Abstract
Several studies propose that nocturia, which is common among obstructive sleep apnea (OSA) patients, might be benefited from continuous positive airway pressure (CPAP) treatment. However, related literature remains obscure and thus needs further consolidation and clarification. The present study aimed to provide further evidence regarding potent correlation between CPAP and nocturia in OSA patients. Five hundred and thirty publications were selected after a search in PubMed, Cochrane Library, ClinicalTrials.gov, Google Scholar databases, as well as in unpublished literature. Study eligibility criteria were fulfilled by 11 studies. A systematic review and meta-analysis with subgroup analyses and meta-regressions examined 11 means regarding nocturia rates before and after treatment with CPAP in a total of 830 patients. Mean differences (MD) and confidence intervals (CI) were estimated using random effects model. The study was registered to PROSPERO database (ID: 160600). Nocturia rates are diminished after CPAP treatment, when compared with nocturia rates before CPAP treatment: Overall MD = − 1.13, 95% CI: [− 1.48, − 0.78], P < 0.001; however, increased heterogeneity (I2 = 93%, P = < 0.001) was observed. No statistically significant publication bias was detected (Eggers’ regression P = 0.095; Begg and Mazumdar’s P = 1.000). Meta-regression revealed that the beneficial impact of CPAP treatment regarding nocturia episodes is independently enhanced in patient groups with ≤ 60% severe OSA cases (P = 0.001), ≤ 50 years old (P = 0.001), and > 27 mg/m2 BMI (P = 0.012). Nocturia is alleviated in CPAP-treated OSA patients; CPAP beneficial effect is independently correlated with younger age, increased BMI, and less severe cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of partial or complete collapse of the upper airway during sleep. The ensuing reduction or cessation of breathing often leads to acute derangements in gas exchange and recurrent arousals from sleep. The prevalence of obstructive sleep apnea associated with accompanying daytime sleepiness is approximately 3 to 7% for adult men and 2 to 5% for adult women in the general population [1].
Nocturia, awaking from sleep to void, negatively affects health and well-being. Nocturia is more prevalent among the elderly. Although nocturia traditionally has been regarded as a predominantly male condition, it is just as prevalent in women as in men. OSA has been recognized as a major non-urologic cause of nocturia [2].
Several studies propose that OSA patients, when treated with continuous positive airway pressure (CPAP), might have a subsidiary beneficial effect by reducing nocturia [3,4,5,6,7,8,9,10,11]. On the contrary, the only randomized control trial (RCT) published up to date failed to demonstrate similar results [12, 13]. A systematic review and meta-analysis including the latter RCT and four previously published studies [4, 6,7,8] concluded that CPAP treatment produced a statistically significant reduction in the mean number of nocturia incidents [14].
The present study aimed to provide further evidence regarding potent correlation between CPAP and nocturia in OSA patients by identifying all relevant studies and summarize their results.
Materials and Methods
Literature Search
A systematic literature review was conducted using PubMed/MEDLINE, EMBASE, Cochrane Library, and ClinicalTrials.gov databases from December 01, 1999, until September 15, 2020, to identify all studies that reported data concerning frequency of nocturia before and after the use of CPAP in patients with obstructive sleep apnea. The Google Scholar and ResearchGate databases were used as an additional pool of published data, dissertations, and other unpublished work; an iterative search was performed until no additional publication could be traced. Personal communication was followed where needed. The relevant protocol was registered in PROSPERO database on April 28, 2020 (ID: 160600); a revision submitted on September 29, 2020, was emerged during the review process.
Study Selection
The review was independently conducted by two authors (VP and NA) using a search strategy that included the PubMed search terms (nocturia) AND (continuous positive airway pressure) or (nocturia) AND (apnea). A third author (DF) was responsible for any discordance. No software was used for study retrieval. Sources of financial support were traced where possible.
Outcome Measures
The present study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to formulate the basis of pre-specified eligibility criteria using the PICO (P—Populations/People/Patient/Problem: OSA patients, I—Intervention(s): CPAP, C—Comparison: frequency of nocturia episodes before and after the use of CPAP, O—Outcome: mean differences) worksheet and search strategy [15]. The AMSTAR checklist was used to confirm the high quality of the present meta-analysis [16].
Eligible studies were all that (1) are at least partly written in English (e.g., report an abstract in English); (2) are either RCTs or non-RCTs (cohorts) with NOS ≥ 6; (3) report frequency of episodes of nocturia, either as recorded in the 7th question (Q7) of the International Prostate Symptom Score questionnaire (IPSS) or referred separately, before and after the use of CPAP, in both male and female patients with obstructive sleep apnea; (4) report a relevant measure of statistical significance; (5) report either a relevant effect estimate (means accompanied by their standard deviations) or enough data to compute it; and (6) are not duplicates. The two lead authors (VP and NA) determined publication eligibility; a third author (DF) was responsible for any discordance.
Data Extraction
A structured data collection was used to extract the following data from each eligible study: title of the study, name of the first author, year of publication, country where the study was conducted, duration of the study (in months), loss of follow-up (LOF) percentage, female ratio in patient sample, age, BMI, apnea-hypopnea index (AHI), percentage of severe OSAs, sample size, episodes of nocturia before and after CPAP, adjustment for confounders, and Newcastle-Ottawa quality assessment scale (NOS) score. The two lead authors (VP and NA) performed data extraction; a third author (DF) was responsible for any discordance.
Quality Assessment of the Studies
For all eligible studies, the following were performed: (1) Cochrane risk of bias tool (RoB 2.0 tool) evaluating selection, performance, detection, attrition, reporting, and other biases was estimated for RCTs; (2) quality assessment for non-RCTs (either before-after (pre-post) studies without a separate control group or cohorts) was approached using the Newcastle-Ottawa scale (NOS) which evaluates selection, comparability, and outcome, mainly focusing on risk of bias; in detail, selection item was given a maximum of 4 stars, comparability item a maximum of 2 stars, and exposure (for case-control studies) or outcome of interest (for cross-sectional studies) a maximum of 3 stars; (3) the USA National Institute of Health (NIH) quality assessment-based tool for before-after (pre-post) studies with no control group available on https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools was additionally used for all studies with an adaptation for the RCT intervention group when appropriate; quality rate was arbitrarily described as good, fair, and poor when a positive record was attributed to all 12, 9–11, and ≤ 8 criteria, respectively [17]. The two lead authors (VP and NA) performed quality assessment; kappa statistics were used for the evaluation of inter-rater agreement for quality assessment tools. A third author (DF) was responsible for any discordance.
Data Synthesis and Statistical Analysis
Data synthesis was performed using RevMan 5.3 software from the Cochrane Collaboration (London, UK). As effect estimates, mean differences (MD) and confidence intervals (CI) expressed in nocturia episodes per night were extracted from each study and combined together using the random effects, generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study in the pooled analysis inversely to its variance [18]. Random effects model allows generalizing common effect size beyond the (narrowly defined) population included in the analysis [19]. As data between before and during treatment are paired, pooled SD was used as approached by the formula [SDbefore2 + SDafter2 – 2*r*SDbefore*SDafter]½, where SDbefore, SDafter, and r denote SD before initiation of treatment with CPAP, SD after treatment with CPAP, and correlation coefficient between data consequently. In cases that r was unknown, it was arbitrary given a value of 0.7 if statistical significance; this is consistent with Rosenthal’s conservative approach [20]. In cases that only median, range, and sample size was provided, means and SD were estimated as described by Luo et al. [21] and Wan et al. [22], respectively.
Statistical Analysis
Analysis of publication bias was performed through Eggers’ regression, Begg and Mazumdar’s rank correlation test, funnel plot (Precision vs MD) with trim-and-fill analysis, Rosenthal failsafe-N test for number of unpublished studies with the aid of Comprehensive Meta-Analysis software, version 3.3.070.
Heterogeneity was based on Q test and I2; Q test P value < 0.10 and/or I2 > 50% was indicative of significant heterogeneity and was further analyzed. Analysis of heterogeneity was performed through sensitivity analysis, meta-regressions, and subgroup analysis focusing on study characteristics, biases, and confounders.
Sensitivity analysis was used to explore the impact of excluding or including individual studies. Subgroup analysis was used to seek whether qualitative or quantitative interaction exists. Meta-regression was conducted separately for study characteristics and quality assessment as described by NOS items (selection, comparability, and outcome).
OSAs patients with AHI > 30 were regarded as severe. Years passed since publication, sample size, duration of CPAP treatment, female ratio, mean age, mean BMI, percentage of severe OSAs, and percentage of LOF were treated as arbitrarily defined binary variables in both univariate and multivariate analysis (meta-regression). All parameters analyzed in univariate analysis were also included in meta-regression independently of the level of statistical significance in the former analysis; this was supposed to be the safe and informative process given that the linear regression model used was based on bootstrapping (number of samples, 1000; CI level, 95%; sampling, simple) thus compensating for instability that might result from small sample size. Variables with variance inflation factor (VIF) > 2.5 (or tolerance < 0.4) were discarded to avoid collinearity. Missing cases were listwise excluded for multivariate analysis. All statistical tests were carried out using SPSS 20.0 software (IBM Corp ©).
Results
Study Characteristics
We identified 245 and 284 publications in PubMed and EMBASE databases, respectively. ClinicalTrials.gov and Cochrane Library failed to contribute any additional publications. One more publication of interest was identified through Google Scholar. One unpublished pre-print was provided by the second author through personal communication [Apergis, 2019].
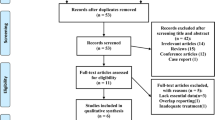
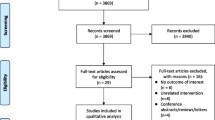
Summing up, all publications taken under consideration for eligibility were 530. Of these, 326 were duplicates, and 190 were excluded from title or abstract. The remaining 14 publications were assessed for eligibility through full-text; three of them were excluded for various reasons, and 11 were found to satisfy all inclusion criteria thus providing 11 means regarding nocturia incidents before and after treatment with CPAP in 830 OSA patients (Fig. 1).
Quality Assessment and Risk of Bias
Cochrane risk of bias (RoB) tool did not reveal any selection, performance, detection, attrition, reporting, or other bias in the single RCT included in the present meta-analysis. All detailed data concerning quality assessment for cohorts based on NOS items, mainly focusing on risk of bias, are provided in Table 1. Furthermore, quality assessment based on USA NIH Quality assessment-based tool for before-after (pre-post) studies with no control group is analytically presented at Table 2. The overall inter-rater agreement for NOS as well as for NIH tool was kappa = 0.89 and 1.00, respectively.
Primary Outcome
In general, nocturia incidents are decreased at a statistically significant extent (P < 0.001) when compared before and after CPAP treatment; the relevant overall MD is − 1.13 (95% CI: − 1.48, − 0.78) clearly favoring CPAP treatment (P < 0.001); however, increased heterogeneity (I2 = 93%, P = <0.001) was observed (Fig. 2).
Publication Bias
No significant publication bias was suspected as funnel plot was not indicative of lack of symmetry, and trim-and-fill analysis produced no imputed data points (Fig. 3), and Rosenthal failsafe-N test rejected ad hoc rule (failsafe-N = 2055). Moreover, Eggers’ regression (P = 0.095) as well as Begg and Mazumdar’s rank correlation test (P = 1.000) yielded a non-statistically significant result.
Analysis of Heterogeneity
Sensitivity analysis was used to explore the impact of excluding or including studies. Interestingly, excluding studies one by one failed to compromise heterogeneity as yielded to comparable levels (88–94%).
Subgroup analysis revealed differences in MD thus indicating potent sources of heterogeneity regarding source (gray literature vs publications, P = 0.005), type of study (non-RCTs vs RCTs, P < 0.001), year of publication (2009 and before vs 2010 and after, P < 0.001), origin of study (Europe/USA vs Asia, P = 0.090), sample size (> 50 vs ≤ 50 patients, P = 0.020), female ratio (no females vs mixed sample, P < 0.001), age (≤ 50 years old vs > 50 years old, P = 0.002), body mass index (BMI) (> 27 mg/m2 vs ≤ 27 kg/m2, P = 0.005), severe OSA cases (≤ 60% vs > 60%, P = 0.003), and quality assessment for both NOS and NIH tool; on the contrary, no differences were detected regarding duration of observation period, loss of follow-up, and adjustment of effect estimates for potent confounders as age and BMI (Table 3).
Meta-regression analysis yielded to a robust model (n = 8, overall P < 0.001, adjusted R2 = 0.971, VIF ≤ 1.5 for all included variables) concluding that the beneficial impact of CPAP treatment in nocturia episodes of OSA patients is independently enhanced in groups of OSA patients that had ≤ 60% severe OSA cases (b = 0.810, P = 0.001), were ≤ 50 years old (b = 0.800, P = 0.001), and had > 27 mg/m2 BMI (b = 0.415, P = 0.012). Detailed data are presented at Table 3.
Discussion
As nocturia is frequent among OSA patients and CPAP has been proposed to ameliorate the relevant morbidity, we have conducted a systematic review and meta-analysis including the only RCT published previously on the topic along with ten relevant cohort studies that met all eligibility criteria. We have demonstrated that the use of CPAP decreases nocturia incidents at a mean of − 1.13 episodes of nocturia/night (95% CI: − 1.48, − 0.78) at a statistically significant extent (P < 0.001). The augmented heterogeneity observed (I2 = 93%, P = <0.001) has been mainly attributed to non-publication bias factors; meta-regression analysis suggests that the profile of patients who might benefit mostly by the use of CPAP includes those who suffer from moderate OSA, are young, and have increased BMI.
One can argue that the present meta-analysis is redundant since Wang et al. first published a meta-analysis on the same topic [14]. However, it is our strong belief that a number of issues concerning the latter study could justify an alternative approach on the field. First, the inevitable limited number of studies included (n = 5), prevented from broader evaluation of the available literature under less strict inclusion criteria and concrete investigation of the potent causes of substantial heterogeneity, other than publication bias, which might be clinically useful. Second, the use of fixed effects model could be replaced with random effects model, as there is urge to enlighten the increased (and unaccountable) heterogeneity. Third, the computation of MDs has to be performed over paired data, as the opposite approach introduces reporting bias; of note, if we apply random effects model in MDs computed for paired samples with r = 0.7 in the meta-analysis of Wang et al., overall MD is computed to be − 1.14 (95% CI: − 1.85, − 0.43), namely, the same as the one reported hereby by ours, with an I2 of 96% (P < 0.001). Fourth, the data of Guilleminault et al. [4] reported in the meta-analysis of Wang et al. disregard all OSA patients who did not exhibit nocturia, thus introducing a strong selection bias. Lastly, the authors failed to search for unpublished material.
Our current approach has the advantage of triple analysis towards potent sources of heterogeneity: sensitivity analysis, subgroup analysis, and meta-regression. Sensitivity analysis illustrated that no single study account for the substantial heterogeneity was observed, as one-by-one exclusion of published studies does not limit heterogeneity more than 5%. This is also true for the introduction of unpublished material, whose exclusion yields to comparable I2 (94%); therefore, even if data from Apergis were eliminated to avoid an additional publication bias linked to the fact that he is also one of the authors of the present study, no substantial difference regarding heterogeneity would be produced. Furthermore, as far as subgroup analysis is concerned, qualitative interaction, a rare phenomenon that may be used as an argument that the most appropriate result of a meta-analysis is the overall effect across all subgroups, was not observed in our case; however, quantitative interaction exists as the size of the effect varies but not the direction, thus indicating that the intervention, hereby CPAP, is beneficial to different degrees in different subgroups. In detail, source (publications or gray literature), study design, year of publication, origin of study, sample size, female ratio in patients group, age, BMI, and severity of OSA cases have been accounted as potent heterogeneity sources according to subgroup analysis; however, multivariate analysis (meta-regression) confirmed the former proposal only for age, BMI, and severity of OSA cases.
All already published data derived from cohorts are qualitatively in keeping with our results, as they all report that the use of CPAP limits nocturia episodes to a variable extent. However, the only RCT included [12, 13], based on intention to treat, fails to exhibit a beneficial role of CPAP in OSA patients regarding nocturia; this could be explained by the fact that the trial refers to elderly patients and thus is not at all representative as far as the total age spectrum of the disease.
Interestingly, no publication bias was detected as implied by results derived from funnel plot with trim-and-fill analysis, Eggers’ regression, Begg and Mazumdar’s rank correlation test, and Rosenthal failsafe-N test; this result could reflect that no clear-cut pre-defined or pre-judged size or even direction of difference was suspected in the scientific community as a whole.
The major limitation of the present study might be the combination of data from different kind of studies, namely, an RCT and ten non-RCT studies (mainly cohorts). However, both univariate analysis and multivariate analysis (meta-regression) did not prove any statistically significant difference regarding overall effect estimates. Thus, this practice might be considered non-misleading. Moreover, the small size of samples analyzed in meta-regression (n = 8) might result to obtain spurious results without any clear indication of there being a problem [23].
A serious query could focus on the decision to proceed to the meta-analysis despite the high heterogeneity. However, several reasons might support our approach: (1) there was little evidence of publication bias (as trim-and-fill analysis suggested no imputed studies) or small size studies effect (as Egger’s regression was not statistically significant), (2) there was no considerable qualitative interaction, (3) although quantitative interaction existed, the direction did not vary, (4) sensitivity analysis did not reveal any particular study to account for the increased heterogeneity, and (5) the vast proportion of the heterogeneity could be explained by the performed meta-regression, as deduced by the relevant adjusted R2 (0.971).
In conclusion, the present meta-analysis illustrated that CPAP may well ameliorate nocturia by reducing nocturia rates at a mean of 1.13 episodes per night; the extent of CPAP beneficial intervention is independently correlated with less severe OSA cases, young age, and increased BMI. Future, cumulative evidence are welcome to further enlighten this field.
References
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. https://doi.org/10.1513/pats.200709-155MG.
Van Kerrebroeck P, Andersson KE. Terminology, epidemiology, etiology, and pathophysiology of nocturia. Neurourol Urodyn. 2014;33(Suppl 1):S2–5. https://doi.org/10.1002/nau.22595.
Fernández-Pello S, Gil R, Escaf S, Rodríguez Villamil L, Alzueta A, Rodríguez C, et al. Lower urinary tract symptoms and obstructive sleep apnea syndrome: urodynamic evolution before and after one year of treatment with continuous positive airway pressure. Actas Urol Esp. 2019;43(7):371–7. https://doi.org/10.1016/j.acuro.2019.03.004.
Guilleminault C, Lin CM, Gonçalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res. 2004;56(5):511–5. https://doi.org/10.1016/S0022-3999(04)00021-2.
İrer B, Çelikhisar A, Çelikhisar H, Bozkurt O, Demir Ö. Evaluation of sexual dysfunction, lower urinary tract symptoms and quality of life in men with obstructive sleep apnea syndrome and the efficacy of continuous positive airway pressure therapy. Urology. 2018;121:86–92. https://doi.org/10.1016/j.urology.2018.08.001.
Liu S, Liu L. Effect of treatment with continuous positive airway pressure on nocturnal polyuria in patients with obstructive sleep apnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2001;24(3):158–60.
Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006;67(5):974–7. https://doi.org/10.1016/j.urology.2005.11.054.
Miyauchi Y, Okazoe H, Okujyo M, Inada F, Kakehi T, Kikuchi H, et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology. 2015;85(2):333–6. https://doi.org/10.1016/j.urology.2014.11.002.
Miyazato M, Tohyama K, Touyama M, Nakamura H, Oshiro T, Ueda S, et al. Effect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndrome. Neurourol Urodyn. 2017;36(2):376–9. https://doi.org/10.1002/nau.22936.
Yu CC, Huang CY, Kuo WK, Chen CY. Continuous positive airway pressure improves nocturnal polyuria in ischemic stroke patients with obstructive sleep apnea. Clin Interv Aging. 2019;14:241–7. https://doi.org/10.2147/CIA.S193448.
Vrooman OPJ, van Balken MR, van Koeveringe GA, et al. The effect of continuous positive airway pressure on nocturia in patients with obstructive sleep apnea syndrome. Neurourol Urodyn. 2020;39(4):1124–8. https://doi.org/10.1002/nau.24329.
McMillan A, Bratton DJ, Faria R, PREDICT Investigators, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–12. https://doi.org/10.1016/S2213-2600(14)70172-9.
McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. A multicentre randomised controlled trial and economic evaluation of continuous positive airway pressure for the treatment of obstructive sleep apnoea syndrome in older people: PREDICT. Health Technol Assess. 2015;19(40):1–188. https://doi.org/10.3310/hta19400.
Wang T, Huang W, Zong H, Zhang Y. The efficacy of continuous positive airway pressure therapy on nocturia in patients with obstructive sleep apnea: a systematic review and meta-analysis. Int Neurourol J. 2015;19(3):178–84. https://doi.org/10.5213/inj.2015.19.3.178.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. https://doi.org/10.1136/bmj.j4008.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. https://doi.org/10.1186/s40779-020-00238-8.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. https://doi.org/10.1016/j.cct.2015.09.002.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. https://doi.org/10.1002/jrsm.12.
Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage Publications; 1993. ISBN: 9780803942462
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Jaki T, Kim M, Lamont A, George M, Chang C, Feaster D, et al. The effects of sample size on the estimation of regression mixture models. Educ Psychol Meas. 2019;79(2):358–84. https://doi.org/10.1177/0013164418791673.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Papadopoulos, V.P., Apergis, N. & Filippou, D.K. Nocturia in CPAP-Treated Obstructive Sleep Apnea Patients: a Systematic Review and Meta-Analysis. SN Compr. Clin. Med. 2, 2799–2807 (2020). https://doi.org/10.1007/s42399-020-00584-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00584-7