Abstract
Wastewater generated by dyeing industries is of great threat to the globe and is major area of concern. In present paper, we deals with oxidation of brilliant green (BG) by catalytic route in alkaline medium at 298 K. Osmium(VIII) used as catalyst and the reaction showed first-order and zero-order behavior with respect to [BG] and [CAT] respectively. The reaction followed fractional-order kinetics with respect to [NaOH] and [Os(VIII)]. Negligible effect of [Cl−], [PTS] and ionic strength of the medium on the rate of oxidation have also been noted. Decrease in the rate of reaction with decrease in the dielectric constant of the medium was also observed. Comparative studies between catalyzed and uncatalyzed reaction have also been reported. It is found that catalyzed reaction is four fold faster than uncatalyzed reaction. Activation parameter and catalytic constant (KC) have also been calculated at different temperatures. GC–MS analysis identified the oxidation products of the BG i.e. N, N-diethylaminobenzophenone and p-N, N-diethylamino phenol while the stoichiometry of the reaction was found to be 1:1. On the basis of kinetic results, a plausible mechanism has been proposed and verified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Harmful chemicals, dyes, oils etc. pollute the water. Among the pollutants of water, a major role is played by dyes. Most of the dyes released from the pharmacy and textile industries, as they are mutagenic and teratogenic can cause serious health hazards to humans and livestock [1]. Dyes released into aquatic environment decrease or stop capacity of water re-oxygenation by blocking sunlight thereby increasing BOD valueand it can also impart color to water [2, 12, 13]. Therefore these conditions generally disturb or prevent the growth of aquatic plants and animals [3, 4]. BG being carcinogenic in nature has been banned in many countries [5]. BG, besides being a compound of biological interest [6] has a major use in biological staining, modern textile and leather industries [7]. It is also used in antiseptic preparations active against gram-positive bacteria. It is irritating to skin, respiratory system and gastrointestinal tract [8, 9] and can cause serious damage to eyes. It can also produce serious health damage [10] on inhalation and it is also investigated as a mutagen in microorganism. BG causes some degree of carcinogenicity, hypersensitivity reactions, microbial and fish toxicity [11].
For the oxidation of variety of organic compounds, aromatic N-halosulphonamides, a group of mild oxidizing agents, have been extensively used [14,15,16]. These oxidants have strongly polarized N-linked halogens which are in the + 1 state. They undergo two electrons change which forms halide ions and corresponding sulphonamides. Sodium N-chloro-p-toluenesulphonamide (CAT or RNClNa) is a prominent member of this class of oxidant. In both acidic and alkaline media it behaves as an oxidizing agent. It is versatile oxidizing agent and due to formation of its various oxidizing species depending upon pH of the medium, it has shown a variety of kinetics result [17,18,19,20,21,22,23]. Depending on the reaction conditions, it can behave as both electrophiles and nucleophiles [24]. Extensive literature survey depicts only tworeports on the kinetics of oxidation of triphenylmethane dyes by CAT [25, 26].
Transition metals due to theirmultiple oxidation state are known to catalyze many oxidation–reduction reactions [27]. In recent years either alone or binary mixtures, the use of transition metal ions, as catalysts in various redox processes have attracted considerable interest [28]. For different metal ion catalysts like Cr(III) [29], Ru(III) [30,31,32,33,34,35,36,37,38,39,40], Ir(III) [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55], Pd(II) [52, 56,57,58,59] and Os(VIII) [59,60,61,62,63], Au(II) [64] different oxidizing agents have been used in the oxidation of organic compounds. The mechanism of the reaction relies upon the nature of the substrate, oxidant and moreover upon the ways in which transition metal complex ions play their role in order to promote the reactant molecules to the activated state before finally changing into products under experimental conditions. The main purpose of the present study are: (i) to confirm real reactive species of oxidant and catalyst (ii) to elaborate the plausible reaction mechanism (iii)to form compatibility of rate law with kinetic results and to calculate activation parameters (iv) to identify the oxidation products (v)to calculate thermodynamic parameters (vi) to find the catalytic efficiency of Os(VIII) and (vii) to compare and contrast the catalyzed reactivity with that of uncatalyzed one. Overall, the removal of BG dye from aqueous system is the main concern for both human health and ecosystem, thus it encouraging us the search for low cost and better efficient method which helps in removing color contaminant from waste water. So we have developed a catalytic transformation route for degradation of BG dye and its kinetic study to ensure its feasibility.
2 Experimental
2.1 Materials
During the experiment, all chemicals used were of reagent grade and water was triple distilled. BG (Sd. Fine Chem.) was used without any further purification and was 99.8% pure. Purification of CAT (E-Merck) was done by the method of Morris et al. [20]. Firstly, an aqueous solution of CAT was prepared then it was standardized iodometrically and finally stored in amber colored stoppered bottles until further use. By dissolving OsO4 (Johnson Mathey) in 0.50 mol dm−3 NaOH a standard stock solution of Os(VIII) was prepared. The concentration was confirmed [69] by determining the unreacted [Fe(CN)2]4− solution with standard Ce(IV) solution in an acidic medium. Without further purification KNO3 and NaOH were used.
2.2 Kinetic measurements
By using an UV–visible spectrophotometer (Digital Spectrophotometer 166, Systronic, India) kinetic measurements were carried out. The kinetic experiments in the present study were carried out between 293 and 318 K. To conduct this experiment, a Ragga Ultra Cold chamber with digital temperature control (India) was operated, thereby keeping the temperature as constant with an accuracy ± 0.1 ºC. In the present study, kinetic runs were performed under pseudo-first-order conditions with a known excess of the [CAT] over [BG] at 298 K. Glass-stoppered Pyrex boiling tubes whose outer surfaces coated black were used to perform the reactions to eliminate any photochemical effects. To keep the total volume constant for all runs, the oxidant as well as requisite amounts of BG, NaOH, Os(VIII) and water were taken in separate tubes and were thermostatted for 30 min at 298 K. To initiate the reaction, a measured amount of oxidant was rapidly added to the stirred reaction mixture. Instantly, 4 ml aliquot of the solution was pipetted into a cuvette positioned in the spectrophotometer. For nearly three half-lives, absorbance (Abs) measurements were made at λmax = 622 nm for BG. To evaluate the pseudo-first-order rate constants (k1) which were found reproducible within ± 5%, plots of log Abs versus time were made. To see the effect of temperature and to calculate the thermodynamic activation parameters, the reaction was also performed at various temperatures (298 K, 303 K, 308 K, 313 K and 318 K). In order to understand the effect of dissolved O2 on the rate of the reaction kinetic runs were also carried out in N2 atmosphere. No prominent difference in the results was observed under a N2 atmosphere and in the presence of air.
2.3 Stiochiometry and product analysis
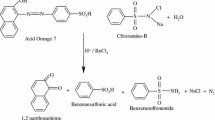
Different sets of reaction mixtures containing varying ratios of CAT to BG in presence of constant amount of NaOH and Os(VIII) were kept for 72 h in a closed vessel at 298 K. Estimation of unconsumed CAT in each set revealed that for the oxidation of 1 mol of BG, 1 mol of CAT was consumed. Accordingly, the following stiochiometry equation may be expressed as (Scheme 1).
The reaction mixture was neutralized with dilute HCl and the products were extracted with ethyl acetate after completion of the reaction. The organic products were identified and separated by using TLC technique and column-chromatography respectively. Methanol was used to recrystallize the purified compounds. p -toluenesulfonamide (PTS) was identified [65] as the reduction product of CAT by paper chromatography using PhCH2OH saturated with H2O as the solvent with 0.5% vanillin in 1% HCl solution in C2H5OH, as the spray reagent. Furthermore the result was confirmed by its melting point 139 ℃, which was within close proximity to the reported temperature of 137–140 ℃ in previous works [66]. N, N-diethylaminobenzophenone and p-N, N-diethylamino phenol were identified as oxidation products of BG. By gas chromatography coupled with mass spectral techniques (GC–MS) these compounds were confirmed. The mass spectra showed N, N-diethylaminobenzophenone and p-N, N-diethylamino phenol as oxidation products having molecular ion peak 253 amu and 165 amu respectively. It was also observed that under the present reaction conditions, there was no further oxidation of these products.
3 Results and discussion
The detailed kinetic investigations were performed under pseudo-first-order conditions of [CAT] ≫ [BG] (Table 1).
3.1 Dependence of rate on the concentration of BG
Under pseudo-first-order condition of [CAT] > [BG], the effect of BG was studied in the range (2.5 × 10−5 to 8 × 10−5). The rate constants of the reaction in each kinetic run was determined by plotting of the log Abs versus time (Fig. 1, Table 2), which formed straight line and shows that the reaction under the chosen conditions follows pseudo-first-order kinetics.
3.2 Dependence of rate on the concentration of NaOH
The effect of NaOH was studied in the range of (1 × 10−4 to 10 × 10−4) at constant concentrations of BG, CAT andOs(VIII). The rate constants increased with an increase in [NaOH] (Table 3) and plots of log k1 versus log [OH−] were linear with fractional slope (0.848), showing a fractional-order dependence of the rate on [NaOH] (Fig. 2).
3.3 Dependence of rate on the concentration of CAT
The effect of CAT was studied in the range of (2 × 10−4 to 12 × 10−4) at constant concentrations of BG, NaOH and Os(VIII). The value of k1 remains constant (Table 2), showing a zero-order with respect to [CAT].
3.4 Dependence of rate on the concentration of Os(VIII)
To study the effect of Os(VIII) concentration on the rate of reaction, the reaction concentration varied in the range of (1.16 × 10−4 to 11.65 × 10−4) at constant [BG], [CAT] and [NaOH] at 298 K. The result elucidates that rate of reaction increase with increasing [Os(VIII)] (Table 3). Plots of log k1 versus log [Os(VIII)] were linear with fractional slope (0.783), confirming fractional-order dependence on [Os(VIII)](Fig. 3).
3.5 Dependence of rate on PTS and Cl− concentrations
To study the effect of PTS (reduction product of CAT) concentration on the rate of reaction, the reaction concentration varied in the range of (3 × 10−5 to 15 × 10−5) at constant [BG], [CAT], [NaOH] and [Os(VIII)] at 298 K. The value of k1 remains constant showing a zero-order with respect to [PTS] (Table 2). Variation of [Cl−] did not bring about any significant change in k1 values under the constant experimental conditions.
3.6 Dependence of rate on ionic strength
Keeping the other experimental conditions constant, the effect of ionic strength (µ) of the medium was studied in the range of (6 × 10−4 to 24 × 10−4), using KNO3 solution. It is observed that the µ had no effects on the rate of oxidation (Table 2), which indicates the involvement of a non-ionic species in the rate determining step.
3.7 Dependence of rate on the variation of temperature
The reaction was carried out at five different temperatures (298–313 K) while the other experimental conditions were kept constant. From the linear Arrhenius plots of log k1 versus 1/T (Fig. 4), activation energy (Ea) and other activation parameters (ΔH≠, ΔS≠, ΔG≠ and log A) for the overall reaction have been computed. The rate constants corresponding to different temperatures and the calculated activation parameters are given in (Table 4) for uncatalyzed and Os(VIII) catalyzed reaction.
3.8 Polymerization study
The reaction mixture with acrylamide was placed in an inert atmosphere for 24 h to test the presence of free radicals in the reaction. It is found that there is no precipitate in the reaction mixture, when the reaction mixture is diluted with methanol. This clearly indicates that the free radicals are not formed in the redox reaction under observation.
3.9 Reactive species of CAT
In both acidic and alkaline media, CAT (ArNClNa) acts as an oxidizing agent. In aqueous solutions CAT behaves as a strong electrolyte and it shows different types of reactive species (Eqs. 1–6) in solutions depending on the pH of the medium [20, 67, 68].
3.10 Reactive species of Os(VIII) catalyst
Osmium(VIII) has been reviewed as a catalyst in some redox reactions. At different OH− concentrations Os(VIII) is known to form different complexes like [OsO4(OH)2]2− and [OsO5(OH)]3−[69]. [OsO5(OH)]3− is significant at higher concentration of OH−. Since the rate of oxidation increased with increase in [OH−], hence at lower concentrations of OH−, as employed in the present study, it is reasonable that [OsO4(OH)2]2− was operative and its formation is important in the reaction. Mechanistically most studies [70] propose that electron is being transferred between the substrate and catalyst in the rate determining steps leading to the products, followed by the rapid oxidation of Os (VI/VII) to Os(VIII) by main oxidants, resulting in zeroth order kinetics with respect to the main oxidant. In view of unit order in each osmium, substrate and oxidant, Os(VIII) is regenerated by Os(VI) intervention [71]. In some other reports [72], it is observed that with the regeneration of the catalyst, Os(VIII) forms complex with substrate, in view of apparent less than unit order in substrate concentrations which is oxidized by the oxidant. Hence the study of the behavior of Os(VIII) becomes prominent. First order dependency in [BG] and fractional order in [OH−], [Os(VIII)] and zero order in [CAT] was observed in the present investigation.
3.11 Spectral evidence for the Os(VIII) catalyzed oxidation of BG by CAT in alkaline medium
The color imparted by BG is due to its chemicalstructure which consists of a central carbon bonded to three aromatic rings, one of which is in the quinoid form (the chromophore) and the oxochrome is –NR2. The changes in the absorption spectra of BG solution (3 × 10−5 mol dm−3) during the oxidation process byCAT (3 × 10−4 mol dm−3) in alkaline media (4 × 10−3 mol dm−3) at 298 K at different times are shown in (Fig. 5). A rapid degradation of the BG is indicated by the decrease of the absorption peak of the dye at λmax = 622 nm. Decrease in Abs intensity of the bond at λmax during oxidation also express loss of conjugation. The decrease also indicates that the most active site for oxidative attack is central carbon attached to the aromatic ring (quinoid form) [26].
3.12 Reaction scheme
On the basis of observed kinetic results and taking [OsO4(OH)2]2− as the most active species of OsO4, the reaction steps in Scheme 1 are being proposed for the oxidation of BG by CAT in an alkaline medium catalyzed by Os(VIII).
According to the reaction Scheme 1 and considering the fact that 1 mol of BG is oxidized by 1 mol of CAT, the rate can be expressed as:
On the basis of Scheme 1, equilibrium steps (i–ii), Eqs. 8 and 9 can be obtained in the following forms, and taking S as BG and C2 as [OsO4(OH)2]2− respectively
Substitute the expression for [C1] (Eq. 8) into Eq. 9 to obtain the expression for Eq. 10
By substituting for [C3] from Eq. 10 into Eq. 7, we get
At any time in the reaction, the total concentration of S that is [S]T, can be expressed as
By substituting for [C1] and [C3] from Eqs. 8 and 10, respectively into Eq. 12 and solving for [S], we get
Substitute the expression for [S] (Eq. 15) into Eq. 11 to obtain the expression for Eq. 16
Equation 16 is the rate law based on the observed kinetics orders with respect to each reactant involved in the reaction.
The rearrangement of Eq. 16 gives Eq. 17
Equation 18 indicates that if a plot is made between 1/k1 vs 1/[Os] (Fig. 6) and 1/k1 vs 1/[OH−] (Fig. 7), a straight line with positive intercept on y-axis will be obtained and this proves the validity of the rate law (Eq. 16) and the proposed reaction scheme 1, on the basis of which the rate law Eq. 16 has been derived. For the oxidation of BG the values obtained for k, K1 and K2 have been calculated and found as 3.52 × 10−3, 96.27 and 22.7 × 103 respectively.
3.13 Effect of dielectric constant and calculation of the size of the activated complex
Given equation explains the dependence of rate constant on the dielectric constant of medium:
where the rate constant in a medium of infinite dielectric constant is given by ko, ZA and ZB are the charges of reacting ions, dAB refers to the size of activated complex, T is absolute temperature and D is the dielectric constant of the medium. This equation shows that a straight line having slope equal to − ZAZBe2N/2.303 (4πε0)dABRT will be obtained if plot is made between log k1 versus 1/D (Fig. 8). A plot of log k1 versus 1/D gives a straight line, for the limiting case of zero angle approach between two dipoles or an ion dipole system, with a negative slope for a reaction between a negative ion and a dipole or between two dipoles, while a positive slope for a positive ion–dipole interaction. The former concept agrees with the last observations. With the help of slope of straight line the value of dAB has been evaluated and is found to be 0.73 Aº. The proposed mechanism is also supported by the negative values of dielectric constant, moderate values of energy of activation and other thermodynamic parameters. Highly solvated transition state is indicated by the fairly high positive values of free energy of activation and enthalpy of activation. Formation of the compact activated complex with fewer degrees of freedom is suggested by large negative value of entropy of activation.
3.14 Comparision of Os(VIII) catalyzed and uncatalyzed reactions
In order to evaluate the catalytic efficiency of Os(VIII) it was thought worthwhile to compare the reactivity of BG with CAT in the absence of Os(VIII) catalyst under identical experimental conditions. Consequently, the reactions were studied at different temperatures (293–313 K) and values of activation parameters for the uncatalyzed reactions were computedfrom the Arrhenius plots of log k1 versus 1/T ((Table 4) (Fig. 4). As compared to the uncatalyzed reactions, Os(VIII) catalyzed reactions were found to be about four times faster. This was also confirmed by the calculated activation parameters (Table 4). Thus the observed rates of oxidation of BG by CAT in the presence of Os(VIII) catalyst justify the need of a catalyst for a facile oxidation. Further, the results also suggests that Os(VIII) has been an efficient catalyst in effecting the facile oxidation of BG dye by CAT in alkaline medium. The reaction path is altered by the catalyst Os(VIII)by stabilizing the transition state, which inturn provides an alternative pathway having lower activation energy for the reaction.
3.15 Catalytic activity
As pointed out by Moelwyn-Hughes [73] in the presence of catalyst, the uncatalyzed and catalyzed reactions proceed simultaneously, so that,
Here the observed pseudo-first-order rate constant obtained in the presence of Os(VIII) is represented by k1, k0 is that for the uncatalyzed reaction, KC is the catalytic constant and × is the order of the reaction with respect to Os(VIII). × value for the standard run was found to be 0.783 in the present investigation. Then KCvalue is calculated using the equation.
The value of KC was found to be varying with temperature when evaluated for BG at different temperatures (293–313 K). The values of energy of activation and other activation parameters for the catalyst were computedby plotting log KC versus 1/T, which were found to be linear (Fig. 9). The summarized results are shown in Table 5.
3.16 Correlation coefficient
A correlation coefficient can be defined as a quantitative measure of some type of correlation and dependence, meaning statistical relationships between two or more random variables or observed data values. The correlation coefficient represented by r which means how closely data in a scatter plot fall along a straight line. If the value of r is near to one, it indicates that given data is expressed by a linear equation and data with values of r near to zero, show non linear relationship.
In our present study, the correlation coefficients between variables are given in Table 6. Allcalculated values are closely related to one indicating a positive linear relationship between variables.
3.17 Multiple regression analysis
In order to arrive at a conclusion, while discovering the relationship between dependent, that is, pseudo-first-order variable rate constant k1 and two independent variable [OH−] and [Os(VIII)], whether or not the proposed mechanism is well in accordance with our experimental kinetic data, a multivariate regression analysis using the computer package STAT GRAPHICS have proved to be very helpful. A relationship between the observed pseudo-first-order rate constant k1 and the concentration of all the reactants of the reaction, except BG and CAT was determined with the aid of a multivariate regression analysis.
where k is 3.08 × 10−2. The R-squared statistic indicated that the model as fitted explains 99.70% of the variability in [OH−] and [Os(VIII)]. The adjusted R-squared statistic, which is more suitable for comparing models with different numbers of independent variables, is 99.65%. The standard error of the estimate shows the standard deviation of the residuals to be .0155. This supports the validity of the rate law given in Eq. 16. The proposed reactions in Scheme (1) were used to calculate the rate based on the multiple regression analysis Eq. 22 and hence they are also valid. The validity of the rate law expressed in Eq. 16 and the proposed reaction mechanism is clearly supported by the similarity among the three rates that is, the observed (experimentally), calculated (from the rate law), and predicted (from regression analysis).
4 Comparative studies
An attempt has been made to compare our experimental findings with the results reported earlier for Pd (II) catalyzed oxidation of triphenylmethane dyes (p-Rosanilne, Crystal Violet (CV), Ethyl Violet (EV)) by CAT [25] in an alkaline medium and uncatalyzed oxidation of BG by CAT [26] in an acidic medium. As far as the kinetic order with respect to oxidant is concerned, it is zero-order [26] in the oxidation of BG, first-order in the Pd (II) catalyzed oxidation of triphenylmethane dyes [25] and zero-order in the present study. The reported [25, 26] first-order kinetics in [dye] seems to be similar with the present study as far as order with respect to dye is concerned. As far as the kinetic order with respect to medium is concerned, it is first-order in the uncatalyzed oxidation of BG, fractional-order in Pd (II) catalyzed oxidation of triphenylmethane dyes by CAT and in the present study. Negligible effects of [Cl−] have also been noted in the reported [25, 26] and in present study. The reported [26] negligible effect of ionic strength of the medium seems to be similar with the present study, but dissimilar for Pd (II) catalyzed oxidation of triphenylmethane dyes by CAT [25]. The dielectric constant showed a negative effect on the reaction rate in the uncatalyzedoxidation of BG by CAT [26] and in the present study. Addition of p-toluenesulfonamide (PTS) retards the rate for triphenylmethane dyes [25] and had no significant effect on rate of uncatalyzed oxidation of BG by CAT [26] and in the present study.
5 Conclusion
The oxidation of BG by CAT experienced a slow reaction rate in alkaline media, but increased in rate in the presence of the Os(VIII) catalyst. The reactive species involved was only the [OsO4(OH)2]2−for the Os(VIII) catalyzed oxidation of BG by CAT. Os(VIII) catalyzed reactions were found to proceed nearly four faster than the uncatalyzed reactions and justifies the use of Os(VIII) catalyst for the facile oxidation of the BG by CAT in alkaline medium. The observed results were explained by plausible mechanisms and the related rate law was deduced. It can be stated that Os(VIII) acts as an efficient catalyst for the oxidation of BG by CAT in alkaline medium.
References
Mekkawy HA, Ali MO, El-Zawahry AM (1998) Toxic effect of synthetic and natural food dyes on renal and hepatic functions in rat. Toxicol Lett 95:150–155
Munusamy S, Aparna RSL, Prasad RGS (2013) Photocatalytic effect of TiO2 and the effect of dopants on dedragation of BG. Sustain Chem Process 1:1–8
Song YL, Tai J, Bai B (2010) TiO2-assisted photodegradtion of direct blue 78 in aqueous solution in sunlight. Water Air Soil Pollut 213:311–317
Wang S (2008) A comparative study of fenton and fenton-like reaction kinetics in decolourisation of wastewaster. Dyes Pigments 76:714–720
Gogete RP, Bhosale GS (2013) Comparison of effectiveness of acoustic cavitation and hydrodynamic cavitation in combined treatment schemes for degradation of dye wastewater. Chem Eng Process 71:59–69
Leitch A (1996) Brilliant green as an antiseptic. Br Med J 1:236–237
Sood S, Umar A, Mehta SK, Sinha ASK, Kansal SK (2015) Efficient photocatalytic degradation of brilliant green using Sr-doped TiO2 nanoparticles. Ceram Int 41:3533–3540
Mane VS, Vijay Babu PV (2011) Studies on the adsorption of BG dye from aqueous solution onto low cost NaOH treated saw dust. Desalination 273:321–329
Mane VS, Mall ID, Shrivastava VC (2007) Use of bagasse fly ash as an adsorbent for the removal of BG from aqueous solution. Dyes Pigments 73:269–278
Mittal A, Kaur D, Mittal J (2008) Applicability of waste materials-bottom ash and deoiled soya-as adsorbents for the removal and recovery of a hazardous dye, brilliant green. J Colloid Interface Sci 326:8–17
Vachalkova A, Novotny L, Blesova M (1996) Polarographic-reduction of some triphenylmethane dyes and their potential carcinogenic activity. Neoplasma 43:113–117
Khera MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res 39:5135–5141
Ahmed R, Kumar R (2010) Kinetic and thermodynamic studies of brilliant green adsorption onto Carbon/Iron oxide nano composite. J Korean Chem Soc 54:125–130
Rangappa KS, Raghavendra MP, Mahadevappa DS, Gowda DC (1998) Kinetics and mechanism of oxidation of erythro-series pentoses and hexoses by N-chloro- p- toluenesulfonamide. Carbohydr Res 306:57–67
Rangappa KS, Manjunathaswami H, Raghavendra MP, Gowda DC (1998) Oxidation of threose-series pentoses and hexoses by sodium-N- chloro-p-toluenesulfonamide. Carbohydr Res 307:253–262
Mahadevappa DS, Rangappa KS, Gowda NMM, Gowda BT (1981) Kinetics and mechanism of oxidation of L- threonine in acid media by sodium-N-chloro p-toluenesulfonamide. J Phys Chem 85:3651–3658
Ramkrishna S, Kandlikar S (1988) Kinetics of oxidation of mono, di and tri-chloro acetic acids by chloramine-T in acid medium: catalysis by Ir(III). Indian J Chem 27A:27–32
Jha S, Sharma PD, Gupta YK (1983) Kinetics and mechanism of the copper(II)-catalyzed oxidation of lactic acid with chloramine-T in alkaline solutions. Inorg Chem 22:1393–1395
Sailani R, Bhasin M, Khandelwal CL, Sharma PD (2014) Kinetics and mechanism of electron transfer reaction: oxidation of sulfanilic acid by N-chloro p-toluenesulfonamide in acid perchlorate medium. Bull Korean Chem Soc 35:111–116
Morris JC, Salazar JA, Winemann MA (1948) Equilibrium studies on N-chloro compounds. J Am Chem Soc 70:2036–2041
Kambo N, Upadhyay SK (2004) Kinetics and mechanism of platinum(IV) catalyzed oxidation of some hexoses by chloramine-T. Indian J Chem 43A:1210–1215
Ramchandra H, Mahadevappa DS, Rangappa KS, Gowda NMM (1997) Ru(III) catalyzed mechanistic studies of oxidation of benzhydrols by sodium-N-chloro-p-toluenesulfonamide in HCl medium. Int J Chem Kinet 29:773–780
Shukla A, Gupta S, Upadhyay SK (1991) Pd(II) complexes of amino alcohols and their reaction with chloramine-T: a kinetic study. Int J Chem Kinet 23:279–288
Kulkarni RM, Bilehal DC, Nandibewoor ST (2002) Oxidative degradation of paracetamol by diperiodateonickelate (IV) in aqueous alkaline medium. J Chem Res 40:147–148
Vinod KN, Gowda KNN (2009) Oxidative decolorization of triphenylmethane dyes by chloramine-T in alkaline medium catalyzed by Pd(II): a comparative spectrophotometric kinetic and mechanistic approach. J Mol Catal A Chem 298:60–68
Singh AK, Bano S (2014) Mechanistic aspects for the oxidation of brilliant green dye by chloramine-T in the presence of perchloric acid:a spectrophotometric kinetic approach. Res Chem Intermed 40:605–617
Abrar JC, Shweta JM, Nandibewoor ST (2010) Osmium(VIII) catalyzed and uncatalyzed oxidation of a hemorheologic drug pentoxifyline by alkaline copper(III) periodate complex: a comparative kinetic and mechanistic approach. Polyhedron 29:2875–2883
Singh SP, Singh AK, Singh AK (2008) First and novel oxidation of d-fructose by potassium iodate using [IrCl3(H2O)OH]− complex as homogeneous catalyst in alkaline medium. J Mol Catal A Chem 293:97–102
Das AK (2001) Kinetic and mechanistic aspects of metal ion catalysis in cerium(IV) oxidation. Coord Chem Rev 213:307–325
Song WY, Jiang QM (2005) Kinetics and mechanism of Cr(III) catalyzed oxidation of tetrahydrofurfuryl alcohol by cerium (IV) in aqueous sulfuric acid medium. Acta Chim Sinica 63:109–113
Das AK, Das M (1995) Kinetics and mechanism of Ru(III) catalyzed oxidation of ethanol by cerium(IV) in aqueous sulfuric acid media. Int J Chem Kinet 27:7–16
Singh AK, Singh AK, Singh V, Rahmani S, Singh B (2006) Ru(III) catalyzed oxidation of diethanolamine and triethanolamine by Br(V) in presence of perchloric acid. J Chem Res 8:56–63
Singh AK, Singh V, Singh AK, Gupta N, Singh V (2002) Kinetics and mechanism of Ru(III) and Hg(II) co-catalyzed oxidation of d-galactose and d-ribose by N-bromoacetamide in perchloric acid. Carbohydr Res 337:345–351
Singh AK, Jain B, Negi R, Katre Y, Singh SP, Sharma VK (2009) A novel oxidation of valine by N-bromophthalimide in the presence of Ru(III) chloride as a homogeneous catalyst. Catal Lett 131:98–104
Singh AK, Negi R, Jain B, Katre Y, Singh SP, Sharma VK (2009) Kinetics and mechanism of Ru(III) catalyzed oxidation of paracetamol by chloramine-T in aqueous acidic medium. Catal Lett 132:285–291
Singh AK, Jain B, Negi R, Katre Y, Singh SP, Sharma VK (2009) Kinetics and mechanism of oxidation of β-alanine by N-bromophthalimide in the presence of Ru(III) chloride as homogeneous catalyst in acidic medium. Transit Met Chem 34:521–528
Singh AK, Jain B, Negi R, Katre Y, Singh SP, Sharma VK (2010) Kinetic study of Ru(III)- catalyzed oxidation of glycine by N-bromophthalimide in acidic medium. Transit Met Chem 35:407–414
Khan AAP, Khan A, Asiri AM, Singh AK (2015) Homogeneous catalysis of Ru(III) for the oxidation of thiamine by CAT in acidic medium. Int J Electrochem Sci 10:759–774
Kushwaha U, Singh A, Kumar A, Singh AK, Khan F (2012) A kinetic and mechanistic study of tarteric acid by potassium bromate in perchloric acid medium catalyzed by Ru(III). J Chem Pharm Res 4:3144–3153
Shetti NP, Hosamani RR, Nandibewoor ST (2009) Mechanistic investigations of Ru(III) catalyzed oxidation of l-trptophan by diperiodatocuprate(III) in aqueous alkaline media: a kinetic study. Open Catal J 2:130–139
Singh AK, Sachdev N, Shrivastava A, Katre Y, Singh SP (2010) A novel and facile oxidation of d-glucose by N-bromophthalimide in the presence of chloro-complex of Ru(III). Synth React Inorg Met Org Nano Met Chem 40:947–954
Das AK, Das M (1995) Kinetics and mechanism of Ir(III) catalyzed oxidation of formic acid by cerium(IV) in aqueous sulfuric acid media. Indian J Chem 34A:866–872
Singh AK, Negi R, Katre Y, Singh SP (2008) Mechanistic study of oxidation of paracetamol by chloramine-T using micro-amount of chloro-complex of Ir(III) as a homogeneous catalyst in acidic medium. J Mol Catal A Chem 302:36–42
Singh AK, Jain B, Katre Y (2009) Kinetics and mechanism of oxidation of glycine by N-bromophthalimide in the presence of chlorocomplex of Ir(III) as homogeneous catalyst. Oxid Commun 32:355–370
Singh SP, Singh AK, Singh AK (2009) Kinetics of Ir(III)-catalyzed oxidation of d-glucose by potassium iodate in aqueous alkaline medium. J Carbohydr Chem 28:278–292
Singh AK, Jain B, Negi R, Katre Y, Singh SP, Sharma VK (2010) Kinetic study of oxidation of valine by N-bromophtalimide in presence of Ir(III) chloride as homogeneous catalyst. Synth React Inorg Met Org Nano Met Chem 40:71–77
Tandon PK, Sahgal S, Singh AK, Kumar S, Dhusis M (2006) Oxidation of cyclic ketones by cerium (IV) in presence of Ir(III) chloride. J Mol Catal A Chem 258:320–326
Singh AK, Srivastava S, Srivastava J, Singh R (2007) Kinetics and mechanism of the Ir(III) catalyzed oxidation of xylose and maltose by potassium iodate in aqueous alkaline medium. Carbohydr Res 342:1078–1090
Tandon PK, Purwar M, Singh S, Srivastava N (2008) Uncatalyzed and Ir(III) catalyzed oxidation of p-methoxybenzaldehyde by cerium(IV). J Mol Chem A Chem 284:120–126
Singh AK, Rahmani S, Singh B, Singh RK, Singh M (2004) Mechanism of Ir(III) and Hg(II) co catalyzed oxidation of reducing sugars by N-bromoacetamide in acidic medium. J Phys Org Chem 17:249–256
Singh AK, Rahmani S, Singh VK, Gupta V, Kesarwani D, Singh B (2001) Irridium (III) catalysis of N-bromosuccinimide oxidation of reducing sugars in aqueous acid. Indian J Chem 40A:519–523
Tandon PK, Gayatri SS, Srivastav M, Singh SB (2007) Catalysis by Ir(III), Rh(III) and Pd(II) metal ions in the oxidation of organic compounds with H2O2. Appl Organomet Chem 21:135–138
Yong-Qing Z, Hong-Mei L, Lin Y, Guo-Zhong Y, Wen-Yu S, Yu-Kai L (2007) Kinetics and mechanism of Ir(III)-catalyzed oxidation of ethanol amine by cerium (IV) in sulfuric acid media. Chem Res Chin 23:338
Tandon PK, Sahgal S, Gayatri PM, Dhusia M (2006) Oxidation of ketones by cerium(IV) in presence of iridium(III) chloride. J Mol Chem A Chem 250:203–209
Tandon PK, Singh AK, Sahgal S, Kumar S (2008) Oxidation of cyclic alcohols by cerium(IV) in acidic medium in the presence of iridium(III) chloride. J Mol Chem A Chem 282:136–143
Ashish SSP, Singh AK, Singh B (2007) Mechanistic study of Pd(II) catalyzed oxidation of crotonic acid by periodate in aqueous perchloric acid medium. J Mol Catal A Chem 266:231–235
Singh AK, Singh V, Rahmani S, Singh AK, Singh B (2003) Mechanism of Pd(II) and Hg(II) co-catalyzed oxidation of d-mannose and maltose by acidic solution of N-bromoacetamide. J Mol Catal A Chem 197:91–100
Singh AK, Jain B, Negi R, Katre Y, Singh SP (2009) Oxidation of valine by N-bromophthalimide in presence of chloro complex of Pd(II) as homogeneous catalyst: a kinetic and mechanistic study. Open Catal J 2:12–20
Ashish SAK, Singh AK, Singh B (2004) Kinetics of oxidation of crotonic acid by sodium N-chloro-p-toluenesulphonamide in the presence of Pd(II) and Os(VIII) as homogeneous catalysts. Indian J Chem 43A:1645–1653
Shetti NP, Hegde RN, Nandibewoor ST (2009) Mechanistics aspects of uncatalyzed and Os(VIII) catalyzed oxidation of 5-flourouracil- an anticancer drug by alkaline diperiodatoargentate. Inorg Chim Acta 362:2270–2278
Tripathi R, Upadhyay SK (2004) Kinetics of oxidation of reducing sugars by catalytic amount of Os(VIII) in presence of periodate. Int J Chem Kinetics 36:441–448
Tripathi R, Kambo N, Upadhyay SK (2004) Kinetic and mechanism of Ru(III) catalyzed oxidation of some reducing sugars by sodium metaperiodate in alkaline medium. J Bulg Chem Indian 75:18–26
Malode SJ, Shetti NP, Nandibewoor ST (2012) Mechanistic aspects of Os(VIII) catalyzed oxidation of loop diuretic drug furosemide by Ag(III) periodate complex in aqueous alkaline medium. J Chem Sci 124:421–430
Saxena OC (1967) New titrimetric microdetermination of antipyrine. Microchem J 12:542–546
Puttaswamy JRV, Nirmala V, Radhakrishna A (2005) Ru(III) catalyzed oxidation of some N-hetero cycles by chloramine-T in hydrochloric acid medium: a kinetic and mechanistic study. J Mol Catal A Chem 229:211–220
Mulla RM, Gurubasavaraj NST (2006) Kinetics of Ru(III) catalyzed oxidation of paracetamol by diperidatonickelate (V) in aqueous alkaline medium. Appl Catal A 314:208–215
Bishop E, Jennings VJ (1958) Titrimetric analysis with chloramine-T: the status of chloramine-T as a titrimetric reagent. Talanta 1:197–212
Hardy FE, Johnston JP (1973) The interaction of N-bromo-N-sodiobenezenesulphonamide with p-nitrophenoxide ion. J Chem Soc Perkin Trans II 2:742–746
Sirasamath KT, Hiremath CV, Nandibewwor ST (2006) Kinetic, mechanistic and spectral investigations of Ru(III)/Os(VIII) catalyzed oxidation of paracetamol by alkaline diperiodatoargentate-(III). J Appl Catal A Gen 305:79–89
Mohanty RK, Das M, Das AK (1997) Kinetics and mechanism of Os(VIII) mediated cerium(IV) oxidation of dimethylsulfoxide in aqueous sulfuric acid. Transit Met Chem 22:484–491
Sharma K, Mehrotra RN (2008) Kinetics and mechanism of the Os(VIII) catalyzed oxidation of hypophosphiote and phenyl phosphinite ion by alkaline hexacyanoferrate (III) ion. Polyhedron 27:3425–3432
Puttaswamy SA, Shubha JP (2009) Kinetics and reactivities of Ru(III) and Os(VIII) catalyzed oxidation of ornidazole with chloramine-T in acid and alkaline media: a mechanistic approach. J Mol Catal A Chem 310:24–33
Moelwyn-hughes EA (1947) The kinetics of reactions in solutions, vol 297. Oxford University Press, London
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A.K., Bano, S. & Jain, B. Mechanistic investigation of osmium(VIII)catalyzed oxidation of brilliant green dye by chloramine-T in alkaline medium: a spectrophotometric kinetic study. SN Appl. Sci. 2, 245 (2020). https://doi.org/10.1007/s42452-020-2030-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2030-y