Abstract
The dissipation behaviour and left-over residues of Hexaconazole fungicide and Lambda-Cyhalothrin pesticide in potato plant and soil were analysed by gas chromatography mass spectrometry (GC-MS). At fortified levels of 10, 100 and 500 μg/kg, the recoveries of Lambda-Cyhalothrin and Hexaconazole were in the range 81.66–93.25% and 76.11–93.92% with standard deviations of 0.87–8.13% and 0.88–7.68%, respectively. The half-life ranges for all matrices (tuber, stem, leaf and soil) were 13.8–17.3 days for Hexaconazole and 11.5–17.3 days for Lambda-Cyhalothrin. The final concentration of Hexaconazole and Lambda-Cyhalothrin in tuber at harvest was compared with the maximum residual limit (MRL) of CODEX (0.01 mg/kg) and EU (0.02 mg/kg) and found to be higher at dosages of 30 g/ha and 40 g/ha, respectively. There was no residual concentration of either pesticide in the control plot. The results obtained from risk evaluation showed that the risk of Lambda-Cyhalothrin at dosage of 7–15 g/ha and Hexaconazole at dosage of 5–30 g/ha was negligible to humans while a dose of 40 g/ha for Lambda-Cyhalothrin resulted in a health hazard to humans. This study may prove helpful in ascertaining the MRL and also provide direction on the appropriate use of Lambda-Cyhalothrin and Hexaconazole in potato farming as the waiting period from last application to harvest is 25 days for Hexaconazole and 38 days for Lambda-Cyhalothrin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum) ranks third as a food crop in the world and in India is the fourth food crop after rice, wheat and maize. Furthermore, India is the second largest producer of potato worldwide (Scott and Suarez 2011). Chemical control of pests and diseases is a common practice in potato cultivation.
Hexaconazole [(R, S)-2-(2, 4-dichlorophenyl)-1-(1H-1, 2, 4-triazole-1-yl) hexane-2-ol] is a broad spectrum synthetic triazole fungicide for the control of diseases like early blight and late blight in potato (Government of India, Insecticide Act, 1968 (2009), by both preventive and curative action (Kumar et al. 2004). Lambda-Cyhalothrin [3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2,2-dimethyl-cyano(3-phenoxyphenyl) methylcyclopropanecarboxylate] is a synthetic pyrethroid insecticide used for control of aphids in the potato crop. It disrupts the normal functioning of the nervous system of an insect leading to paralysis or death (World Health Organization (WHO 1990). Multiresidue analytical procedures for detecting chemical residue concentration in potato tuber and other vegetables were developed by using GCECD (Gas Chromatography with Electron Capture Detector) (Srivastava et al. 2011). Additionally, many more analytical procedures have been used for analysis of Lambda-Cyhalothrin and Hexaconazole residue concentration from different matrices by using gas chromatography mass spectrometry (Balinova et al. 2007; Wang et al. 2011; Walorczyk et al. 2011; Vuković et al. 2012; Zayats et al. 2013), low pressure gas chromatography (LP-GC-MS-MS) detection (Arrebola et al. 2003a, 2003b), GCMS/MS detection (Arrebola et al. 2003a, 2003b; Walorczyk and Drożdżyński 2012; Banerjee et al. 2012) and gel permeation chromatography gas chromatography mass spectrometry (GPC-GC-MS) detection (Xu et al. 2012). The degradation of Hexaconazole residue in tea, tomato and soil has been reported (Kumar et al. 2004; Liang et al. 2012). The degradation of Lambda-Cyhalothrin residue in zucchini, soil and paddy has also been reported (Tariq et al. 2006; Barik et al. 2010; Lofty et al. 2013). However, the degradation behaviour of Hexaconazole and Lambda-Cyhalothrin residue in potato plant has not been investigated and reported in literature.
Several government agencies in the world have established the maximum residual limits (MRLs) of Lambda-Cyhalothrin and Hexaconazole in potato to ensure food safety and environmental protection. In European Commission (2012), the MRLs of Hexaconazole and Lambda-Cyhalothrin are set at 0.02 mg/kg for potato tuber. In CODEX alimentarius commission (2016), the MRL of Lambda-Cyhalothrin is set at 0.01 mg/kg for potato tuber. There is no directive on MRLs for Lambda-Cyhalothrin and Hexaconazole in India for potato tuber, although it is one of the most widely used food crops and limited work has been done to estimate the degradation rate and the final residue concentration under field conditions.
In this article, a GC-MS procedure has been used to analyse the residual concentration of Lambda-Cyhalothrin and Hexaconazole in soil and different parts of potato plant (leaf, stem and tuber) when grown on agricultural land. The study examined the dissipation kinetics and final residual concentration in all these matrices. A health risk evaluation study was also done on the basis of data obtained from field experiments. This study should allow the farmer to follow strategies for the appropriate and secure utilization of Lambda-Cyhalothrin and Hexaconazole in potato cultivation field.
Material and Methods
Chemicals
Standards of analytical grade Hexaconazole (purity 99.3%) and Lambda-Cyhalothrin (purity 97.8%) were purchased from Sigma-Aldrich Chemicals Pvt. Ltd. The liquid formulation 5% SC Hexaconazole and 5% EC Lambda-Cyhalothrin was purchased from Rallis India Limited. HPLC grade acetone and ethyl acetate were purchased from Merck. Primary secondary amine (PSA) Bondesil 40 μm, part no. 12213023, and anhydrous magnesium sulfate, part no. 5982–8082, were purchased from Agilent technology. Sodium chloride and activated charcoal powder were from Sigma-Aldrich and deionized water was used from a laboratory distillation unit. Hexaconazole and Lambda-Cyhalothrin stock standard solutions were prepared by dissolving standard pesticides with ethyl acetate and stored at − 20 °C.
Field Experiments
A field experiment for pesticides dissipation kinetics and residue in potato plant and field was conducted from October 2014 to February 2015 within the academic premises of Motilal Nehru National Institute of Technology, Allahabad, India. There were five (A, B, C, D and E) types of plot, each with three rows and an area of 1.486 m2. Plot E was worked as control without fertilizers and pesticides treatment. All the plots were treated with both organic (20 t/ha) and inorganic fertilizers (N:P2O5:K2O at 180:80:110 kg/ha) except plot A which was treated with only organic fertilizer. Plots A and B were given a number of treatments of pesticides as recommended by the manufacturer (10 g/ha for Hexaconazole and 15 g/ha for Lambda-Cyhalothrin). Plot C was given the same number of treatments but with lower dosages and plot D was given same number of treatments but with higher dosages (Table 1). The first application of both the pesticides in potato field was done 54 days after planting and followed by two more sprays at intervals of 9 days. A liquid solution of pesticides was applied with hand held sprayer onto potato plants and the soil. The first irrigation was done 10 days after planting and was continued until 110 days after planting at 15-day intervals.
On each sampling date, the samples of soil and potato plants were collected from the field for pesticides residue dissipation. The sampling dates were 0, 5, 10, 15, 25 and 38 days after last application. All the samples (leaf, stem, tuber and soil) were kept in a deep freezer at − 20 °C for analysis.
Analytical Procedure
Potato plant (leaf, stem and tuber) and soil samples were collected randomly from different plots. Tuber, leaf and stem were washed, cleaned, chopped and ground in food mixer separately and kept in the dark at − 20 °C pending analysis. The soil samples were air-dried at room temperature, homogenized and set aside in the dark awaiting analysis.
Finally, 10 g of each homogenized sample of tuber, stem and leaf were weighed into a 50-ml Teflon centrifuge tube for pesticides residue analysis by quick, easy, cheap, effective, rugged and safe (QuEChERS) method. Afterwards, 10 ml ethyl acetate (additional 5 ml water in soil sample) was added. After the tube was vortexed, 4 g of anhydrous magnesium sulfate and 1 g activated sodium sulfate were added and shaken for 10 min at 50 rpm on rotospin and then centrifuged for 10 min at 10,000 rpm. Then 1 ml of extract was mixed with the mixture of 50 mg PSA, 150 mg anhydrous magnesium sulfate and 10 mg activated charcoal. This mixture was again shaken for 10 min at 50 rpm on rotospin and centrifuged for 10 min at 10,000 rpm. The supernatant was collected in 2-ml vial and mixed with 5 μl acidified ethyl acetate (ethyl acetate acidified with 5% formic acid). Cleaned extract was collected in auto sampler vial for pesticides residue analysis on GC-MS.
GC-MS Analysis
An Agilent 6890 N network GC system coupled with an Agilent 5973 inert mass selective detector, equipped with an Agilent 7683B auto sampler injector and capillary column DB-5MS, 30 m × 0.250 mm I.D. × 0.25 μm film thickness was used. ChemStation 1.1 data system software was applied for collection and processing data. The carrier gas (helium) was of purity greater than 99.995%.
GC-MS conditions: splitless injection of 1 μl was carried out at 250 °C with solvent delay time 6 min. The following temperature program was applied: initial temperature 180 °C, hold 2 min, then a positive gradient of 10 °C/min was applied to 200 °C, hold 2 min, then a positive gradient of 10 °C/min was applied to 270 °C, hold 2 min. The mass spectrometer was operated in electron impact ionization mode (ionization energy 70 eV) with transfer line set at 300 °C and ion source temperature set at 230 °C. The carrier gas flow rate was 1.1 ml/min. Scan and SIM mode were run separately.
Statistical Analysis
The dissipation kinetics in the leaf, stem, tuber and soil were analysed by plotting the residual pesticides concentration versus time (days after last application), fitting non-linear curves and calculating the coefficient of determination (R2). The best fitting curve was the first order exponential kinetic equation: Ct = C0e-kt, where Ct is residue concentration (μg/kg) of pesticides at time t (days after last application), k is the dissipation rate constant (days−1) and C0 is initial pesticides concentration (μg/kg). The half-life (t1/2) of pesticides was determined from k value for every experiment, defined as the time requisite for dissipation of pesticides concentration to half of the initial concentration (t1/2 = 0.693/k). Final residual pesticides concentration in tuber was obtained at 38 days after last application which represented the maximum risk to humans through consumption of tuber. Then, the preharvest interval (PHI) for safe use of these pesticides was determined and the maximum residual pesticides concentrations were compared with MRL value.
Hazard Risk Index Analysis
It is essential to compare the estimated exposure to established toxicological criteria such as estimated average daily intake (EADI). The EADI of pesticides should be less than established acceptable daily intake (ADI) value (WHO 1997). EADI of pesticides residues for each pesticide and commodity was calculated by using the following equation documented by Darko and Akoto (2008).
Where PI is tuber consumption rate (kg/day), C is residual concentration of pesticides (mg/kg) and bw is the average body weight.
The hazard index (HI) is as follows:
Hazard indices of pesticides residue were calculated by using the data obtained from tuber consumption assumption. Consumption rate of potato tuber in India in 2013 was 24.40 kg/capita/year (FAOSTAT 2013). According to Australian Government Acceptable Daily Intake for Agricultural and Veterinary Chemicals (2016), the ADI values of Hexaconazole and Lambda-Cyhalothrin are 0.005 mg/kg and 0.001 mg/kg, respectively. From the above calculations, hazard indices values that are greater than 1 indicate that the risk of pesticide for human is harmful and the pesticides applied in the potato plant are not safe for humans. On the other hand, if hazard indices value is less than 1, the risk is acceptable to humans and use of pesticides on potato is safe.
Results and Discussion
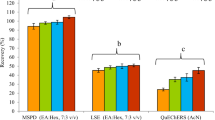
For analysis of Lambda-Cyhalothrin and Hexaconazole residues in samples (leaf, stem, tuber and soil), a matrix-matched calibration curve was obtained by plotting the response against the concentration ranges from 10 to 1000 μg/kg. Linearity was obtained for every sample and showed good correlation coefficient range between 0.987 and 0.999. Validation of analytical method was performed by recovery, limit of detection (LOD) and limit of quantification (LOQ). For recovery, all the samples (leaf, stem, tuber and soil) were fortified with three different levels (10, 100, 500 μg/kg) with two replicates. The mean recoveries of Lambda-Cyhalothrin and Hexaconazole ranged from 81.66 to 93.25% and 76.11 to 93.92%, respectively. The relative standard deviations for Lambda-Cyhalothrin and Hexaconazole were 0.87 to 8.13% and 0.88 to 7.68%, respectively, which was less than 20%. The limit of quantification for pesticides was determined using a signal to noise ratio of 10, with reference to the background noise obtained from the blank sample and was 10 μg/kg for all matrices for both pesticides. LOD (lowest detected value) was 3 μg/kg and 5 μg/kg for Hexaconazole and Lambda-Cyhalothrin, respectively. According to EU ( 2013), the results indicated that the extraction methods for Lambda-Cyhalothrin and Hexaconazole were suitable for leaf, stem, tuber and soil.
Behaviour of Lambda-Cyhalothrin and Hexaconazole in Leaf, Stem, Tuber and Soil
The dissipation studies of pesticides in potato plant and soil were done in experimental plots, sprayed three times with the formulation of Lambda-Cyhalothrin (5% EC) and Hexaconazole (5% SC) at the dosage mentioned in Table 1. Leaf, stem, tuber and soil samples were analysed and pesticide dissipation curves were plotted. The results obtained from the dissipation processes followed the first order kinetic equation (Ct = C0e-kt) with correlation coefficients for Lambda-Cyhalothrin of 0.982–0.854 and for Hexaconazole of 0.999–0.901. The degradation of Hexaconazole and Lambda-Cyhalothrin in leaf, stem, tuber and soil was not more than 88.2% at 38 days following last application of pesticides in the plots. The pesticides residues in leaf, stem, tuber and soil were observed to decrease exponentially with time.
The kinetic equation, correlation coefficient and half-life of Lambda-Cyhalothrin and Hexaconazole for leaf, stem, tuber and soil are shown in Table 2. The dissipation curves of Hexaconazole for leaf, stem, tuber and soil are shown in Fig. 1. After last application, the initial concentrations of Hexaconazole in plot A and plot B were 18.36 and 15.23 μg/kg in tuber, 42.65 and 47.91 μg/kg in stem, 49.67 and 49.58 μg/kg in leaf and 96.11 and 99.21 μg/kg in soil, respectively. On the other hand, the half-life in plot A and plot B were 15.75 and 16.9 days for tuber, 13.07 and 14.43 days for stem, 14.43 and 16.11 days for leaf and 13.86 and 14.14 days for soil, respectively. The dissipation curves of Lambda-Cyhalothrin for leaf, stem, tuber and soil samples are shown in Fig. 2. The initial concentrations of Lambda-Cyhalothrin after last application in plot A and plot B were 23.66 and 25.66 μg/kg in tuber, 30.12 and 31.18 μg/kg in stem, 30.92 and 31.58 μg/kg in leaf and 118.53 and 119.32 μg/kg in soil, respectively. The half-life in plot A and plot B was 13.88 and 13.86 days in tuber, 11.36 and 11.17 days in stem, 11.56 and 11.53 days in leaf and 15.4 and 14.74 days in soil, respectively.
Based on the dissipation kinetics data obtained from plot A and plot B, the initial concentration and half-life of Lambda-Cyhalothrin and Hexaconazole were different for leaf, stem, tuber and soil. The initial concentration and half-life were affected by many complicated factors. The organic matter content and pH probably exerted special influences on pesticides residue concentrations in soil (Arias-Estévez et al. 2008) as plot A was treated with organic fertilizers while plot B was treated with both organic and inorganic fertilizers. Dissipation of pesticides in soil also depends on the properties of the pesticides (Paterio-Moure et al. 2008). The dissipation rate of Hexaconazole in plot A was faster than in plot B while for Lambda-Cyhalothrin it was faster in plot B than plot A, probably due to the higher organic matter content in plot A.
Final residues of Hexaconazole and Lambda-Cyhalothrin in leaf, stem, tuber and soil are summarized in Tables 3 and 4, respectively. Final residual concentrations of Hexaconazole depended on dosage, being highest at 30 g/ha, 51.76 μg/kg in tuber; 110.1 μg/kg in stem; 110.5 μg/kg in leaf and 80.4 μg/kg in soil in plot D at 38 days after the last application. At the lowest dosage (5 g/ha), the final concentration of pesticide in tuber was below detection limit and in leaf, stem and soil were below the quantification level. The final concentration of Hexaconazole in tuber at harvest was below the EU MRL (0.02 mg/kg) in plots A, B and C, but not in plot D which received the highest dosage.
The final concentration of Lambda-Cyhalothrin also depended on the dosage, being highest at 40 g/ha, 85.5 μg/kg in tuber; 78.42 μg/kg in stem; 79.54 μg/kg in leaf and 103.5 μg/kg in soil at 38 days after the last application. At the lowest dosage (7 g/ha), the final concentration of pesticide was not detected in tuber, leaf and stem while in soil it was below the quantification level. The final concentration of Lambda-Cyhalothrin in tuber at harvest was less than the MRLs of CODEX (0.01 mg/kg) and EU (0.02 mg/kg) in plots A, B and C but not in plot D which received the highest dosage.
Risk Assessment
Maximum residual limits of Lambda-Cyhalothrin and Hexaconazole for potato tuber have not yet been established in India. The hazard indices value was calculated with the equation mentioned earlier.
Final residual concentration was used for estimation of EADI value for tuber, as shown in Table 5. The range of HI values for Hexaconazole was 0.84–0.00 and for Lambda-Cyhalothrin was 7.54–0.00. Hence, the risk to humans is negligible when the formulation of Hexaconazole (5% SC) at dosage of 5–30 g/ha and Lambda-Cyhalothrin (5% EC) at dosage of 7–15 g/ha is used in potato cultivation. On other hand, a Lambda-Cyhalothrin dosage of 40 g/ha poses a health hazard to humans.
Conclusions
A method was developed using GC-MS for detection of Hexaconazole and Lambda-Cyhalothrin in potato plants (leaf, stem and tuber) and also in the soil. The degradation and final concentration of pesticides in tubers were obtained to ensure human health security and realistic use of these pesticides in potato cultivation. The final residual concentrations of Hexaconazole and Lambda-Cyhalothrin in tubers were above the MRLs of EU at dosages of 30g/ha and 40g/ha, respectively. The final residual concentration of Hexaconazole in tuber was found to be below the MRLs of EU while Lambda-Cyhalothrin concentration was higher. The half-lives were estimated as 16.9–15.75 days in tuber, 13.07–14.43 days in stem, 14.43–16.11 days in leaf and 13.8–14.14 days in soil for Hexaconazole and 13.88–13.86 days in tuber, 11.36–11.17 days in stem, 11.56–11.53 days in leaf and 15.4–14.74 days in soil for Lambda-Cyhalothrin. Risk assessment results indicate that the risk to humans from Hexaconazole and Lambda-Cyhalothrin use in potato cultivation at dosages of 5–30 g/ha and 7–15 g/ha, respectively, is negligible, while a dosage of 40 g/ha of Lambda-Cyhalothrin does pose a risk to humans. Therefore, both Hexaconazole and Lambda-Cyhalothrin can be used as pesticides in potato field at respective dosages of 30 g/ha and 15 g/ha, without much risk, with application of each pesticide restricted to three times during entire cultivation period.
References
Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J, Mejuto J, García-Río L (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123:247–260. https://doi.org/10.1016/j.agee.2007.07.011
Arrebola FJ, Martínez Vidal JL, González-Rodríguez MJ, Garrido-Frenich A, Morito S (2003a) Reduction of analysis time in gas chromatography application of low pressure gas chromatography tandem mass spectrometry to the determination of pesticide residues in vegetables. J Chromatogr A 1005:131–141. https://doi.org/10.1016/S0021-9673(03)00887-2
Arrebola FJ, Martínez Vidal JL, Mateu-Sánchez M, Álvarez-Castellón FJ (2003b) Determination of 81 multiclass pesticides in fresh foodstuffs by a single injection analysis using gas chromatography chemical ionization and electron ionization tendem mass spectrometry. Anal Chim Acta 484:167–180. https://doi.org/10.1016/S0003-2670(03)00332-5
Australian Government (2016) Acceptable daily intakes for agricultural and veterinary chemicals. Office of Chemical Safety, Department of Health, Canberra, pp 1–119 http://www.health.gov.au/internet/main/publishing.nsf/content/6279C451E3D11E89CA257BF0001DAAE7/$File/ADI%20List_updated%20to%2031%20Mar%202016.pdf
Balinova A, Mladenova R, Shtereva D (2007) Solid phase extraction on sorbents of different retention mechanisms followed by determination by gas chromatography mass spectrometric and gas chromatograpgy electron capture detection of pesticide residues in crop. J Chromatogr A 1150:136–144. https://doi.org/10.1016/j.chroma.2007.02.002
Banerjee K, Utture S, Dasgupta S, Kandaswamy C, Pradhan S, Kulkarni S, Adsule P (2012) Multiresidue determination of 375 organic contaminants including pesticides, polychlorinated biphenyls and polyaromatic hydrocarbons in fruits and vegetables by gas chromatography triple quadrupole mass spectrometry with introduction of semi quantification approach. J Chromatogr A 1270:283–295. https://doi.org/10.1016/j.chroma.2012.10.066
Barik SR, Ganguly P, Kunda SK, Kole RK, Battacharyya A (2010) Persistence behavior of thiamethoxam and Lambda Cyhalothrin in transplanted paddy. Bull Environ Contam Toxicol 85:419–422. https://doi.org/10.1007/s00128-010-0101-2
Codex Alimentarius Commission (2016) Pesticide residues in food, FAO/WHO food standard. http://www.fao.org/fao-who-codexalimentarius/standards/pestres/commodities-detail/en/?lang=en&c_id=350
Darko G, Akoto O (2008) Dietary intake of organophosphorous pesticide residues through vegetables from Kumasi, Ghana. Food Chem Toxicol 46:3703–3706. https://doi.org/10.1016/j.fct.2008.09.049
European Commission (2012) Pesticide EU-MRLs Regulation (EU) No 899/2012. http://ec.europa.eu/sanco_pesticides/public/index.cfm?event=substance.historic
European Union (2013) Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. EU, Document No. SANCO/12571/2013. http://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2013_12571.pdf
FAOSTAT (2013) Potato consumption statistics in India. http://www.potatopro.com/india/potato-statistics. data retrieved on July, 2015
Government of India, Indian horticulture database (2013). Ministry of Agriculture, National horticulture board. http://nhb.gov.in/area-pro/Indian%20Horticulture%202013.pdf
Government of India, Insecticide Act-1968 (2009) Ministry of Agriculture, Department of Agriculture & Co-operation, New Delhi. http://www.cibrc.nic.in/mupi.pdf
Kumar V, Ravindranath SD, Shanker A (2004) Fate of hexaconazole residues in tea and its behavior during brewing process. Chem Health Saf 11:21–25. https://doi.org/10.1016/j.chs.2003.09.018
Liang H, Li L, Li W, Wu Y, Liu F (2012) The decline and residues of hexaconazole in tomato and soil. Environ Monit Assess 184:1573–1579. https://doi.org/10.1007/s10661-011-2061-3
Lofty HM, El-Aziz A, El-Aleem A, Monir HH (2013) Determination of insecticides malathion and lambda-cyhalothrin residue in zucchini by gas chromatography. Bull Fac Pharm Cairo Univ 51:255–260. https://doi.org/10.1016/jbfopcu.2013.08.001
Paterio-Moure M, Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J (2008) Occurrence and downslope mobilization of quaternary herbicide residues in vineyard-devoted soils. Bull Environ Contam Toxicol 80:407–411. https://doi.org/10.1007/s00128-008-9403-z
Scott GJ, Suarez V (2011) Growth rates for potato in India and their implication for industry. Potato J 38:100–112. ISSN 0970-8235
Srivastava AK, Trivedi P, Srivastava MK, Lohani M, Srivastava LP (2011) Monitoring of pesticide residues in market basket samples of vegetable from Lucknow city, India: QuEChERS method. Environ Monit Assess 176:465–472. https://doi.org/10.1007/s10661-010-1597-y
Tariq MI, Afzal S, Hussain I (2006) Degradation and persistence of cotton pesticides in sandy loam soil from Punjab, Pakistan. Environ Res 100:184–196. https://doi.org/10.1016/j.envres.2005.05.002
Vuković G, Shtereva D, Bursić V, Mladenova R, Lazić S (2012) Application of GC-MSD and LC-MS/MS for the determination of priority pesticides in baby foods in Serbain market. LWT – Food Sci Technol 49:312–319. https://doi.org/10.1016/j.lwt.2012.07.021
Walorczyk S, Drożdżyński D (2012) Improvement and extension to new analytes of a multi-residue method for the determination of pesticides in cereals and dry animal feed using gas chromatography tandem quadrupole mass spectrometry revisited. J Chromatogr A 1251:219–231. https://doi.org/10.1016/j.chroma.2012.06.055
Walorczyk S, Drożdżyński D, Gnusowski B (2011) Multiresidue determination of 160 pesticides in wines employing mixed mode dispersive solid phase extraction and gas chromatography tandem mass spectrometry. Talanta 85:1856–1870. https://doi.org/10.1016/j.talanta.2011.07.029
Wang Y, Jin H, Ma S, Lu J, Lin R (2011) Determination of 195 pesticide residues in Chinese herbs by gas chromatography mass spectrometry using analyte protectants. J Chromatogr A 1218:334–342. https://doi.org/10.1016/j.chroma.2010.11.036
WHO (1990) Cyhalothrin, Environmental Health Criteria, 99; Geneva, Switzerland
WHO (1997) Guidelines for predicting dietary intake of pesticide residues (revised) global environment monitoring system – food contamination monitoring and assessment programme (GEMS/Food) in collaboration with Codex Committee on pesticide residues. Programme of Food Safety and Food Aid, pp. 1–44
Xu X, Yu S, Li R, Fan J, Chen S, Shen H, Han J, Huang B, Ren Y (2012) Distribution and migration study of pesticides between peel and pulp in grape by online gel permeation chromatography gas chromatography mass spectrometry. Food Chem 135:161–169. https://doi.org/10.1016/j.foodchem.2012.04.052
Zayats MF, Leschev SM, Petrashkevich NV, Zayats MA, Kadenczki L, Szitás R, Szemán Dobrik H, Keresztény N (2013) Distribution of pesticides in n-hexane/water and n-hexane/acetonitrile system and estimation of possibilities of their extraction isolation and preconcentration from various matrices. Anal Chim Acta 774:33–43. https://doi.org/10.1016/j.aca.2013.03.003
Acknowledgements
The authors are thankful to the director and Head of the Civil Engineering department Motilal Nehru National Institute of Technology Allahabad, India, for providing the necessary laboratory facilities for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study is part of Raginee Devi’s Ph. D program with support from MNNIT Allahabad, India.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R. P. Singh and A. K. Sachan are co-second authors.
Rights and permissions
About this article
Cite this article
Devi, R., Singh, R.P. & Sachan, A.K. Dissipation Kinetics of Hexaconazole and Lambda-Cyhalothrin Residue in Soil and Potato Plant. Potato Res. 62, 411–422 (2019). https://doi.org/10.1007/s11540-019-9418-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-019-9418-3