Abstract
In the binary mixtures of 2-ethyl-1-hexanol with various functional groups (benzyl chloride, 3-methyl aniline, 3-methoxy aniline and 2,6-dimethyl cyclohexanone) densities, speeds of sound and viscosities including those of pure liquids were measured over the entire composition range at different temperatures (303.15, 308.15 and 313.15) K and atmospheric pressure 0.1 MPa. Using this experimentally determined data, the excess/deviation parameters (molar volume, isentropic compressibility and deviation in viscosity) partial molar volumes, partial molar isentropic compressibilities of these components at infinite dilution were calculated. The results are discussed in terms of intermolecular forces between different component molecules in the binary mixture are more than the average intermolecular forces existing between the similar molecules of pure components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of thermodynamic properties of binary mixtures contributes to an understanding of the behavior of different liquids and their functional groups. This information is very useful in the design of industrial process and in the development of theories for the liquid state and predictive methods. Excess thermodynamic parameters of different mixtures are useful in the study of molecular interactions and arrangements.
The knowledge of physicochemical properties of non-aqueous binary liquid mixtures has relevance in theoretical and applied areas of research, and such results are frequently used in design process (flow, mass transfer or heat transfer calculations) in many chemical and industrial processes [1]. The excess properties derived from these physical property data reflect the physicochemical behavior of the liquid mixtures with respect to the solution structure and intermolecular interactions between the component molecules of the mixture [2, 3].
Study of hydrogen-bonded systems is essential and helpful as hydrogen bond plays a vital role in chemical, physical and biological process. Organic compounds containing electronegative group can interact with compounds containing active hydrogen through hydrogen bond. This type of hydrogen bond takes part in role in the stability of biologically important molecules. Alkanols are polar and self-associated liquids and the dipolar association of alkanols decreases when they are mixed with polar compounds containing various functional groups due to formation of hydrogen bonds between 2-ethyl-1-hexanol and various functional groups in the mixtures over the rupture of hydrogen bonds present in pure 2-ethyl-1-hexanol.
We report here a study of excess/deviation functions in four non-component molecules with 2-ethyl-1-hexanol systems. This is in continuation of our earlier work [4,5,6] on excess/deviation functions of polar liquids with amines. However, in this work a systematic study is reported on excess/deviation functions of non-component molecules with 2-ethyl-1-hexanol over the entire composition range and at temperature (T) ranging from 303.15 to 313.15 K. The results are discussed in terms of intermolecular forces between the different components molecules in the binary mixture are more than the average intermolecular forces existing between the similar molecules of pure components.
2 Procedure
2.1 Materials
The 3-methoxyaniline, benzyl chloride and 3-methylaniline liquids were sigma Aldrich samples. Table 1 contains information regarding their source, purification method, final purity and analysis method. Values of density, speed of sound, and viscosity are presented in Table 2. These values are in good agreement with the data available in the literature [7,8,9,10,11,12,13,14].
2.2 Apparatus and procedure
All the binary liquid mixtures were prepared by weighing required amounts of pure liquids in an electric balance (ER-120A, Afoset) with a precision of ± 0.01 mg by syringing each component into air-tight stopper bottles to minimize evaporation losses. The uncertainty of the mole fraction was ± 1 × 10−4.
The details of the density, speed of sound and viscosity methods and their measurement techniques were described elsewhere [15]. The uncertainty of density measurement for liquid mixtures is ± 0.2 × 10−4 g cm3. The uncertainty in the measured speed of sound is ± 0.053 m s−1. The experimental uncertainty of viscosity estimated as ± 1.13%. The temperature of the liquids during the measurements was maintained within an uncertainty of ± 0.01 K in an electronically controlled thermostatic water bath.
2.3 Theory
The excess thermodynamic/deviation functions were calculated by using the following equations
where η, η1,η2, V, V1 and V2 are viscosities and mixture molar volumes and pure components respectively.
The isentropic compressibilities were calculated from the relation
where \(\rho\) is the density and \(u\) is the speed of sound of the binary mixture.
Further, the excess isentropic compressibilities (\(k_{s}^{E}\)) are calculated from the following relations recommended by Benson and Kiyohara [16]
where \(\varphi_{\text{i}}\), \(C_{{p\text{,}i}}\), \(V_{i}\), \(\kappa_{{\text{s,}i}}\) and \(\alpha_{i}\) are the volume fraction, molar heat capacity, molar volume, isentropic compressibility and coefficient of isobaric thermal expansion of pure components respectively.
Excess/deviation functions (VE, ∆η and κ Es ) values are fitted to a Redlich–Kister polynomial equation [17]
where YE is the VE, ∆η and \(\kappa_{s}^{E}\). Coefficientsn values Ai are determined by using the method of least-squares. The standard deviation σ (YE) were calculated by the formula as follows
where m is the total number of experimental points and n is the number of parameters. The coefficients, Ai and corresponding standard deviation values (σ) are presented in Table 3.
3 Results and discussion
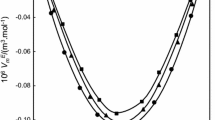
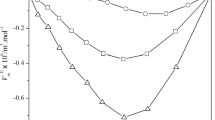
The experimental density, speed of sound and viscosity data and their excess/deviation values for the 2-ethyl-1-hexanol with various functional groups at various temperatures are given in Tables 2 and 4. All the excess/deviation values of these parameters VE, κ Es Δη and G*E at various temperatures are represented graphically in Figs. 1, 2, 3 and 4.
All the excess/deviation values of these parameters may be explained qualitatively in terms of
-
1.
molecular interactions between like/unlike molecules and the difference in size and shape of the unlike components
-
2.
Possible H-bond interaction between 2-ethyl-1-hexanol and various functional groups in the mixtures having 2-ethyl-1-hexanol as a proton donor
-
3.
Dipole–dipole interactions between polar–polar components
The magnitude of excess values of VE and κ Es from ideality of the systems that can be negative, positive, or zero may be explained as a balance between positive contributions (hydrogen bond rupture and dispersive interactions between unlike molecules) and negative contributions (intermolecular dipolar interactions, geometrical fitting between components, intermolecular association complexes between unlike molecules)
The experimental values indicate that the negative effects are dominant over positive ones in all the mixtures, since the excess values of VE and κ Es for all the binary systems is negative over the whole concentration range at various temperatures. In the present systems, negative sign of VE shows that the predominance of formation of hydrogen bonds between 2-ethyl-1-hexanol and various functional groups in the mixtures over the rupture of hydrogen bonds present in pure 2-ethyl-1-hexanol [18, 19].
The negative values of VE for the four systems are in the following order:
-
2-Ethyl-1-hexanol + 2, 6-dimethylcyclohexanone > 2-ethyl-1-hexanol + 3-methoxyaniline > 2-ethyl-1-hexanol +3-methylaniline > 2-ethyl-1-hexanol + benzyl chloride
The value at the minima for the system (2-ethyl-1-hexanol + 2,6-dimethylcyclohexanone) is slightly more negative than that of remaining various functional groups, and this may be due to the ability of 2,6-dimethylcyclohexanone to form stronger hydrogen bonds with 2-ethyl-1-hexanol than various functional groups with 2-ethyl-1-hexanol. Hence, above order was justified. In the present systems, negative values of excess isentropic compressibilities shows that the interaction between the unlike molecules exceed the structure breaking effect between the like molecules.
The negative values of κ Es for the four systems are in the following order:
-
2-Ethyl-1-hexanol + 3-methoxyaniline > 2-ethyl-1-hexanol + 3-methylaniline > 2-ethyl-1-hexanol + benzyl chloride > 2-ethyl-1-hexanol + 2, 6-dimethylcyclohexanone
Specific interactions are strong (more effect that is inductive) between 2-ethyl-1-hexanol and 3-methoxyaniline molecules thereby showing the highest negative excess isentropic compressibilities value for their binary mixture.
The sign and magnitude of Δη and G*E depend on the combined effect of factors such as molecular size, shape, and intermolecular forces [20]. The positive values ofΔη and G*E indicates the presence of specific interactions such as the formation of hydrogen bond between unlike molecules and negative values indicates that mutual loss of specific interactions in like molecules outweigh the specific interactions between unlike molecules [18, 21].
In the present study, the positive values of Δη and G*E for these systems indicates that the strength of interaction between the components in binary mixtures and the formation of an association complex [22, 23]. This results in a liquid structure where the flow is rather difficult than would be expected on the basis of the viscosities of the pure components.
3.1 Partial molar properties
The interpretations of excess partial molar properties (\(\overline{V}_{\text{m,1}}^{\text{E}}\), \(\overline{V}_{\text{m,2}}^{\text{E}}\), \(\overline{K}_{\text{s,m,1}}^{\text{E}}\) and \(\overline{K}_{\text{s,m,2}}^{\text{E}}\)) and excess partial molar properties at infinite dilution(\(\overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}}\), \(\overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}}\), \(\overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}}\) and \(\overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}}\)) of components 2 have previously been described [24]. Tables 5 and 6 shows that the values of \(\overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}}\), \(\overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}}\), \(\overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}}\) and \(\overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}}\) are negative over the whole composition range at experimental temperatures. The observed negative values suggest that the hetero molecular association interactions are stronger than the self-association of molecular interactions of like molecules in the mixtures [25, 26].
4 Conclusions
Densities, viscosities and speeds of sound of binary mixtures of 2-ethyl-1-hexanol with benzyl chloride,3-methylaniline,3-methoxyaniline and 2,6-dimethylcyclohexanone have been measured at different temperatures and derived parameters along with their excess/deviations values, and also excess partial molar properties at infinite dilution (\(\overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}}\), \(\overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}}\), \(\overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}}\) and \(\overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}}\)) were calculated. The results are analyzed in terms of the specific interactions through the hetero molecular association between the components of the mixtures, resulting in the formation of association complexes.
References
Prasad N, Prakash S (1976) Solute–water interactions and the solubility behaviour of long-chain paraffin hydrocarbons. Acustica 36:313–319
Deshpande DD, Bhatgadde LG (1968) Sound velocities, adiabatic compressibilities and free volumes in aniline solutions. J Phys Chem 72(1):261–266
Snyder WJ, Snyder JR (1974) Velocity of sound in binary mixtures of benzene, hexane, and methanol at 0–65 Deg. J Chem Eng Data 19(3):270–274
Naga Babu P, Venkata Lakshmi V, Gowrisankar M, Raveendra M (2019) Investigation of molecular interactions in binary mixtures of 2-methoxyaniline with isomeric cresols at various temperatures through thermo physical properties. Phys Chem Liq. https://doi.org/10.1080/00319104.2019.1594225
Sulthana SP, Gowrisankar M, Babu S, Santos D (2019) Investigation of ketonic effect in molecular interactions of 2-methylcyclohexanone with aniline, N-methyl aniline and N,N-dimethyl aniline at various temperatures. Int J Ambient Energy. https://doi.org/10.1080/01430750.2019.1673816
Mukesh B, Sreekanth T, Srinivasa Krishna T, Gowrisankar M (2019) Thermodynamic and transport properties of 2-methoxyaniline with substituted ethanols at various temperatures. Int J Ambient Energy. https://doi.org/10.1080/01430750.2019.1636873
Bhatia SC, Sangwa J, Rani R, Kiran V (2013) Densities, viscosities, speeds of sound, and refractive indices of binary mixtures of 2-ethyl-1-hexanol with benzene and halobenzenes. Int J Thermophys 34(11):2076–2088
Zorȩbski E, Dzida M, Wysocka E (2011) Acoustic and thermodynamic properties of 2-ethyl-1-hexanol by means of high-pressure speed of sound measurements at temperatures from (293 to 318) K and pressures up to 101 MPa. J Chem Eng Data 56(5):2680–2686
Zábranský M, Růžička V Jr (2004) Estimation of the heat capacities of organic liquids as a function of temperature using group additivity: an amendment. J Phys Chem 33:1071–1081
Pandiyan V, Oswal SL, Vasantharani P (2011) Thermodynamic and acoustic properties of binary mixtures of ethers. III. Diisopropyl ether or oxolane with o- or m-toluidines at 303.15, 313.15 and 323.15 K. Thermochim Acta 516:64–73
Reid RC, Prausnitz JM, Poling BE (1987) The properties of gases and liquids. McGraw Hill Inc., New York, p 139
Riddick JA, Bunger WB, Sakano TK (1986) Organic solvents physical properties and methods of purification, 4th edn. Wiley Interscience, New York
Kalavathi E, Venkatesulu A, Gowrisankar M (2019) Influence of carbonyl group on thermodynamic and transport properties of binary liquid mixtures of 2-methoxyaniline with ketones at various temperatures. Chem Data Collect 23:100260
Ali A, Nain AK, Chand D, Lal B (2005) Molecular interactions in binary mixtures of anisole with benzyl chloride, chlorobenzene and nitrobenzene at 303.15 K: an ultrasonic, volumetric, viscometric and refractive index study. Ind J Chem 44A:511–515
Venkateswara Rao P, Gowrisankar M, Venkatramana L, Srinivasa Krishna T, Ravindhranath K (2016) Studies on the importance of nature of substituent on the thermodynamic and transport properties of liquid mixtures at various temperatures. J Chem Therm 101:92–102
Benson GC, Kiyohara O (1979) Evaluation of excess isentropic compressibilities and isochoric heat capacities. J Chem Therm 11:1061–1064
Redlich O, Kister AT (1948) Algebraic representation of thermodynamic properties and the classification of solutions. J Ind Eng Chem 40:345–348
Fort RJ, Moore WR (1966) Viscosities of binary liquid mixtures. Trans Faraday Soc 62:1112–1119
Nigam RK, Singh PP, Singh M, Singh KC (1980) Excess enthalpies of mixing of aniline + isomeric xylenes & Barker’s theory of associated mixtures. Indian J Chem 19A(3):192–194
Nath J, Srivastava AK (1986) Excess volumes for binary liquid mixtures of trichloroethylene with anisole, pyridine, quinoline and cyclohexane at 298.15 and 308.15 K. Fluid Phase Equili 28(1):97–101
Iloukhani H, Reddy KD, Prabhakara Rao MV (1985) Excess volumes of trichloroethylene with some aliphatic and alicyclic ketones at 303.15 K and 313.15 K. Phys Chem Liq 14(3):181–188
Reed TM III, Taylor TE (1959) Viscosities of liquid mixtures. J Phys Chem 63(1):58–67
Meyer R, Meyer M, Metzer J, Peneloux A (1971) Thermodynamic and physicochemical properties of binary solvent. J Chem Phys 62:405
Gowrisankar M, Venkateswarlu P, Kumar KS, Sivarambabu S (2012) Thermodynamics of amine + ketone mixtures 3. Volumetric, speed of sound data and viscosity at (303.15 and 308.15 K) for the binary mixtures of N,N-dimethylaniline + propiophenone, + p-methylacetophenone, + p-chloroacetophenone. Mol Liq 173:172–179
Fernandez J, Andrade MP, Pintos M, Sarmiento F, Bravo R (1983) Excess enthalpies of (secondary amine + alcohol) at 298.15 K. J Chem Therm 15(6):581–584
Rao DN, Naidu PR (1981) Excess volumes of (ethylenediamine + an alcohol). J Chem Therm 13(7):691–694
Busygina GI, Maslova VA, Shvetsova KG, Babinkov AG, Rabinovich IB, Karyakin NV (1987) Specific heat and thermodynamic functions of phthalic anhydride and 2-ethylhexanol at 13–350 K. Zhur Fiz Khim 61:2347–2351
Smith RH, Andrews DH (1931) Thermal energy studies. I. Phenyl derivatives of methane, ethane and some related compounds. J Am Chem Soc 53:3644–3660
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sulthana, S.P., Gowrisankar, M., Babu, S. et al. Binary mixtures of 2-ethyl-1-hexanol with various functional groups (benzyl chloride, 3-methylaniline, 3-methoxyaniline and 2,6-dimethylcyclohexanone). SN Appl. Sci. 2, 960 (2020). https://doi.org/10.1007/s42452-020-2751-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2751-y