixtures are shown in Table 1. The pH, electrical conductivity (EC) and TOC of PS and RS all increased after biochar addition, while H/C ratio decreased, which is in accordance with the results of Martin et al. (2012). Concentrations of DOC and TSN increased with the addition of 300-PT biochars in PS and RS, while the addition of 700-PT biochars led to opposite results. This indicated that 300-PT biochars released higher concentrations of DOC and TSN, while 700-PT biochars fixed DOC and TSN due to their strong sorption ability (Mukherjee and Zimmerman 2013). Available N content decreased in biochar–soil mixtures except PB3 and the declining extent was more obvious with high-PT biochars, which was mainly ascribed to the varied sorption capacity of biochars (Table S3). The SA of biochar–soil mixtures increased after the addition of 500-PT and 700-PT biochars, which is consistent with the results of Jin et al. (2016) and Zhang et al. (2018b).
3.2 Sorption of IMI and CLO to biochars, soils and their mixtures
It could be seen from Table 2 that sorption isotherms of IMI and CLO on biochars and PS and RS with or without biochars could be well described by the Freundlich equation. The sorption affinity (Kf) on biochars increased with the PTs of biochar, being 188–4460 (mg/kg)(mg/L) − n and 50.0–2090 (mg/kg)(mg/L) − n for biochars derived from maize straw (Mns) and pig manure (Pns), respectively. These values are similar with those in the literature, for example, Mandal and Singh (2017) reported a Kf value of IMI of 3410 (mg/kg)(mg/L) − n on a biochar obtained by pyrolyzing rice straw at 600 °C. Kf of IMI and CLO on the biochar–soil mixtures also increased with the increasing PTs of biochar, which were 7.57–48.9 (mg/kg)(mg/L) − n for IMI and 3.65–43.3 (mg/kg)(mg/L) − n for CLO, respectively. The sorption affinities of PS to IMI and CLO were greater than those of RS. Compared to the bare soil, the Kf values of IMI were enhanced by 5.92- to 51.9-fold with addition of 2% M3-7 and 3.09- to 23.9-fold with addition of 2% P3-7, and those of CLO were promoted by 2.75- to 39.3-fold by 2% M3-7 and 1.62- to 19.8-fold by 2% P3-7, respectively. This indicated that biochar amendment greatly enhanced soil sorption capacities for IMI and CLO. Moreover, the enhancement on IMI and CLO sorption in biochar-amended soil increased with the PTs of biochars, which is consistent with the results gained from biochars derived from rice straw, wheat straw and swine manure at 300, 450 and 600 °C (Jin et al. 2016).
To compare the sorption capacities of various biochar–soil mixtures, the sorption distribution coefficient (Kd) of IMI and CLO was calculated at Ce = 0.05 mg/L (Table 2). Similar to Kf, the enhancements of Kd were gained after biochar amendment. The Kd values of IMI and CLO for soils amended with low-PT (300 °C) biochars were obviously lower than those for soils with high-PT (500–700 °C) biochars, which indicated that biochars produced at high-PTs were more powerful adsorbents for IMI and CLO, being consistent with other studies (Zhang et al. 2018a; Khorram et al. 2016). Our previous study has proved that the sorption of IMI and CLO on bare soils was mainly governed by soil organic carbon content via the hydrophobic partitioning and specific interactions like H-bonding (Zhang et al. 2018c). However, in this paper, the Kd values (Ce = 0.05 mg/L) of IMI and CLO on biochar–soil mixtures had a significant positive relationship with SA and H/C (Table 3 and Fig. S1). This indicated that SA and aromaticity (as indicated by H/C) controlled the sorption of IMI and CLO on biochar–soil mixtures. This suggested that the p/π–π EDA interactions between the aromatic ring (or the lone electron pairs on O/N atoms) in pesticide molecules (Table S1) and the aromatic structure in biochar may contribute to the adsorption of IMI and CLO on biochars and biochar–soil mixtures. Additionally, the difference in Kd among various biochar–soil mixtures was smaller than those among various biochars, possibly due to the low dosage of biochar as well as the interactions between biochar and soil.
To further elucidate the interaction between soil and biochar on the sorption of IMI and CLO, Kd values (Ce = 0.05) between the experimental values and predicted values were compared (\({K}_{\text{d}}^{\prime}\), Table 2). The experimental Kd values of IMI and CLO were greater than predicted \({K}_{\text{d}}^{\prime}\) for 300-PT biochar–soil mixtures, while their predicted \({K}_{\text{d}}^{\prime}\) were higher than the experimental Kd for 700-PT biochar–soil mixtures especially for M7 with a greater SA. These results indicated that soil and biochar constituents did not exist independently. Due to the great OC content, aromatic intensity, and SA, 700-PT biochars show stronger adsorption affinities for DOC and inorganic salts (Ren et al. 2018a). The loss of DOC and available N in 700-PT biochar–soil mixtures observed in this current study confirmed the interactions of soil constitutes and 700-PT biochar (Table 1), and these interactions competed for the surface sorption sites or blocked biochar meso- and micro-pores (Ren et al. 2018b), thus decreasing available sorption sites for IMI and CLO. Moreover, the attachment of soil mineral particles could also decrease the accessibility of micro-pores and inner sorption sites (Yavari et al. 2015; Ren et al. 2016a). But for the soils amended with 300-PT biochars, the available N and DOC increased due to the release from the biochar, which may change the biochars’ surface properties and improve the sorption of IMI and CLO by exposing the interior sorption sites (Zhang et al. 2013, 2018a). In the acidic PS and RS, the surface ash of biochar could be cleared up in the acidic conditions, and more micro-pores and inner sorption sites could be exposed to sorbate (Zhang et al. 2013), which resulted in a greater experimental Kd of IMI and CLO than predicted \({K}_{\text{d}}^{\prime}\) for 300-PT biochar–soil mixtures. Thus, although the sorption capacity of 700-PT biochar for IMI and CLO was decreased by the interactions with soil, the biochar amendment increased their sorption in PS and RS, and the enhancement increased with biochar PTs. The enhanced sorption of IMI and CLO in biochar-amended soils could play an important role in their immobilization in acidic soils. However, it should be noted that the differences in Kd of the two insecticides between soils amended with 700-PT biochars and those with 500-PT biochars were not as great as those between 500-PT biochars and 300-PT biochars, and hence, from the view of energy saving, 500-PT biochars are the best option for adsorbents.
3.3 Degradation of IMI and CLO in soils and biochar–soil mixtures
The degradation of IMI and CLO were examined in soil slurries with or without biochars under sterile and unsterile conditions to clarify the effects of biochar on chemical and biological degradations of IMI and CLO (Table 4). The 60-day removal rates of IMI and CLO in unsterile bare PS were higher than the ones in bare RS, being 42.3% and 53.4% in PS for the two insecticides, respectively. All the biochar amendments enhanced k value of IMI in the PS. The removal rate of IMI in PS amended with biochars increased in the order of P7 > P5 ≈ M5 ≈ M7 ≈ P3 ≈ M3 for sterile treatments, and P7 > M5 ≈ M3 ≈ M7 ≈ P5 ≈ P3 for unsterile ones. The IMI were removed by 68.5% and 81.4% in PS amended with P7 under sterile and unsterile conditions, being significantly higher than other biochar treatments (p < 0.05). There was a similar trend for the removal rates of CLO with IMI in biochar-PS mixtures, however, the removals of CLO were much higher, being up to 90.5% in P7 amended unsterile PS. For RS, the amendment of P7 also had higher removal rates of IMI and CLO than the treatments of other five biochars both under the sterile and unsterile conditions. Furthermore, the k values of IMI and CLO in the unsterile soils were all greater than those in the sterile soils, and correspondingly, their removal rates under the unsterile condition were also greater than those under the sterile condition. This suggests that IMI and CLO degradation in soils could occur via both biotic and abiotic processes. In the unsterile soils, the chemical degradation and biological degradation coexisted, and chemical degradation contributed more than biological degradation.
3.4 Degradation of IMI and CLO in biochar suspensions and insight into effects of biochar on chemical degradation
The chemical degradation is the dominating pathway for IMI and CLO removals in soils, and biochar amendments could enhance their chemical degradation. To investigate the mechanisms for biochar enhanced chemical degradation of IMI and CLO, the degradation of the two insecticides in various biochar solutions and suspensions were examined. IMI and CLO degraded greatly in the biochar suspension (Table 5). The 10-day degradation percentages of IMI in biochar suspension ranged from 16.7% to 53.5% for Mns and 13.9–45.0% for Pns, and those of CLO ranged from 12.5% to 50.2% for Mns and 18.6–73.9% for Pns, respectively. Greater degradation percentages of IMI and CLO were acquired in suspensions with 700-PT biochar. The improvement in insecticide degradation could first be ascribed to the enhanced pH, which was confirmed by the results that the IMI and CLO degradation percentages in background solution at pH 6.5 were 1.16% and 2.52%, and pH 11.0 (the pH of biochar ash suspension with M7) for 45.7% and 22.3%, respectively. The hydrolysis is a dominating pathway of chemical degradation of IMI and CLO (Liu et al. 2006), and OH– can catalyze hydrolysis (Morrissey et al. 2015). However, in this paper, the pH values of PS and RS amended with high-PT biochars were still acidic or neutral (pH < 6.8, Table 1) due to the great buffer capacity of soil, and hence, elevated pH could not explain the enhanced chemical degradations of the two insecticides in the biochar-amended soils. To eliminate the effects of pH, the pH of biochar suspensions was adjusted to 6.5. At this neutral pH, the degradation percentages of IMI and CLO declined obviously, but still remained at levels higher than those in deionized water with the same pH. This indicates that factors other than pH were included in the catalytic degradation of IMI and CLO. In biochar leachate with pH adjusted to neutral, the degradation percentages of IMI had no significant differences among the treatments (all below 3.5%) except those of CLO having a percentage degradation of 5.0–10.0%. This suggests that the dissolved metal ions might contribute to the chemical degradation of the two pesticides but with limited extents, contributing more to the catalyzed degradation of CLO than IMI. To determine the main active biochar component for IMI and CLO-catalyzed degradation, deashed biochars and biochar ashes were all prepared (Zhang et al. 2013, 2018b). The degradation percentages of IMI and CLO in biochar ash suspensions were generally higher than those in the corresponding biochar suspensions even at an adjusted pH value of 6.5, suggesting that mineral ash is the main component for the catalyzed degradation of IMI and CLO. It was reported that metal-saturated clays in soil, and surface metal ions or oxides and organic anions in biochar played active roles in elevating interlayer latent pH, which could further facilitate hydrolysis (Liu et al. 2006; Shainberg 1973). The chemical removal percentages of IMI and CLO on PS and biochar–PS mixtures had a significant positive correlation with Kd (Table S4; rIMI = 0.851, rCLO = 0.844; p < 0.05). This could be due to that high contents of oxygen-containing functional groups (e.g., –COO− and –O−) and alkali metal minerals (e.g., whitlockite, calcite, sylvite and cyclowollastonite shown in Fig. S2) in high-PT biochars released latent OH−, which is able to catalyze hydrolysis (Yuan et al. 2011). Moreover, metal oxides on ash can catalyze the hydrolysis of many pesticides by increasing the positive charge density on the reaction center atom in pesticides (Zhang et al. 2013), and there are abundant mineral active groups such as metal oxides in ash (Yuan et al. 2011). Therefore, mineral active groups were the key biochar components leading to high degradation percentages of IMI and CLO in biochar ash suspensions and contributed to the enhanced IMI and CLO chemical degradation in biochar-amended acidic soils and high-PT biochars contained higher ash and hence showed greater capacity to prompt hydrolysis.
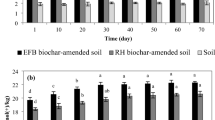
The degradation percentages of IMI and CLO in deashed biochar suspensions were also quite high, even greater than those in biochar suspensions (Table 5), suggesting that organic moiety could also catalyze their chemical degradation. Recently, EPFRs in biochar and their catalytic capacity to degrade contaminants by generating ·OH and other free radicals have gained extensive attention (Zhang et al. 2018b; Fang et al. 2015a). In this paper, the intensity of EPFRs in biochars and the degradation percentages of IMI and CLO catalyzed by ·OH generated by biochars were determined (Text S3). The EPFR signals from 700-PT biochars were stronger than those from other biochars (Table S5). The degradation percentages of IMI and CLO had a significant positive correction with the intensity of EPFRs in biochars (rIMI = 0.539, rCLO = 0.662; p < 0.05). After the suspensions were added with tertiary butanol, the degradation percentages of IMI and CLO all decreased (Fig. 1). This suggested that part of the chemical degradation of IMI and CLO could be ascribed to their reaction with ·OH, and this has also been observed in other studies (Zhang et al. 2018b; Fang et al. 2015a). In addition, other free radicals (e.g., superoxide radical anion and singlet oxygen) could also be generated by EPFRs in biochars (Fang et al. 2015b). After the addition of tertiary butanol, the degradation percentages of IMI and CLO in neutral suspensions were still consistently greater than those in deionized water with the same pH (Fig. 1). Therefore, it could be concluded that the biochar amendments provided mineral active groups and generated ·OH and other free radicals, which caused high chemical degradation percentages of IMI and CLO in biochar-amended acidic soils.
3.5 Effects of biochar on IMI and CLO biodegradation
The addition of biochar could improve soil fertility via several ways such as enhancing nutrient retention, CEC, water-holding capacity and pH (Hartley et al. 2016), and hence influence biological community composition and microbial abundance (Andert and Mumme 2015; Xu et al. 2016). In this paper, the biotic removal rates of IMI and CLO were roughly estimated as the difference of the removal rates between unsterile soils and the corresponding sterile soils. The biotic removal rates of IMI and CLO in bare PS were higher than those in bare RS (Fig. 2). Ren et al. (2015, 2016b) found that the dissipation potential of organic pollutants varied with soil type which is associated with bacterial community. In this paper, the lesser biodegradation of the two insecticides observed in RS could be due to its poor native microbial community caused by lower organic matter content (Table S1). The biotic removal rate of IMI and CLO in the two soils significantly increased after being amended with M3 and P3, while those with M7 and P7 amendment showed significant reductions. Low-PT biochars contained greater amounts of DOC, available N and P (Table 1) than high-PT biochars, which in turn contributed to enhance the quantity and biodegrading function of microorganisms (Chen et al. 2016). A significant positive correlation between the biotic removal rates of IMI and CLO and DOC values of biochar-amended soils was found (Fig. 3; rIMI = 0.655, rCLO = 0.787; p < 0.05), confirming the above conclusion. Furthermore, our previous study (Zhang et al. 2020) showed that DOM derived from low-PT biochars mainly consisted of aliphatic and fulvic acid-like compounds and could increase the relative abundances of Proteobacteria and Bacteroidetes (e.g., Dyadobacter, Sphingobacterium, Novosphingobium, Pedobacter and Mucilaginibacter), which have strong ecological linkages with the carbon cycles, biological electron transfer and organic pollutant degradation (Jiao et al. 2016). For example, Sphingobacterium could degrade the neonicotinoid insecticide via nitrate reduction and chloropyridinyl dechlorination (Zhang et al. 2018c; Wang et al. 2016). Besides, biochar amendments could also change nutrient retention and pH (Table 1), which would lead to corresponding changes of microbial communities and affect the contaminant biodegradation.
Besides the activity of microbial communities, chemical bioavailability is also a key aspect influencing the biodegradation. In this current study, biochar amendments increased the sorption of IMI and CLO, and their sorption affinity increased with the increasing biochars’ PTs for a given raw material (Table 2). As a whole, a negative correlation between the biodegradation percentages of IMI and CLO and their Kd was found (Fig. 3; rIMI = -0.522, rCLO = − 0.774; p < 0.05), which indicated that the sorption reduced the concentrations of IMI and CLO in soil pore water and consequently reduced their bioavailability. High-PT biochar had greater sorption capacity compared to low-PT biochar, and hence, led to a lower biodegradation of the two insecticides due to the reduced bioavailability of the insecticides.
Therefore, biochars as soil amendments could affect chemical and biological degradation of IMI and CLO. The soils amended with high-PT biochars had greater chemical degradation percentages of IMI and CLO, and the lowest biodegradation percentages, which could be ascribed to the mineral active groups and generation of ·OH and other free radicals in high-PT biochars; whereas, soils amended with low-PT biochars had the opposite trend, which could be due to the increasing DOC, available N for microbial communities and low adsorption capacity. Biochar amendments could not only catalyze the chemical degradation of IMI and CLO, but also decrease their biodegradation by reducing their bioavailability.
4 Conclusions
The results gained from this current study suggested that soil properties of two acidic soils (PS and RS) and the sorption and degradation of two insecticides, IMI and CLO therein were all affected by biochar amendments. The pH, EC, TOC and SA of the two soils all increased after biochar additions, while H/C decreased. With increasing biochar PTs, the sorption of the two insecticides in biochar-amended soils was enhanced to greater extents, which could be due to the increasing SA and decreasing H/C. The soil acidic pH favored the sorption in 300-PT biochar–soil mixtures by removing the ash and exposing the inner sorption sites in biochar. Low-PT biochar amendments promoted the biological degradation of IMI and CLO by the enhancing labile C and N sources (DOC and available N) for microbial communities. While, high-PT biochar amendments gained the opposite results that they inhibited the biodegradation by reducing the bioavailability of IMI and CLO, and promoted their chemical degradation by providing mineral active groups and generating ·OH and other free radicals, which could be associated with their higher clay content and organic matter content. Finally, in the view of insecticide dissipation, the optimum biochar was P7, which could shorten the t1/2 by 2.45–3.28 times for IMI and by 4.49–6.40 times for CLO in the two soils. The results of this study together with those in the literature suggest that biochar is a promising material for the remediation of pesticide contamination, however the efficiency is affected by the intrinsic properties of soil and biochar as well as the pesticides, and specific test is needed case by case before the application of biochar technology.
References
Anderson JC, Dubetz C, Palace VP (2015) Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 505:409–422. https://doi.org/10.1016/j.scitotenv.2014.09.090
Andert J, Mumme J (2015) Impact of pyrolysis and hydrothermal biochar on gas-emitting activity of soil microorganisms and bacterial and archaeal community composition. Appl Soil Ecol 96:225–239. https://doi.org/10.1016/j.apsoil.2015.08.019
Botias C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D (2015) Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ Sci Technol 49:12731–12740. https://doi.org/10.1021/acs.est.5b03459
Chai Y, Currie RJ, Davis JW, Wilken M, Martin GD, Fishman VN, Ghosh U (2012) Effectiveness of activated carbon and biochar in reducing the availability of polychlorinated dibenzo-p-dioxins/dibenzofurans in soils. Environ Sci Technol 46:1035–1043. https://doi.org/10.1021/es2029697
Chen J, Sun X, Li L, Liu X, Zhang B, Zheng J, Pan G (2016) Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur J Soil Sci 67:857–867. https://doi.org/10.1111/ejss.12388
Fang G, Gao J, Liu C, Dionysiou DD, Wang Y, Zhou D (2014) Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ Sci Technol 48:1902–1910. https://doi.org/10.1021/es4048126
Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D (2015a) Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour Technol 176:210–217. https://doi.org/10.1016/j.biortech.2014.11.032
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015b) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653. https://doi.org/10.1021/es5061512
Hallmann CA, Foppen RP, van Turnhout CA, de Kroon H, Jongejans E (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:341–343. https://doi.org/10.1038/nature13531
Han D et al (2017) Degradation of dimethyl disulphide in soil with or without biochar amendment. Pest Manag Sci 73:1830–1836. https://doi.org/10.1002/ps.4545
Hartley W, Riby P, Waterson J (2016) Effects of three different biochars on aggregate stability, organic carbon mobility and micronutrient bioavailability. J Environ Manage 181:770–778. https://doi.org/10.1016/j.jenvman.2016.07.023
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908. https://doi.org/10.1021/jf101303g
Jiao S, Liu Z, Lin Y, Yang J, Chen W, Wei G (2016) Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol Biochem 98:64–73
Jin J, Kang M, Sun K, Pan Z, Wu F, Xing B (2016) Properties of biochar-amended soils and their sorption of imidacloprid, isoproturon, and atrazine. Sci Total Environ 550:504–513. https://doi.org/10.1016/j.scitotenv.2016.01.117
Jones DL, Edwards-Jones G, Murphy DV (2011) Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol Biochem 43:804–813. https://doi.org/10.1016/j.soilbio.2010.12.015
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kessler SC et al (2015) Bees prefer foods containing neonicotinoid pesticides. Nature 521:74–76. https://doi.org/10.1038/nature14414
Khorram MS, Zhang Q, Lin D, Zheng Y, Fang H, Yu Y (2016) Biochar: a review of its impact on pesticide behavior in soil environments and its potential applications. J Environ Sci 44:269–279. https://doi.org/10.1016/j.jes.2015.12.027
Lattao C, Cao X, Mao J, Schmidt-Rohr K, Pignatello JJ (2014) Influence of molecular structure and adsorbent properties on sorption of organic compounds to a temperature series of wood chars. Environ Sci Technol 48:4790–4798. https://doi.org/10.1021/es405096q
Liu W, Zheng W, Ma Y, Liu KK (2006) Sorption and degradation of imidacloprid in soil and water. J Environ Sci Health Part B 41:623–634. https://doi.org/10.1080/03601230600701775
Lun L et al (2020) Application of biochar-based materials in environmental remediation: from multi-level structures to specific devices. Biochar. https://doi.org/10.1007/s42773-020-00041-7
Mandal A, Singh N (2017) Optimization of atrazine and imidacloprid removal from water using biochars: designing single or multi-staged batch adsorption systems. Int J Hyg Environ Health 220:637–645. https://doi.org/10.1016/j.ijheh.2017.02.010
Mandal A, Singh N, Purakayastha TJ (2017) Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci Total Environ 577:376–385. https://doi.org/10.1016/j.scitotenv.2016.10.204
Martin SM, Kookana RS, Van Zwieten L, Krull E (2012) Marked changes in herbicide sorption-desorption upon ageing of biochars in soil. J Hazard Mater 231–232:70–78. https://doi.org/10.1016/j.jhazmat.2012.06.040
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303. https://doi.org/10.1016/j.envint.2014.10.024
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193–194:122–130. https://doi.org/10.1016/j.geoderma.2012.10.002
Oleszczuk P, Jośko I, Futa B, Pasieczna-Patkowska S, Pałys E, Kraska P (2014) Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 214–215:10–18. https://doi.org/10.1016/j.geoderma.2013.10.010
Qiang H, Shuang S, Zhe C, Baowei H, Jianrong C, Xiangke W (2019) Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar 1:45–73. https://doi.org/10.1007/s42773-019-00006-5
Ren G, Ren W, Teng Y, Li Z (2015) Evident bacterial community changes but only slight degradation when polluted with pyrene in a red soil. Front Microbiol 6:22. https://doi.org/10.3389/fmicb.2015.00022
Ren X, Zhang P, Zhao L, Sun H (2016a) Sorption and degradation of carbaryl in soils amended with biochars: influence of biochar type and content. Environ Sci Pollut Res Int 23:2724–2734. https://doi.org/10.1007/s11356-015-5518-z
Ren G, Teng Y, Ren W, Dai S, Li Z (2016b) Pyrene dissipation potential varies with soil type and associated bacterial community changes. Soil Biol Biochem 103:71–85. https://doi.org/10.1016/j.soilbio.2016.08.007
Ren X et al (2017) Sorption, transport and biodegradation—an insight into bioavailability of persistent organic pollutants in soil. Sci Total Environ 610–611:1154–1163. https://doi.org/10.1016/j.scitotenv.2017.08.089
Ren X, Wang F, Zhang P, Guo J, Sun H (2018a) Aging effect of minerals on biochar properties and sorption capacities for atrazine and phenanthrene. Chemosphere 206:51–58. https://doi.org/10.1016/j.chemosphere.2018.04.125
Ren X, Sun H, Wang F, Zhang P, Zhu H (2018b) Effect of aging in field soil on biochar's properties and its sorption capacity. Environ Pollut 242:1880–1886. https://doi.org/10.1016/j.envpol.2018.07.078
Rundlof M et al (2015) Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80. https://doi.org/10.1038/nature14420
Shainberg I (1973) Rate and mechanisms of Na-montmorillonite hydrolysis in suspension. Soil Sci Soc Am Proc 5:689–694
Sigmund G, Gharasoo M, Hüffer T, Hofmann T (2020) Deep learning neural network approach for predicting the sorption of ionizable and polar organic pollutants to a wide range of carbonaceous materials. Environ Sci Technol 54:4583–4591. https://doi.org/10.1021/acs.est.9b06287
Sohi S (2010) Carbon storage with benefits. Science 338:1034–1035
Sun K et al (2013) Impact of deashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene. Environ Sci Technol 47:11473–11481. https://doi.org/10.1021/es4026744
Tong H, Hu M, Li FB, Liu CS, Chen MJ (2014) Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil. Soil Biol Biochem 70:142–150. https://doi.org/10.1016/j.soilbio.2013.12.012
Trigo C, Spokas KA, Cox L, Koskinen WC (2014) Influence of soil biochar aging on sorption of the herbicides MCPA, nicosulfuron, terbuthylazine, indaziflam, and fluoroethyldiaminotriazine. J Agric Food Chem 62:10855–10860. https://doi.org/10.1021/jf5034398
Wang J, Chen J, Zhu W, Ma J, Rong Y, Cai Z (2016) Isolation of the novel chiral insecticide paichongding (IPP) degrading strains and biodegradation pathways of RR/SS-IPP and SR/RS-IPP in an aqueous system. J Agric Food Chem 64:7431–7437. https://doi.org/10.1021/acs.jafc.6b02862
Wang N, Chang ZZ, Xue XM, Yu JG, Shi XX, Ma LQ, Li HB (2017) Biochar decreases nitrogen oxide and enhances methane emissions via altering microbial community composition of anaerobic paddy soil. Sci Total Environ 581–582:689–696. https://doi.org/10.1016/j.scitotenv.2016.12.181
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8. https://doi.org/10.1016/j.ejsobi.2016.02.004
Yang J, Pan B, Li H, Liao S, Zhang D, Wu M, Xing B (2016) Degradation of p-nitrophenol on biochars: role of persistent free radicals. Environ Sci Technol 50:694–700. https://doi.org/10.1021/acs.est.5b04042
Yavari S, Malakahmad A, Sapari NB (2015) Biochar efficiency in pesticides sorption as a function of production variables—a review. Environ Sci Pollut Res Int 22:13824–13841. https://doi.org/10.1007/s11356-015-5114-2
Yokoyama S, Yuri K, Nomi T, Komine M, Nakamura S, Hattori H, Rai H (2017) The high correlation between DNA and chloroform-labile N in various types of soil. Appl Soil Ecol 117–118:1–9. https://doi.org/10.1016/j.apsoil.2017.04.002
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Zhang P, Sun H, Yu L, Sun T (2013) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 244–245:217–224. https://doi.org/10.1016/j.jhazmat.2012.11.046
Zhang P, Zhang X, Zhao Y, Wei Y, Mu W, Liu F (2016) Effects of imidacloprid and clothianidin seed treatments on wheat aphids and their natural enemies on winter wheat. Pest Manag Sci 72:1141–1149. https://doi.org/10.1002/ps.4090
Zhang P, Sun H, Ren C, Min L, Zhang H (2018a) Sorption mechanisms of neonicotinoids on biochars and the impact of deashing treatments on biochar structure and neonicotinoids sorption. Environ Pollut 234:812–820. https://doi.org/10.1016/j.envpol.2017.12.013
Zhang P, Sun H, Min L, Ren C (2018b) Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ Pollut 236:158–167. https://doi.org/10.1016/j.envpol.2018.01.030
Zhang P, Ren C, Sun H, Min L (2018c) Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci Total Environ 615:59–69. https://doi.org/10.1016/j.scitotenv.2017.09.097
Zhang P, Huang P, Xu X, Sun H, Jiang B, Liao Y (2020) Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses. Sci Total Environ 708:134619. https://doi.org/10.1016/j.scitotenv.2019.134619
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1802001), the special fund of platform for innovation of Tianjin Science and Technology Commission (19PTZWHZ00040), Science and Technology Major Project of Tianjin (19ZXSZSN00010) and Postdoctoral Science Foundation of China (2019M651018) and Ministry of Education of China (111 program, T2017002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, P., Min, L., Tang, J. et al. Sorption and degradation of imidacloprid and clothianidin in Chinese paddy soil and red soil amended with biochars. Biochar 2, 329–341 (2020). https://doi.org/10.1007/s42773-020-00060-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-020-00060-4