Abstract
Densities (ρ), speeds of sound (u), and viscosities (η) are reported for binary mixtures of 2-methylaniline with substituted ethanols (2-phenylethanol, 2-chloroethanol and 2-aminoethanol) over the entire composition range of mole fraction at T = (303.15–318.15) K and at atmospheric pressure, 0.1 MPa. The excess molar volume, excess isentropic compressibility and deviation in viscosity are calculated from the corresponding experimental densities, speeds of sound and viscosities. The excess properties are correlated using the Redlich–Kister polynomial smoothing equation. Excess partial molar volumes and excess partial molar isentropic compressibilities were calculated for all the binary systems throughout the composition range and also at infinite dilution. The variations in these properties with composition, for all the binary mixtures, suggest that loss of dipolar association, difference in size and shape of the component molecules, dipole–dipole interaction and hydrogen bonding between molecules of 2-methylaniline with 2-phenylethanol, 2-chloroethanol and 2-aminoethanol are involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of molecular interactions in mixed solvent systems is of great significance owing to the practical applications of these systems in various technologies, as they provide a wide choice of solutions with appropriate properties. The excess thermodynamic properties of binary liquid mixtures have been very useful for obtaining information on the intermolecular interactions in the systems. The negative or positive deviations from the ideal value depend on the type and extent of the interactions between unlike molecules, as well as on the composition and the temperature [1].
Knowledge of the physico-chemical properties of non-aqueous binary liquid mixtures has relevance in theoretical and applied areas of research and such results are frequently used in the design process (flow, mass transfer or heat transfer calculations) in many chemical and industrial processes. The excess properties derived from these physical property data reflect the physico-chemical behavior of the liquid mixtures with respect to the solution structure and intermolecular interactions between the component molecules of the mixture [2].
This work is part of our program to give information/data for the characterization of molecular interactions between solvents in binary systems [3]. The liquids were chosen for the present study on the basis of their industrial importance. 2-Methylaniline is an important compound used in the manufacturing of dyes and of rubber vulcanization accelerators. It is also used in the fabrication of hypnotic and anesthetic pharmaceuticals and pesticides. On the other hand, 2-chloroethanol is a polar, bi-functional compound, consisting of both a hydroxyl group as a proton donor and halogen atom as a proton acceptor. It is a versatile solvent used in many industrial areas and is also a mutagenic chemical. 2-Aminoethanol is a widely used agent in the carbon dioxide and hydrogen sulfide removal processes. 2-Phenylethanol has found usage in artificial essences and as a base solvent for some flavor compounds.
In the present study, our focus is on the study of liquid mixtures of substituted ethanols with 2-methylaniline because there have been a few studies on these mixtures [4, 5]. It is expected that there will be a significant degree of H-bonding in these binary mixtures because 2-methylaniline and substituted ethanols both have a proton donor and a proton acceptor group [6]. To understand the possible associations between 2-methylaniline and substituted ethanols through –OH···NH and –NH···OH bonds, we report the densities, speeds of sound and viscosities for three binary systems (2-methylaniline with 2-phenylethanol, with 2-chloroethanol, and with 2-aminoethanol) at T = (303.15–318.15) K and under 0.1 MPa pressure. The experimental data has been used to compute the excess volumes (VE), excess isentropic compressibilities (\( \kappa_{S}^{\text{E}} \)) and deviations in viscosity (∆η). The results are used to qualitatively discuss specific interactions between unlike molecules.
2 Experimental Methods
2.1 Materials
Chemicals used in the present study are 2-methylaniline (Sigma–Aldrich), 2-phenylethanol (Sigma–Aldrich), 2-chloroethanol and 2-aminoethanol. These chemicals were purchased from S.D. Fine Chemicals Ltd. 2-chloroethanol and 2-aminoethanol chemicals were further purified by standard methods [7, 8] like distillation and fractional distillation under reduced pressure, and only the middle fractions were collected. Before use, the chemicals were stored over 0.4 nm molecular sieves for about 72 h to remove water and gas. The purity of the liquid samples was checked by gas chromatography. The water contents were determined by the Karl–Fischer method. The details of the chemicals and purification methods are presented in Table 1.
2.2 Apparatus and Procedure
All the binary liquid mixtures were prepared by weighing appropriate amounts of the pure liquids on an Afcoset-ER-120A electric balance, using a syringe, in a narrow mouth stoppered bottle. The resolution of electronic balance was ± 0.01 mg while the accuracy of the mole fraction was ± 1 × 10−4.
Densities and speeds of sound were measured with a digital oscillating Density and Sound Analyzer (DSA 5000 M, Anton Parr, Austria) with a reproducibility of ± 1 × 10−6 g·cm−3 for the density and ± 1 × 10−2 m·s−1 for the speed of sound. The speed of sound was measured using a propagation time technique at the frequency 3 MHz. The densimeter was calibrated randomly with dry air at atmospheric pressure and doubly-distilled, freshly degassed and deionized water (ρ = 997.075 kg·m−3 at 298.15 K) supplied by Anton-Paar as described elsewhere. After each measurement, distilled water and anhydrous ethanol were used to clean the vibrating tube. The combined expanded uncertainties associated with the measurements for temperature, density and speed of sound are estimated to be within 0.01 K, 0.8 × 10−3 g·cm−3 and 0.5 m·s−1, respectively, at the 95% confidence level.
The viscosities of the pure liquids and their mixtures were determined at atmospheric pressure at T = (303.15–318.15) K by using an Ubbelohde viscometer, which was calibrated with benzene, carbon tetrachloride, acetonitrile and doubly distilled water. The Ubbelohde viscometer bulb capacity was 15 mL and the capillary tube had a length of about 90 mm with 0.5 mm internal diameter. The viscometer was thoroughly cleaned and perfectly dried, filled with the sample liquid by fitting the viscometer to about 30° from the vertical, and its limbs were closed with Teflon caps to avoid evaporation. The viscometer was kept in a transparent walled bath with a thermal stability of ± 0.01 K for about 20 min to obtain thermal equilibrium before making the measurement. An electronic digital stopwatch with an uncertainty of ± 0.01 s was used for flow time measurements. The experimental uncertainty of viscosity of pure liquids was estimated at 0.17 mPa·s and the uncertainty of temperature ± 0.01 K. The combined expanded uncertainty of viscosity was estimated as 0.2 mPa·s. The purities of all these solvents were compared with the measured densities, speeds of sound and viscosities of the pure liquids with literature [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] and these comparisons are listed in Table 2 and graphically compared with the average absolute deviation (AAD) with literature values in Supplementary Material as Fig. 1S–9S. Density, speed of sound and viscosity values of 2-methylaniline were taken from our previous papers [29, 30].
3 Results and Discussion
The experimental densities and sound velocities for all the binary systems at various compositions were used to calculate the excess thermodynamic functions, using the following equations:
Deviation in viscosity were be calculated by following equation,
where ρ, η and V are the density, viscosity and molar volume of the binary mixture. Also x1, M1, ρ1, η1, V1 and x2, M2, ρ2, η2, and V2 are the mole fraction, molar mass, density, viscosity and molar volume of pure components 1 and 2, respectively.
The experimental data were used to compute the isentropic compressibility (κ S ) by using the following relation:
The method used for calculating \( \kappa_{S}^{\text{E}} \) (Benson–Kiyohara approach) was outlined previously [31]:
where \( \kappa_{S} \) is the isentropic compression and \( \kappa_{S}^{\text{id}} \) is the isentropic compression of the ideal mixture,
where ϕ i is the volume fraction of component i, and \( \kappa_{S} \), \( V_{\text{m}} \), \( \alpha_{p} \) and \( C_{p} \) are the isentropic compressibility, molar volume, coefficient of isobaric thermal expansion and molar heat capacity, respectively, and R is the gas constant and T is the absolute temperature. The values of ϕ i , \( V_{\text{m}}^{\text{id}} \), \( \alpha_{p}^{\text{id}} \) and \( C_{p}^{\text{id}} \) were calculated using the following relations:
and
Values of C p were obtained from the literature [32]. α p was calculated from measured densities by the relation,
The VE, \( \kappa_{S}^{\text{E}} \) and ∆η values were fitted with a Redlich–Kister [33] polynomial equation,
where YE represents VE, \( \kappa_{S}^{\text{E}} \) and ∆η. Values of the coefficients A i have been determined by using the method of least squares. The standard deviations σ(YE) have been calculated by using the formula
where m is the total number of experimental points and n is the number of parameters. The coefficients, A i and corresponding standard deviation values (σ) are presented in Table 6.
The observed VE values can be discussed in terms of several effects, which are (i) physical, (ii) chemical and (iii) geometrical contributions [34]. (i) The physical interactions involve expansion due to mutual breaking of OH···O and N–H···N bonds present in the self-associated substituted ethanol and the amine; (ii) the chemical or specific interactions involves contraction due to complex formation between unlike molecules, which include hydrogen bond (HNH···OH and OH···N); and (iii) structural contributions resulting from geometrical fitting of one component into other, due to the difference in the free volumes between components, resulting in negative contributions to VE.
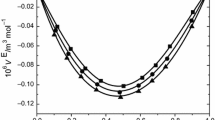
The excess volume data reported in Tables 3, 4, and 5 at T = 303.15 K are graphically represented in Fig. 1 and in Fig. 10S at 313.15 K. The excess volume has negative values for all the studied systems over the whole composition range and at all experimental temperatures. The negative value of VE may be due to the predominance of chemical or specific interactions and structural contributions dominating over physical contributions. Hence, the OH···N bond is stronger than the OH···O and N–H···N bonds in the mixtures [35]. The existence of strong O–H···N bond was also confirmed through NMR, IR and UV studies [36]. The same trend is observed for the system water + 2-aminoethanol [37].
The values of VE for the binary mixtures of 2-methylaniline with the substituted ethanols are in the following order:
The more negative excess volume in the system 2-methylaniline + 2-aminoethanol reveals that more efficient packing and/or attractive interactions occur between these two components when mixed together. Consequently, its structure and smaller size lead to easier interstitial accommodation with 2-methylanilinemolecules compared to 2-methylaniline with 2-chloroethanol or with 2-phenylethanol. Similar results have been reported earlier [38, 39]. Hence the above order is justified.
Further, inspection of Tables 3, 4, 5 and Fig. 1 indicates that as the temperature increases from 303.15 to 318.15 K, the values of VE for all these binary systems decrease. An increase in temperature increases the kinetic energy and therefore the breaking up of associate species present in the pure liquids, releasing more free dipoles of unlike molecules into the mixture, which results in interactions with each other and the formation of greater numbers of H-bonds between 2-methylaniline and the substituted ethanol’s monomer. This has already been described in the literature for 1-alkanol + hexane systems [40], alcohol + triethylene glycol systems [41] and 1-hexanol + ether systems [42].
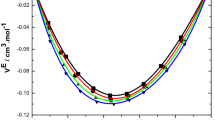
Excess isentropic compressibility (\( \kappa_{S}^{\text{E}} \)) data for the mixtures of 2-methylaniline with substituted ethanols are graphically depicted in Fig. 2 at 303.15 K and Fig. 11S at 313.15 K and the data are given in Tables 3, 4, 5, and they show that the \( \kappa_{S}^{\text{E}} \) values are negative over the entire mole fraction range at T = (303.15–318.15) K and become more negative with increasing temperature for all three binary mixtures. Thus, the mixtures are less compressible than the pure components, i.e., a greater resistance to compression (enhanced rigidity) is observed. The three systems show both enhanced rigidity (\( \kappa_{S}^{\text{E}} \) < 0) and contraction (VE < 0) over the entire composition range. In other words, the volume decreases (more compact packing of molecules), and simultaneously the whole system becomes more rigid (less compressible) [43]. As shown in Fig. 2, in the mixtures of 2-methylaniline and 2-phenylethanol, the trend of \( \kappa_{S}^{\text{E}} \) is identical to that of the excess molar volumes. The same trend can be observed for other two systems. Interpretation of the \( \kappa_{S}^{\text{E}} \) data is generally not simple because the \( \kappa_{S}^{\text{E}} \) < 0 values are affected by both the molecular packing and the patterns of molecular aggregation induced by the molecular interactions. However, in these three binary systems it seems that the interpretation of negative \( \kappa_{S}^{\text{E}} \) values will be the same as for the negative VE values.
The values of \( \kappa_{S}^{\text{E}} \) for the binary mixtures of 2-methylaniline with substituted ethanols are in the following order:
An examination of the curves in Fig. 3 at 303.15 K and Fig. 12S at 313.15 K indicates that the negative values for binary mixture containing 2-methylaniline and 2-phenylethanol are smaller than those of the other binary systems, because the phenyl group (–C6H5) has steric hindrence with the –NH2 group. 2-Methylaniline has a free NH2 group and easily interacts with the neighboring molecules [44,45,46]. Hence the above order is justified. The negative values of \( \kappa_{S}^{\text{E}} \) increase with increasing temperature, which suggests that specific interactions increase due to the enhanced thermal energy. Comelli et al. [47] have also reported similar behavior for \( \kappa_{S}^{\text{E}} \).
A correlation between the signs of Δη and VE has been observed for a number of binary solvent systems [48, 49], i.e., Δη is positive when VE is negative and vice versa. In general, for systems where dispersion and dipolar interactions are operating, the Δη values are found to be negative, whereas donor–acceptor and hydrogen-bonding interactions lead to the formation of complex species between unlike molecules and thereby result in positive Δη values [50]. The viscosity deviation data of the liquid mixtures are graphically given in Fig. 3 and viscosity deviation data are given in Tables 3, 4, 5. The positive values of viscosity deviation for the binary systems investigated suggest that the hetero-molecular complexes between unlike molecules are relatively more numerous than those present in the pure components [51]. The effect of temperature increase is to disrupt hetero and homo association of the molecules resulting in an increase in fluidity of the liquids, giving higher Δη values at higher temperatures. The viscosity deviation values are found to be opposite to the sign of excess molar volumes for all binary mixtures, which is in agreement with the view proposed by Brocos et al. [52, 53].
As Tables 3, 4 and 5 show, the excess properties obtained are negative over the entire composition range and absolute values of VE, \( \kappa_{S}^{\text{E}} \), and \( \Delta \eta \) increase (become more negative) with increasing temperature from T = (303.15–318.15) K. This effect can be attributed to the fact that the attractive interactions between like molecules become weaker with increasing temperature. Also, increasing temperature expands the volume of the mixture so that more space occurs between larger molecules that will become available for the smaller molecules to fill upon mixing. This packing effect will decrease the mixture volume.
4 Partial Molar Properties
The interpretations of excess partial molar properties (\( \overline{V}_{\text{m,1}}^{\text{E}} \), \( \overline{V}_{\text{m,2}}^{\text{E}} \), \( \overline{K}_{\text{s,m,1}}^{\text{E}} \) and \( \overline{K}_{\text{s,m,2}}^{\text{E}} \)) and excess partial molar properties at infinite dilution (\( \overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}} \), \( \overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}} \) \( \overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}} \) and \( \overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}} \)) of components 2 have previously been described [54].
In general, negative values of excess partial molar volume of component 1 and excess partial molar volumes of component 2 (\( \mathop {\bar{V}_{{_{\text{m,1}} }}^{\text{E}} }\limits^{{}} \),\( \bar{V}_{\text{m,2}}^{\text{E}} \), \( \overline{K}_{\text{s,m,1}}^{\text{E}} \) and \( \overline{K}_{\text{s,m,2}}^{\text{E}} \)) indicate the presence of significant solute–solvent interactions between unlike molecules, whereas a positive excess partial molar volume of component 1 and excess partial molar volume component 2 data indicate the presence of solute–solute/solvent–solvent interactions between like molecules in the mixtures [55, 56].
A close perusal of Supplementary Table 1S and Fig. 4 indicates that the values of \( \overline{V}_{\text{m,1}}^{\text{E}} \) and \( \overline{V}_{\text{m,2}}^{\text{E}} \) are negative for all the binary mixtures over the whole composition range. Negative values may be attributed to hydrogen bonded complex formation between the components of the mixtures (Table 6).
From the Supplementary Table 2S and Fig. 5 it may be inferred that the values of \( \overline{K}_{\text{s,m,1}}^{\text{E}} \) and \( \overline{K}_{\text{s,m,2}}^{\text{E}} \) are negative for all the binary mixtures over the whole composition range. The negative values indicate donor–acceptor interactions between the components of the mixtures.
From Table 7, it can be seen that the values of \( \overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}} \) and \( \overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}} \) are negative for all the binary mixtures over the whole composition range. The negative \( \overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}} \) and \( \overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}} \) values indicate strong specific interactions through a hetero association complex formation between 2-methylaniline and a substituted ethanol molecule.
It is seen from Table 8 that the values of \( \overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}} \) and \( \overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}} \) are negative for all the binary systems at each investigated temperature. The negative values may be due to the strong chemical and specific interactions through charge transfer complex formation between the components of the binary mixtures.
5 Conclusion
This paper reports experimental data of densities, speeds of sound and viscosities of binary blends of 2-methylaniline with substituted ethanol (2-phenylethanol, 2-chloroethanol and 2-aminoethanol) binary mixtures over the entire composition range at T = (303.15–318.15) K with 5 K interval. From the experimental data, various physicochemical parameters, viz., \( V_{\text{m}}^{\text{E}} \), \( \kappa_{S}^{\text{E}} \) and Δη of the mixtures, the excess partial molar properties (\( \overline{V}_{\text{m,1}}^{\text{E}} \), \( \overline{V}_{\text{m,2}}^{\text{E}} \), \( \overline{K}_{\text{s,m,1}}^{\text{E}} \) and \( \overline{K}_{\text{s,m,2}}^{\text{E}} \)) and excess partial molar properties at infinite dilution (\( \overline{V}_{\text{m,1}}^{{^\circ {\text{E}}}} \), \( \overline{V}_{\text{m,2}}^{{^\circ {\text{E}}}} \) \( \overline{K}_{\text{s,m,1}}^{{^\circ {\text{E}}}} \) and \( \overline{K}_{\text{s,m,2}}^{{^\circ {\text{E}}}} \)) of the components have been calculated.
References
Hassan, M., Shirude, D.F., Hiray, A.P., Sawant, A.B., Kadam, U.B.: Densities, viscosities and ultrasonic velocities of binary mixtures of methylbenzene with hexan-2-ol, heptan-2-ol and octan-2-ol at T = 298.15 and 308.15 K. Fluid Phase Equilib. 252, 88–95 (2007)
Kharat, S.J., Nikam, P.S.: Density and viscosity studies of binary mixtures of aniline + benzene and ternary mixtures of (aniline + benzene + N,N-dimethylformamide) at 298.15, 303.15, 308.15, and 313.15 K. J. Mol. Liq. 131, 81–85 (2007)
Gowrisankar, M., Venkatesulu, A., Srinivasa Krishna, T., Ravindhranath, K.: Studies on the Importance of chain length of alkanols on the thermodynamic and transport properties of liquid mixtures at various temperatures. J. Chem. Thermodyn. 107, 104–113 (2017)
Yadav Dimple, J.S., Singh, K.C., Sharma, V.K.: Molar excess volumes and excess isentropic compressibilities of 2-methylaniline (i) + benzene (j) + methylbenzene}, {2-methylaniline (i) + benzene (j) + 1,2-dimethylbenzene (k)}, and {2-methylaniline (i) + benzene (j) + 1,4-dimethylbenzene (k) at T = 308.15 K. J. Chem. Eng. Data 54, 2109–2112 (2009)
Saini, N., Yadav, J.S., Sunil, K.J., Sharma, D., Sharma, V.K.: Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J. Chem. Thermodyn. 43, 782–795 (2011)
Kumar, S., Jeevanandham, P.: Densities, viscosities, refractive indices and excess properties of aniline and o-anisidine with 2-alkoxyethanols at 303.15 K. J. Mol. Liq. 174, 34–41 (2012)
Vogel, A.L.: Text Book of Practical Organic Chemistry. Longman Green, London (1989)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents, 4th edn. Wiley, New York (1986)
Sharma, V.K., Solanki, S., Bhagour, S.: Excess heat capacities of binary and ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and anilines. J. Chem. Eng. Data 59, 1852–1864 (2014)
Jangra, S.K., Yadav, J.S., Sharma, V.K.: Thermodynamic investigations of ternary o-toluidine + tetrahydropyran + N,N-dimethylformamide mixture and its binaries at 298.15, 303.15 and 308.15 K. J. Mol. Liq. 163, 36–45 (2011)
Pandiyan, V., Oswal, S.L., Malek, N.I., Vasantharani, P.: Thermodynamic and acoustic properties of binary mixtures of ethers. V. Diisopropyl ether or oxolane with 2- or 3-chloroanilines at 303.15, 313.15 and 323.15 K. Thermochim. Acta 524, 140–150 (2011)
Papari, M.M., Ghodrati, H., Fadaei, F., Sadeghi, R., Behrouz, S., Soltani Rad, M.N., Moghadasi, J.: Volumetric and ultrasonic study of mixtures of 2-phenylethanol with 1-butanol, 2-butanol, and 2-methyl-1-butanol at T = (298.15–323.15) K and atmospheric pressure: measurement and prediction. J. Mol. Liq. 180, 121–128 (2013)
Kermanpour, F., Jahani, H., IIoukhani, H.: Excess molar volume and derived thermodynamic properties of binary mixtures of 2-methyl-1-butanol and 2-ethyl-1-butanol + different ethers at the temperature range of 293.15 to 313.15 K. J. Mol. Liq. 146, 29–34 (2009)
Alavianmehr, M.M., Sharifi, M., Rad, M.N.S.: Measurement and modeling of volumetric properties and sound speeds of several mixtures of alcohol liquids containing 1-propanol and 2-propanol at T = (298.15–323.15) K and ambient pressure. Fluid Phase Equilib. 376, 181–192 (2014)
Yeh, C.-T., Tu, C.-H.: Densities, viscosities, refractive indexes, and surface tensions for binary mixtures of 2-propanol + benzyl alcohol, + 2-phenylethanol and benzyl alcohol + 2-phenylethanol at T = (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 52, 1760–1767 (2007)
Domanska, U., Zawadzki, M., Lewandrowska, A.: Effect of temperature and composition on the density, viscosity, surface tension, and thermodynamic properties of binary mixtures of N-octylisoquinolinium bis{(trifluoromethyl)sulfonyl}imide with alcohols. J. Chem. Thermodyn. 48, 101–111 (2012)
Dyer, J.R.: Applications of Absorption Spectroscopy of Organic Compounds. Prentice-Hall of India, New Delhi (1978)
Ferrari, G., Foca, G., Manfredini, M., Manzini, D., Marchetti, A., Tassi, L., Ulrici, A.: Density and volume properties of the 2-chloroethanol + 2-methoxyethanol + 1,2-dimethoxyethane ternary solvent system at different temperatures. J. Solution Chem. 32, 93–116 (2003)
Pandey, P.K., Awasthi, A., Awasthi, A.: Acoustic, volumetric and spectroscopic properties of formamide + N-methylformamide + 2-chloroethanol at different temperatures. Phys. Chem. Liq. 52, 320–330 (2014)
Baragi, J.G., Aralaguppi, M.I., Aminabhavi, T.M., Kariduraganavar, M.Y., Kittur, A.: Density, viscosity, refractive index, and speed of sound for binary mixtures of anisole with 2-chloroethanol, 1,4-dioxane, tetrachloroethylene, tetrachloroethane, DMF, DMSO, and diethyl oxalate at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 50, 910–916 (2005)
Alavianmehr, M.M., Shahsavar, S., Ghodrati, H., Hemmati, N.: Measurement and modeling of volumetric properties and speeds of sound of several mixtures of alcohol liquids containing butanediol. J. Chem. Eng. Data 60, 1956–1967 (2015)
Hernandez, J.A., Trejo, A., Flores, B.E.G., Molnar, R.: Viscometric and volumetric behaviour of binary mixtures of sulfolane and N-methylpyrrolidone with monoethanolamine and diethanolamine in the range 303–373 K. Fluid Phase Equilib. 267, 172–180 (2008)
Garcia-Abuin, A., Gomez-Diaz, D., La Rubia, M.D., Navaza, J.M.: Density, speed of sound, viscosity, refractive index, and excess volume of N-methyl-2-pyrrolidone + ethanol (or water or ethanolamine) from T = (293.15 to 323.15) K. J. Chem. Eng. Data 56, 646–651 (2011)
Aralaguppi, M.I., Jadar, C.V., Aminabhavi, T.M.: Density, viscosity, refractive index, and speed of sound in binary mixtures of 2-chloroethanol with methyl acetate, ethyl acetate, n-propyl acetate, and n-butyl acetate. J. Chem. Eng. Data 44, 441–445 (1999)
Hawrylak, B., Burke, S.E., Palepu, R.: Partial molar and excess volumes and adiabatic compressibilities of binary mixtures of ethanolamines with water. J. Solution Chem. 29, 575–594 (2000)
Pouryousefi, F., Idem, R.O.: New analytical technique for carbon dioxide absorption solvents. Ind. Eng. Chem. Res. 47, 1268–1276 (2008)
Han, J., Jin, J., Dag, A.E., Melaaen, M.C.: Density of water (1) + monoethanolamine (2) + CO2 (3) from (298.15 to 413.15) K and surface tension of water (1) + monoethanolamine (2) from (303.15 to 333.15) K. J. Chem. Eng. Data 57, 1095–1103 (2012)
Ervin, V.Q.: Viscosity of ortho-substituted aromatic amines. J. Chem. Eng. Data 25, 387–388 (1980)
Sekhar, M.C., Sankar, M.G., Venkatesulu, A.: Thermodynamic and theoretical study on hydrogen bonded binary mixtures of isomeric butanols with o-toluidine at T = (303.15 to 318.15) K. J. Mol. Liq. 209, 428–439 (2015)
Balaji, R., Sankar, M.G., Venkatesulu, A., Shekar, M.C.: Mesomeric effect on thermodynamic parameters of binary liquid mixtures of N-methyl formamide and o-substituted anilines. J. Mol. Liq. 230, 36–43 (2017)
Benson, G.C., Kiyohara, O.: Evaluation of excess isentropic compressibilities and isochoric heat capacities. J. Chem. Thermodyn. 11, 1061–1064 (1979)
Zábransky Jr., M., Vlastimil, R.: Estimation of the heat capacities of organic liquids as a function of temperature using group additivity: an amendment. J. Phys. Chem. Ref. Data 33, 1071–1081 (2004)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. J. Ind. Eng. Chem. 40, 345–348 (1948)
Oswal, S.L., Desai, H.S.: Studies of viscosity and excess molar volume of binary mixtures.: 1. Propylamine + 1-alkanol mixtures at 303.15 and 313.15 K. Fluid Phase Equilib. 149, 359–376 (1998)
Peterson, R.C.: Interactions in the binary liquid system N,N-dimethylacetamide + water: viscosity and density. J. Phys. Chem. 64, 184–185 (1960)
Nakanishi, K., Ichinose, S., Shirai, H.: Prediction of azeotrope formation based on infrared spectral data in binary solutions containing methanol. Ind. Eng. Chem. Fundam. 7, 381–387 (1968)
Kapadi, U.R., Hundiwale, D.G., Patil, N.B., Lande, M.K.: Viscosities, excess molar volume of binary mixtures of ethanolamine with water at 303.15, 308.15, 313.15 and 318.15 K. Fluid Phase Equilib. 201, 335–341 (2002)
Brocos, P., Calvo, E., Pineiro, A., Bravo, R., Amigo, A.: Heat capacities, excess enthalpies, and volumes of mixtures containing cyclic ethers. 5. Binary systems {1,3-dioxolane + 1-alkanols}. J. Chem. Eng. Data 44, 1341–1347 (1999)
Amigo, A., Bravo, R., Pintos, M.: Excess volumes of binary mixtures containing cyclic ethers + alkanols at 298.15 K. J. Chem. Eng. Data 38, 141–142 (1993)
Heintz, A., Schmittecker, B., Wagner, D., Lichtenthaler, R.N.: Excess volumes of binary 1-alkanol/hexane mixtures at temperatures between 283.15 and 323.15 K. J. Chem. Eng. Data 31, 487–492 (1986)
Valtz, A., Teodorescu, M., Wichterle, I., Richon, D.: Liquid densities and excess molar volumes for water + diethylene glycolamine, and water, methanol, ethanol, 1-propanol + triethylene glycol binary systems at atmospheric pressure and temperatures in the range of 283.15–363.15 K. Fluid Phase Equilib. 215, 129–142 (2004)
Villa, S., Riesco, N., Carmona, F.J., Garcia de la Fuente, I., Gonzalez, J.A., Cobos, J.C.: Temperature dependence of excess properties in alcohols + ethers mixtures: I. Excess molar volumes of 1-propanol or 1-hexanol + ethers at 318.15 K. Thermochim. Acta 362, 169–177 (2000)
Venkatramana, L., Sivakumar, K., Gardas, R.L., Dayananda Reddy, K.: Effect of chain length of alcohol on thermodynamic properties of their binary mixtures with benzylalcohol. Thermochim. Acta 581, 123–132 (2014)
Rauf, M.A., Arfan, M., Aziz, F.: Excess molar volumes of (N,N′-dimethylformamide + an aliphatic alcohol) at 298.15 K. J. Chem. Thermodyn. 15, 1021–1023 (1983)
Ali, A., Nain, A.K., Sharma, V.K., Shmad, S.: Molecular interactions in binary mixtures of tetrahydrofuran with alkanols (C6, C8, C10): an ultrasonic and volumetric study. Indian J. Pure Appl. Phys. 42, 666–673 (2004)
Krestov, G.A.: Thermodynamics of Salvation. Ellis Horwood Limited, England (1991)
Comelli, F., Ottani, S., Francesconi, R., Castellari, C.: Densities, viscosities, and eefractive indices of binary mixtures containing n-hexane + components of pine resins and essential oils at 298.15 K. J. Chem. Eng. Data 47, 93–97 (2002)
Gill, D.S., Cheema, T.S.: Preferential solvation of ions in mixed solvents—I: conductance and viscosity measurements of electrolytes in N,N-dimethylformamide + acetonitrile mixtures 25 °C. Z. Phys. Chem (N.F) 134, 205–214 (1983)
Marcus, Y.: Ion Solvation. Wiley, New York (1985)
Prolongo, M.G., Mesagosa, R.M., Fuentes, H.I., Horta, A.: Viscosities and excess volumes of binary mixtures formed by the liquids acetonitrile, pentyl acetate, 1-chlorobutane, and carbon tetrachloride at 25 °C. J. Phys. Chem. 88, 2163–2167 (1984)
Iloukhani, H., Rezaei-Sameti, M.: Excess molar volumes of the ternary system methylcyclohexane (1) + cyclohexane (2) + n-alkanes (3) at T = 298.15 K. J. Chem. Thermodyn. 37, 1151–1161 (2005)
Brocos, P., Pineiro, A., Bravo, R., Amigo, A.: Refractive indices, molar volumes and molar refractions of binary liquid mixtures: concepts and correlations. Phys. Chem. Chem. Phys. 5, 550–557 (2003)
Pineiro, A., Brocos, P., Amigo, A., Pintos, M., Bravo, R.: Prediction of excess volumes and excess surface tensions from experimental refractive indices. Phys. Chem. Liq. 38, 251–260 (2000)
Venkateswara Rao, P., Gowrisankar, M., Venkatramana, L., Srinivasa Krishna, T., Ravindhranath, K.: Studies on the importance of nature of substituent on the thermodynamic and transport properties of liquid mixtures at various temperatures. J. Chem. Thermodyn. 101, 92–101 (2016)
Wang, H., Liu, W., Huang, J.: Densities and volumetric properties of a (xylene + dimethyl sulfoxide) at temperature from (293.15 to 353.15) K. J. Chem. Thermodyn. 36, 743–752 (2004)
Hawrylak, B., Gracie, K., Palepu, R.: Thermodynamic properties of binary mixtures of butanediols with water. J. Solution Chem. 27, 17–31 (1998)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raghavendra, M., Gowrisankar, M., Krishna, T.S. et al. Studies of Associated Solutions: Evaluation of Thermodynamic Parameters of Blends of 2-Methylaniline and Substituted Ethanols at Various Temperatures. J Solution Chem 47, 684–704 (2018). https://doi.org/10.1007/s10953-018-0749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0749-5