Abstract
Symbiosis is a ubiquitous phenomenon in the biological world. A typical example of mutualism is gut microbiota and their host, especially human. Host use the gut microbiota to consume food to get the energy and nutrients necessary for survival, while the gut microbiota gains habitat and nutrition. Gut microbiota interacts with host to form a balance state that is closely related to host health, and the dysequilibrium can result in many diseases in host such as obesity, high blood pressure, inflammatory bowel disease, and cancer. The gut microbiota participates in host metabolism, organism immunity, gene expression, disease development, and drug efficacy, while it is also affected by diet, antibiotics, lifestyle, inheritance, and time. We systematically reviewed the interaction between host and gut microbiota, analyzed the application status of the gut microbiota in the treatment of host diseases, described the main problems in the treatment, and provided new ideas for the application of gut microbiota in the treatment of human diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Symbiosis refers to the close and mutually beneficial relationship formed between two different organisms. Symbiosis is a very common phenomenon in the biological world and the symbiotic relationship in nature mainly includes parasitism, mutualism, commensalism, amensalism, neutralism, and competitive symbiosis. Mutualism is the most perfect combination of symbiotic relationship for they can provide beneficial help for each other’s survival. Of course, this kind of mutual assistance behavior is passive and unaware for them. They just instinctively choose the most beneficial way to survive for them under pressure based on natural selection of species [1].There is a close symbiotic relationship between humans and microorganisms which are interdependent and coevolved [2]. The host can provide a stable habitat for the microorganisms, while the microorganisms provide the host with the nutrients necessary for growth and development and improve the host’s adaptability to the environment. In vivo symbiotic microorganisms are divided into two types: facultative endosymbiotic bacteria and obligate endosymbiotic bacteria. The obligate endosymbiont bacteria has a long-term coevolution relationship with the host, and it is critical and essential for the survival and growth of the host. The coevolution time of facultative endosymbiotic bacteria and host is more transient; its presence in the host is unstable and its function is not essential for host survival. Many symbiotic relationships may initially be facultative symbiosis and these creature will become more and more dependent on symbiotic relationships after long-term evolution, because symbiotic features have an advantage in the natural selection of survival. In the end, the symbiotic parties will depend entirely on the symbiotic relationship to obtain food, shelter, enzymes, and other means of subsistence [3].
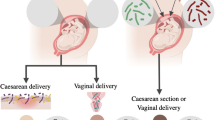
Humans and gut microbiota are a very typical example of mutualism. There are countless bacteria and other microorganisms in the human intestines. In fact, human excretions are mainly composed of bacteria. The human body utilizes the gut microbiota to get more nutrients and calories from the food, while the gut microbiota gets a stable food supply through the body. The normal gut microbiota contains 100 trillion microbes, 500 to 3000 species, and 5 million genes, 100 times more than the human genome. The human gut microbiota is mainly composed of Firmicutes, Bacteroidetes and Actinobacteria, strict anaerobes (Bacteroides, Eubacterium, Bifidobacterium, Fusobacterium, Peptostreptococcus, and Atopobium) are dominant species while facultative anaerobes (Lactobacilli, Enterococci, Streptococci, and Enterobacteriaceae) constitute a minority [4]. Infants receive microbial “seeds” in utero and they are quickly covered by microbes after birth. The microbiota of infants delivered vaginally are similar to mother’s vaginal microbiota, while cesarean section infants are similar to maternal skin microbiota [5]. In the following year, the infant gut microbiota multiplied in large numbers, containing about 1014 microorganisms per milliliter of intestinal contents. Feeding patterns affect the establishment of dominant populations; breast-fed infants contain higher numbers of Bifidobacteria and Lactobacillus while formula-fed infants are mainly Atopobium and Bacteroides. The former has about twice as many gut microbiota as the later, but less diversity. The gut microbiota of infants matures within 3 years after birth and becomes adult microbiome [6].
Gut microbiota is closely related to human life activities and participates in host metabolism, organism immunity, gene expression, disease development, and drug efficacy. It is also affected by diet, antibiotics, lifestyle, inheritance, and time. This article reviews the symbiotic relationship between gut microbiota and humans and analyzes the application prospects of gut microbiota in the treatment of human diseases.
Factors Affecting Gut Microbiota
Modulation of Gut in Early Life
The early gut microbiota determines the characteristics of adult gut microbiota based on the founder effect and plays an important role in the succession direction of the microbial community. The unstable gut microbiota in early life is more sensitive to environmental disturbance and has an important effect on the development of immune system and some diseases in the later life [7]. Exposure to antibiotics in the first year of life increases susceptibility to childhood allergies, asthma, and eczema [8]. A study of 319 children in Canada found that children with asthma developed transient gut microbiota imbalance within 100 days of birth, along with Lachnospira, Veillonella, Faecalibacterium, and Rothia decreased significantly [9]. The sensitivity to chemically induced colitis and asthma reduced after several strains were transplanted to newborn germ-free mice, but the same procedure failed in adulthood [10].Only in early life, the temporarily antibiotic-associated disturbance of gut microbiota-induced metabolic changes and obesity [11]. During a specific period of time before weaning, the gut microbiota is exposed to the immune system to obtain stable immune tolerance, which is persist in the inflammatory environment of adult life. Inhibiting the exposure of microbiota to the immune system or changing the contact time may lead to the loss of immune tolerance to gut microbiota, deterioration of colitis, and inflammatory response to gut microbiota after epithelial cell injury [12]. These results suggest that there may be a “critical period” in the early stage of life, during which microbial exposure or antibiotic disturbances have important and lasting effects on host metabolism, immunity, and other physiological functions.
In addition, gut microbiota varies greatly in different stages of life; the microbiota community of children is obviously more complicated than that of adults, and the adult microbiota community is more uniform, which may be due to the fact that children are developing and the relative stability of adult gut microbiota is higher. Gut microbiota in infants and adults differs in dietary intake and de novo synthesis of nutrients. The infant gut microbiota is rich in genes that de novo synthesis of folic acid, while adults rich in genes utilize dietary folic acid. This is also the case with vitamin B12, and the researchers observed that the gut microbiota of children is enriched in the metabolism of tyrosine, lysine, cysteine, and methionine [13].
Inheritance
Twins and mother-daughter have more similar microbial populations than unrelated individuals, suggesting that inheritance may have an impact on gut microbiota. A study of twins indicated that Christensenellaceae was most genetically affected, which could prevent obesity [14]. However, researchers did not fully understand the intergenerational transfer of gut microbiota until vertically inherited was proved to be the main route of transmission of murine gut microbiota. The main microbiota compositions of germ-free mice were maintained for 10 generations of inbreeding while a fraction of gut microbiota tended to be exchanged, which could be explained by horizontal transmission [15].
The health status or diet habits of parents can affect the disease susceptibility of their offspring through epigenetics. The diet-induced intergenerational disease susceptibility is mainly mediated by gut microbiota. A heavier mother-to-be may affect the gut microbiota in infant and increase obesity susceptibility, which is associated with high levels of Bacteroides and Prevotella. These microbes were higher in children aged 1 and 6 months old [16], indicating intergenerational transfer of microbes associated with obesity. The offspring of dams on the western diet has a high risk of cancer in F2 generation that show increased cancer and accelerated aging, and early exposure to beneficial microorganisms can inhibit intergenerational cancer [17].
The heredity of disturbed microbial community increased the instability of gut microbiota of offspring and decreased the richness. The inheritance of antibiotic-disrupted microbiome not only faithfully replays in the next generation, but also increases the risk of disease. For example, there was a positive correlation between early antibiotic exposure and the risk of IBD in human children [18]. The IBD incidence of offspring increased significantly after the disturbed gut microbiota was transplanted into IL10−/− mice, indicating that inoculants and genotypes could shape the gut microbiota of offspring [19]. Antibiotic-disrupted maternal gut microbiota may have an impact on the gut microecology and long-term disease of offspring, and antibiotic use before and during pregnancy was associated with an increased risk of milk allergy in offspring [20].
Food
It can be seen from Table 1 that the composition and quantity of gut microbiota are affected by food, while that of infants is more obvious. The first food entering the gastrointestinal tract may have a profound effect on the development of the gut microbiota. Breast milk contains protein, maternal bacteria, and nutrients, which can participate in the establishment of neonatal microenvironment such as neonatal immune system, form a dominant population of breast milk-derived microorganisms, and protect against obesity or inflammatory bowel disease (IBD) in adulthood. Dietary shift will significantly change the gut microbiota. The gut microbiota of nursing pigs is mainly Enterobacteriaceae, Bacteroidaceae, Clostridiaceae, Lachnospiraceae, and Lactobacillaceae, the number of Bacteroides and Enterobacteriaceae decreases while Lactobacillaceae, Ruminococcaceae, Veillonellaceae, and Prevotellaceae increased after weaning [32]. Dietary fiber, unsaturated fatty acids, and plant protein foods can increase the diversity of gut microbiota, promote probiotics, and inhibit the proliferation of pathogenic bacteria.
Exercise
Exercise can promote energy metabolism and intestinal peristalsis, increase the number of SCFAs producers, and maintain the dynamic balance of intestinal mucosa immune system. Jiang [33] found that under the same dietary conditions, the population of the exercise group showed an increase in the Lachnospiraceae and Bacteroidaceae, and a decrease in Prevotella and Veillonellaceae. Bifidobacterium adolescentis and Anaerostipes hadrus in Bacteroidaceae can produce abundant SCFAs. This is consistent with the research of Allen [34]; the exercise mice contain more butyric acid in intestines, which is less sensitive to colitis-induced chemical reactions and can accelerate the healing of colitis. The gut microbiota of exercise mice contained more Anaerostipes spp., Akkermansia spp., family Lachnospiraceae genus, Ruminococcus spp. and Parabacteroides spp., and less Prevotella spp. The increase of intestinal butyric acid coincide with the change of SCFAs producers, and the change in lean and obese individuals is different during the experiment, indicating that exercise training can promote the composition and function changes of human gut microbiota and increase the proportion of beneficial bacteria. These changes depend on obesity status and the persistence of exercise, not diet [35].Oral polychlorinated biphenyl (PCB) reduces the level of protease-producing bacteria and significantly alters the abundance of gut microbiota in mice. Exercise can reduce PCB-induced changes in gut microbiota, suggesting that the activity level of mice is associated with significant changes in microbial community abundance, biodiversity, and composition [36].
Antibiotics
Effects of different antibiotics on gut microbiota vary widely due to differences in nature, excretion systems, antibiotic species, doses, and retention times (Table 2). For example, quinolones have weak effects on anaerobe, and they have little effect on gut microbiota no matter given intravenously or orally; aminoglycosides can cause changes in gut microbiota when administered orally, but when administered parenterally, the gut microbiota is less affected because it is mainly excreted through the urine and the concentration in the gut is low; the β-lactam antibiotic has obvious influence on gut microbiota as the drug is excreted through the biliary tract and the concentration of the drug in the gut is high; clindamycin has a strong effect on anaerobe and is mainly excreted through bile, so it has a significant effect on gut microbiota.
The short-term effects of antibiotic intake on the gut microbial community were very obvious and well studied. Take Fluoroquinolones and β-lactams for instance, they could dramatically reduced microbial diversity by 25% and decreased the core phylogenetic microbiota by 58.6% 1 week after treatment. Surprisingly, the proportion of several unknown taxa belonging to the Bacteroides genus was increased in the treatment. With the deepening of the research on gut microbiota, the long-term effect of antibiotics on the gut microbiota has attracted more attention. Some researchers found the influence of clindamycin on Bacteroides in the gut could persist for 2 years after treatment [46].Changes in the gut microbiota lasted 4 years after three patients with dyspepsia were treated with combination therapy of metronidazole, clarithromycin, and omeprazole, without additional antibiotic treatment [47]. Interestingly, antibiotic resistance genes were significantly increased over several years in both cases, indicating that the homeostasis of the gut microbiota formed after antibiotic treatment was elastic and more resilient to the same disturbance again because a greater proportion of the microbiota developed resistance. However, there were studies showed differences in the adaptability of individual microorganisms to antibiotic treatment. Opposite response appeared in the same study which two courses of ciprofloxacin were given to the healthy volunteers. In one group, the initial recovery of the microbiota was slow and incomplete, but recovery was relatively quick after a second treatment. Nevertheless, in other group, a basically fully restored occurred after the first ciprofloxacin treatment, but achieved obvious different steady state after the second treatment, suggesting that the initial antibiotic treatment decreased resilience [48].
Antibiotics can affect the homeostasis of the gut microbiota, making opportunistic species dominant and causing disease. Clostridium bolteae and clostridium difficile, which appear in the intestines of healthy infants, cause disease when the gut microbiota homeostasis is destroyed [49].
Effects of Gut Microbiota on Human Health
Immune
There is a mutually beneficial relationship between human and healthy gut microbiota. It requires avoiding the microbiota-induced infection and suppressing the immune system’s inflammatory response to friendly microbiota. When the beneficial relationship goes wrong, a number of diseases happen (Table 3). In a specific period of time after birth, microbial colonization in vivo can stimulate the development of the infant immune system, while gaining immune tolerance and greatly reducing the incidence of colitis in later life [9]. Maternal microbiota affect the formation of immune system in offspring, and the colonization of microbiota during maternal pregnancy can determine the inherent immune components of intestinal mucosa in offspring.
After the temporary transplantation of wild-type C57BL/6 mice with Escherichia coli (E. coli) HA107 during pregnancy, the number of early postnatal intestinal innate leukocytes was changed, and the proportion and total number of small intestinal innate lymphoid cell (ILC) increased, especially NKp46 + RORgt + ILC3. In addition, maternal colonization reprograms intestinal transcriptional profiles of the offspring, including increased expression of genes encoding epithelial antibacterial peptides and metabolism of microbial molecules. Some of these effects depend on maternal antibodies that may retain microbial molecules and pass them to offspring during pregnancy and in breast milk. Cubs born to mothers who are temporarily colonized during pregnancy are better able to avoid inflammatory reactions to microbial molecules and infiltration of gut microbiota [58].
The gut microbiota interacts with immune system form part of gut-immune-brain signaling axis to regulate the host hypothalamus-pituitary-adrenal hormone and inflammatory stress in an optimal balance to maintain good health. In this way, gut microbiota directly or indirectly affect the proficiency of immune system and the outcome of cancer. Supplementary dietary Lactobacillus reuteri can upregulate the epithelial transcription factor FoxN1 to stimulate the thymus, and continuously reduce the number of circulating neutrophils, which in turn affects the systemic inflammatory process and cancer progression [59]. Gut microbiota can also affect neutrophil homeostasis by regulating the expression of immunoregulatory factor (AIRE) in mouse thymic epithelium, cxcl5-mediated signaling in intestinal cells, and effect of IL-17 [60]. Gut microbiota forms the mucosal immune system by regulating the differentiation and amplification of several types of T cells. Clostridium difficile can induce colon regulatory T cell (Tregs) and increase the number of Tregs in the body. Butyric acid produced by commensal microbiota can promote the differentiation of Tregs in the body and can also regulate the expression of genes involved in the differentiation of natural Tregs—it can increase the histone H3 acetylation of the Foxp3 gene promoter and the conserved non-coding region [61].
Clostridium difficile can provide critical protection against infectious diseases and inhibit the growth of pathogenic bacteria such as Salmonella [62].The metabolite of Clostridium difficile-deamino tyrosine (DAT) protects the host from influenza by activating the amplifying loop of type I interferon signal [63]. Bifidobacterium and Lactobacillus secreted inflammatory inhibitor by downregulation of NF-κB-dependent gene expression, the level of IL-8 secretion, and macrophage-attracting chemokine. They can also directly downregulate the inflammatory response mediated by effector T cells, while upregulating the expression of anti-inflammatory Tregs in mice [64]. Bacteroides fragilis mediates the transmission of polysaccharide (PSA) to dendritic cells via extracellular vesicles, whereas dendritic cells inhibit IBD by mediating the differentiation of Tregs and the secretion of interleukin-10 (IL-10). PSA can also correct systemic T cell defects, TH1/TH2 imbalance, and guide the occurrence of lymphoid tissue [65].
Cancer
Gut microbiota can affect the occurrence, development, and therapeutic efficacy of cancer, and is associated with about 20% of cancers, especially colon cancer (CRC). The bacterial content in colon is 1 million times higher than that in small intestine, and the incidence of cancer is 12 times higher in the former than in the latter, indicating the potential role of gut microbiota in colon cancer [66]. Streptococcus bovis, Helicobacter pylori, Bacteroides fragilis, Enterococcus faecalis, Clostridium septicum, Fusobacterium spp., and E. coli have changed significantly in colon cancer patients. Enterococcus faecalis, Streptococcus faecalis, Helicobacter pylori, and Fusobacterium are found to be enriched in CRC tissues with significant decrease of Roseburia and Eubacterium [67]. Fusobacterium nucleatum can stimulate inflammation by aggregating myeloid cell or activating β-catenin signal to enhance tumorigenesis [68]. Enterotoxigenic Bacteroides fragilis (ETBF) and E. coli carrying pks pathogenicity island can promote tumorigenesis by producing toxins [69].
The initial structure of gut microbiota may determine the host’s susceptibility to colon tumorigenesis. Gram-negative bacteria and positive bacteria seemed to play an opposite role in the susceptibility to tumor. Verrucomicrobia, Bacteroidales, Parabacteroides, Alistipes, and Akkermansia were positively correlated with tumor increase, while Clostridium was negatively correlated with tumor. The number of tumor was negatively correlated with the potential productivity of butyric acid and positively correlated with the host’s glycan degradation capabilities, indicating that the balance between protective butyrate producing bacteria and inflammatory mucin degrading bacteria affected tumorigenesis [70]. Bacteroidales and Akkermansia, which degrade mucin, are most associated with high tumor incidence. Mucin degradants may disrupt the integrity of the mucosal barrier and lead to increased inflammation [71].
Gut microbiota affects the patient’s response to cancer treatment and sensitivity to toxic side effects. After transplantation, fecal microbes from patients who respond to cancer treatment can induce an effective response to cancer treatment in sterile mice [72]. Antibiotic treatment can significantly reduce the therapeutic effect of immunotherapy CpG oligonucleotides and platinum chemotherapy drugs on skin tumors, and supplement Alistipes shahii can enhance the efficacy, whereas Lactobacillus fermentum is opposite [73]. Gut microbiota can metabolize irinotecan to produce toxic substances leading to severe diarrhea, and drugs that selectively target E. coli β-glucuronidase can reduce its side effects [74].
Metabolism
The indigestible carbohydrates in food are mainly decomposed by gut microflora (Fig.1) to produce low molecular weight substances such as SCFAs, which can enter blood circulation through enterohepatic circulation or damaged intestinal barrier. 1/3 of the small molecules in the blood derived from gut microbiota, beneficial such as a variety of transferases, kinases, synthase and coenzyme factors, harmful such as ammonia, phenols, cresols, amines and so on [75].
Gut microbiota regulates the metabolism of glucose, energy, and adipose tissue through a variety of mechanisms, including inflammation. The internal fat of sterile mice was still lower than that of normal mice under the condition of increasing food intake. After transplanting the gut microbiota of obese mice, the internal fat increased obviously and the metabolism of adipose tissue changed [76]. Gut microbiota affects adipose tissue metabolism by endocannabinoids (e-CBs) and lipopolysaccharide (LPS); e-CBs can control lipogenesis while LPS triggers insulin resistance and metabolic disorders [77]. Leptin is another means by which gut microbiota controls fat metabolism [78].
Gut microbiota can affect fat accumulation by regulating the degree of whitening browning [79, 80] and regulating the expression levels of fat metabolism-related genes [81] (Fiaf, Acc1, and Fas). Akkermansia muciniphila can affect the expression of PLD1, regulate intestinal mucus thickness, maintain intestinal barrier integrity, and reduce sugar absorption to control obesity and type 2 diabetes (T2D) [82]. Firmicutes can change the methylation level of gene promoters associated with cardiovascular disease and obesity [83]. Enterobacter cloacae can turn off fat-consuming genes, activate fat-producing genes, produce endotoxins, and cause inflammation and insulin resistance in mice [84]. In addition to participating in amino acid fermentation, gut microbiota can also synthesize many nutritionally essential amino acids from scratch, such as rumen bacterial species (Streptococcus bovis, Selenomonas ruminantium, and Prevotella bryantii) can participate in the de novo synthesis of a variety of amino acids [85].
Lifespan
The delayed onset of apolexis-related diseases is closely related to the changes of gut microbiota; studies have found that the intestinal permeability increases and causes leakage before the death of drosophila, accompanied by changes in the gut microbiota. Antibiotics can improve intestinal function by reducing the number of bacteria associated with apolexis and significantly prolong the lifespan of drosophila [86]. In another study, probiotics and herbal Triphala were fed to drosophila, prolonging their lifespan by 60% and effectively preventing chronic diseases associated with apolexis. This may be achieved by controlling insulin resistance and energy regulation pathways to improve inflammation and oxidative stress [87].
Gut microbiota imbalance can weaken intestinal barrier and lead to intestinal leakage, release inflammatory bacterial products into the body, thereby impairing immune function and reducing lifespan [88]. Transplanting fecal microbiota from young killifish into old killifish can prolong the lifespan of the latter by 41%, compared with the control group. But, young killifish are not affected by the gut microbiota of old killifish [89]. In addition, the ingestion of E. coli has a profound effect on the lifespan of Caenorhabditis elegans (C. elegans), and the colonization degree of bacteria in the intestinal tract is inversely proportional to its lifespan [90]. E. coli may affect the lifespan of C. elegans by controlling the synthesis of folate in the gut [91]. E. coli cannot absorb exogenous folic acid but can hydrolyze pABA-glutamate into the folate precursor (pABA) and glutamate then de novo synthesis folate. Supplementation of shikimate or pABA in the E. coli diet can eliminate the effect of prolonging the lifespan of C. elegans, but other aromatic hydrocarbon products supplementing this pathway are not [92].
Application of Gut Microbiota in Disease Treatment
Engineering Bacteria
Cancer has become a major threat to human life and health, the increasing incidence of cancer and the shortcomings of conventional therapy made new treatment urgently needed, and the emergence of engineering bacteria brings a glimmer of light for cancer treatment. It is known that some bacteria can adhere to the surface of cancer cells as a potential tool for targeted drug delivery. For example, the histone-like protein A (HlpA) of Streptococcus gallolyticus can bind to heparan sulfate proteoglycan (HSPG), particularly syndecan 1 on the tumor surface, is responsible for the microbial infiltration into the tumor. Researchers reprogram symbiotic E. coli Nissle 1917 (ECN), use ice ribozyme protein (INP) tag to bind HlpA to EcN surface to promote cancer cell adhesion, and endow EcN with the ability to secrete myrosinase. Cruciferous vegetables have the effect of preventing cancer on account of containing glucosinolate, while broccoli contains the highest content. Glucosinolates can be transformed to sulforaphane (SFN) by myrosinase to inhibit the growth of cancer cells and promote apoptosis [93]. Feeding a mouse model of colorectal cancer with the engineered EcN and broccoli diets showed significant tumor regression and tumor reduction. After the tumor is cleared, EcN is separated from the colorectal tissue and excreted. This combination results in almost complete inhibition of cancer cells in vitro [94].
Salmonella typhimurium can proliferate in solid tumor and exhibit anti-tumor effect. The Frahm team genetically modifies the LPS structure of Salmonella typhimurium to make it less toxic, and subsequently detects its anti-tumor effect. The results show that the genetically modified Salmonella typhimurium can effectively kill cancer cells and reduce tumor progression without causing other diseases in the body [95]. The Magnetotactic bacterium strain MC-1 can synthesize magnetosomes in vivo and directional migration under the influence of magnetic field. The drug-loaded liposome was attached to the surface of the magnetotactic bacteria to construct a novel nano-drug carrier. Up to 55% of the MC-1, cells infiltrated into the hypoxic region of the tumor by injection and magnetic guidance near the tumor of the immunodeficient mice. This method can effectively overcome the therapeutic resistance in anoxic region of tumor and improve the killing effect of drugs on tumor [96].
Gut Microbiota + Drug
PD-1 inhibitor is a therapeutic agent that can activate the immune system to attack tumor; the clinical response of different patients is vary for the gut microbiota may play an important role in it [97, 98]. Melanoma patients rich in Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium showed better efficacy [99]. The number of Akkermansia muciniphila, Faecalibacterium, and Clostridiales were positively correlated with the therapeutic efficacy, whereas Bacteroides were the opposite [100, 101].The anti-tumor effect of patients taking antibiotics was weakened and their average survival time was shortened.
The antibody targeting CTLA-4 molecule has a significant effect in clinical tumor immunotherapy, and there are also differences in curative effect. Animal experiments have shown that the T cell response to CTLA-4 antibody therapy is related to Bacteroides thetaiotaomicron or Bacteroides fragilis, and the anti-cancer properties of CTLA-4 antibody therapy can be enhanced by the transplantation of Bacteroides fragilis [102]. Cyclophosphamide (CTX) is a significant alkylated anticancer drug, which can destroy intestinal mucosa and cause intestinal wall leakage, make translocations of Lactobacillus and Enterococcus hirae into secondary lymphoid organs, and promote the production of Th17 cells and memory T cells [103]. Gut microbiota by production P-cresol, a competing compound with paracetamol, affects the metabolites of paracetamol-paracetamol sulfate or paracetamol glucuronide, potentially altering its potency and toxicity [104].
Gut microbiota is involved in the metabolic process of some drugs and affects the therapeutic effect of drugs. Further studies on the specific microbes that play a role in drug metabolism can provide a theoretical basis for the treatment mode of “gut microbiota + drugs.”
Supplement Probiotic Bacteria and Probiotics
Fecal microbiota transplantation (FMT) is highly expected in the future for intestinal disorders and gastrointestinal diseases. Ninety-two percent of 317 patients with Clostridium difficile associated diarrhea (CDAD) have disease relief after FMT of healthy donor [11]. Transplantation of Akkermansia muciniphila could reduce the number of Ruminococcus torques, decrease the level of serum endotoxin, promote immune regulation, and delay the development of diabetes mellitus [105]. In a study of 133 patients with ulcerative colitis, the remission rate of FMT was 30.4% and multiple FMT may have better results [106].
Oral probiotics can enhance the beneficial symbiotic population in the gut and prevent immune-mediated pancreatic β-cells destruction. Supplementary cytoderm of Saccharomyces cerevisiae probiotics can increase the number of Bifidobacterium spp., Faecalibacterium spp., and Ruminococcus spp., then reduce blood pressure and improve blood glucose index in patients with T2D. Prebiotic diet can also regulate glucose and liposome homeostasis, leptin sensitivity, and target the activity of endocrine cells [107].
Bifidobacterium and Lactobacillus are recognized as probiotics, which can relieve inflammation, constipation, and diarrhea in children. Oral Lactobacillus increased the number of beneficial symbiotic bacteria in the host, inhibited insulin resistance, reduced glucose and cholesterol concentrations, and prevented the onset of insulin-dependent diabetes in mice [108]. Lactic acid bacteria (LAB) can partly prevent infectious diseases caused by Streptococcus pyogenes and Streptococcus pneumoniae, inhibit oxidative damage induced by streptozotocin, improve antioxidant status, and contribute to better control of T2D. In addition, oral LAB can protect mice from influenza virus infection, improve survival rate, and prolong life expectancy in mice [109]. The heat-killed LAB strain Lactobacillus casei DK128 can reduce the level of virus-induced pro-inflammatory cytokine, regulate viral-induced innate immune cells, induce virus-specific antibodies in early infection, and protect against influenza H3N2 virus. Heat-killed DK128 pretreatment can induce resistance to lethal primary influenza A virus infection in mice and cross-protect mice against secondary lethal infection caused by multiple influenza viruses [110].
Zhao Liping found that dietary fiber can improve insulin secretion and sensitivity in T2D patients by changing gut microbiota structure [111]. The anti-inflammatory effect of dietary inulin-like fructan (ITF) is confirmed under pathological conditions such as obesity, diabetes, or intestinal inflammation. ITF administration increases the number of Bifidobacteria and LAB, the propionate produced by fermentation can reduce systemic inflammation, reduce BaF3 cell infiltration, and counteract malignant cell proliferation in liver tissue [112]. In view of the differences of biological diversity and functional diversity of gut microbiota among individuals, the effects of supplementing probiotics vary from person to person. An Asian diet rich in soybeans can prevent cancer because soybeans can be converted by enzymes produced by gut microbiota into (S)-equol that improves vasomotor disease, osteoporosis, prostate cancer, and cardiovascular disease. The anti-cancer effects of soybeans in the West are reduced because only about 20% of people eating soy-rich foods can produce (S)-equol.
Conclusion and Prospect
The biodiversity and functional diversity of gut microbiota is very important to maintain the healthy physiological state of host. Species-rich microbiota are not easily invaded because they can make more efficient use of limited resources, and different species are specific to each potential limited resource. A microbial community with high functional diversity allows a small but functionally similar species to fill the niche when the dominant species is damaged by environmental disturbances, thereby maintaining the original steady state. Maintain good living and eating habits, reduce unnecessary antibiotic interference, improve gut microbiota during pregnancy, supplement prebiotics in infancy are essential to maintain the homeostasis of gut microbiota, and reduce the incidence of diseases caused by gut microbiota disorder.
The use of gut microbiota to treat related diseases is currently a research hotspot. FMT has been applied to treat CDAD, IBD, and T2D while the use of FMT in clinical treatment still faces many problems: donor’s age, health status and lifestyle, number of donors and FMT, ways of transplantation, ethical issues, short-term complications, and long term potential risks. Duvallet [113] found that a gut microbiota community can be associated with a variety of diseases, whether FMT treatment of a certain disease will affect other related diseases is an urgent problem to be solved in FMT safety evaluation.
Microbial exposure in the “critical period” of early life has an important and long-term effect on host health in later life, and this “critical period” is not easy to determine. Kathryn [49] discovered the immune tolerance time of gut microbiota in mice; however, the results of human experimental studies have not been reported. The risk and ethical issues of gut microbiota intervention in infants are the main challenges facing scientists today. The emergence of microfluidic organs-on-chips provides a glimmer of light for the study of gut microbiota, which can simulate the 3-D environment of human organs and study the interaction between gut microbiota and artificial organs while avoiding the potential risks to the human body.
Gut microbiota are involved in the metabolic process of some drugs and affect the therapeutic effect of diseases. The treatment of “gut microbiota + drugs” provides a new choice for the treatment of human diseases. But, the existing detection methods can only reach the level of families and genera cannot determine the specific microbial species. However, the number of microorganisms, drug conversion rate, drug dosage, and other parameters are not easy to control. High-throughput genetic detection technology combined with microbial databases can detect nearly 3000 microbes and help scientists develop precise research programs. In addition, microfluidic chip technology can be used for single cell screening, analysis, and gene sequencing, with the characteristics of fewer samples, rapid reaction, and automation, is a new microbial detection technology.
Scientists are trying to turn bacteria into targeted drugs using genetic modification technology. Researchers at SYBX use genetically modified E. coli to treat hereditary phenylketonuria. XON Corporation produces a protein that protects the skin by modifying Lactococcus lactis to prevent oral ulcers caused by chemotherapy. However, the therapeutic effect of genetically modified bacteria is slow and the interaction between bacteria and human body is not clear. Transgenic bacteria may transfer the modified genes to other bacteria, causing unknown risks. There is also controversy over the dispose of genetically modified bacteria after treatment. Is there any risk if they continues to colonize the human body? whether the existing technology is capable of completely eliminate bacteria? They will be the next focus of the study.
The prediction of human disease by gut microbiota is another research hotspot. By detecting some metabolites produced by gut microbiota in blood, it may be used as an early warning signal of disease. For example, phenylacetic acid (PAA) may be used as a biomarker in clinical practice, and PAA screening of patients’ blood samples can predict their risk of non-alcoholic fatty liver disease [114]. It was found that some specific changes in bacterial growth rate were related to T2D, while others were related to the occurrence of IBD [115]. This method can be used to predict the incidence of certain diseases or to judge the therapeutic effect of certain diseases. Through long-term monitoring of the composition of gut microbiota or the changes of certain metabolite levels before and after the onset of the disease, after obtaining enough sample data, the BP neural network was used to train and learn the experimental data. It is also a new research direction to construct a mathematical model to predict the risk of related diseases through the change of gut microbiota.
References
Ayres JS. Cooperative microbial tolerance behaviors in host-microbiota mutualism. Cell. 2016;165(6):1323–31.
Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156(1–2):109–22.
Burns AR, Guillemin K. The scales of the zebrafish: host-microbiota interactions from proteins to populations. Curr Opin Microbiol. 2017;38:137–41.
Peterson DA, Frank DN, Pace NR, et al. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–27.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5.
Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. 2014;2:38.
Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–302.
Loewen K1, Monchka B, Mahmud SM, et al. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J. 2018;52(1):1702070.
Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152.
Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Blumberg, microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93.
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21.
Knoop KA, Gustafsson JK, McDonald KG, et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol. 2017;2(18):eaao1314.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99.
Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW. Transmission modes of the mammalian gut microbiota. Science. 2018;362(6413):453–7.
Stanislawski MA, Dabelea D, Wagner BD, Sontag MK, Lozupone CA, Eggesbø M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5(1):113.
Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, et al. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75(7):1197–204.
Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44.
Schulfer AF, Battaglia T, Alvarez Y, Bijnens L, Ruiz VE, Ho M, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol. 2018;3(2):234–42.
Larsen PE, Dai Y. Metabolome of human gut microbiome is predictive of host dysbiosis. Gigascience. 2015;4:42.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, de Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–82.
De Angelis M, Montemurno E, Vannini L, et al. Effect of whole-grain barley on the human fecal microbiota and metabolome[J]. Appl Environ Microbiol. 2015;81(22):7945–56.
Mastrocola R, Ferrocino I, Liberto E, et al. Fructose liquid and solid formulations differently affect gut integrity, microbiota composition and related liver toxicity: a comparative in vivo study. J Nutr Biochem. 2018;55:185–99.
Bai G, Tsuruta T, Nishino N. Dietary soy, meat, and fish proteins modulate the effects of prebiotic raffinose on composition an fermentation of gut microbiota in rats. Int J Food Sci Nutr. 2018;69(4):480–7.
Zhu Y, Shi X, Lin X,et al.Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017 ,8:1395.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22:658–68.
Cuervo A, Hevia A, López P, Suárez A, Diaz C, Sánchez B, et al. Phenolic compounds from red wine and coffee are associated with specific intestinal microorganisms in allergic subjects. Food Funct. 2016;7(1):104–9.
Eid N, Enani S, Walton G, Corona G, Costabile A, Gibson G, et al. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J Nutr Sci. 2014;3:e46.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–9.
Nakata T, Kyoui D, Takahashi H, Kimura B, Kuda T. Inhibitory effects of soybean oligosaccharides and water-soluble soybean fibre on formation of putrefactive compounds from soy protein by gut microbiota[J]. Int J Biol Macromol. 2017;97:173–80.
Frese SA, Kent P, Chris Calvert C, et al. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28.
Xingyu JIANG, Xia ZHAO, Lingyun ZOU, et al. Moderate exercise induces shift in the composition of human gut microbiota. J Third Mil Med Univ. 2017;39(18):1824–31 (in Chinese).
Allen JM, Mailing LJ, Cohrs J, Salmonson C, Fryer JD, Nehra V, et al. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes. 2018;9(2):115–30.
Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–57.
Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121(6):725–30.
Korpela K, Salonen A, Virta LJ, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410.
Korpela K, de Vos WM. Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb Cell. 2016;3(7):296–8.
Jakobsson HE, Jernberg C, Andersson AF, et al. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836.
Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824–31.
Candon S, Perez-Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, et al. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One. 2015;10(5):e0125448.
Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6.
Panda S, El khader I, Casellas F, et al. Short-term effect of antibiotics on human gut microbiota. Plos One. 2014;9(4):e95476.
Raymann K, Shaffer Z, Moran NA. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 15(3):e2001861.
Oh B, Kim BS, Kim JW, et al. The effect of probiotics on gut microbiota during the helicobacter pylori eradication: randomized controlled trial. Helicobacter. 2016;21(3):165–74.
Koido S, Ohkusa T, Kajiura T, Shinozaki J, Suzuki M, Saito K, et al. Long-term alteration of intestinal microbiota in patients with ulcerative colitis by antibiotic combination therapy. PLoS One. 2014;9(1):e86702.
Reese AT, Cho EH, Klitzman B, et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. Elife. 2018;7:e35987.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108:4554–61.
Lozupone CA, Stombaugh JI, Jeffrey I. Gordon, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30.
Giongo A., Gano K. A., Crabb D. B., Etc.Toward defining the autoimmune microbiome for type 1 diabetes [J]. ISME J, 2011, 5(1): 82–91.
He Z, Shao T, Li H, et al. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016;8:64.
Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis[J]. Elife. 2013;2(1629):e01202.
Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease[J]. Sci Rep. 2015;5:8096.
Li J,Zhao F,Wang Y,et al.Gut microbiota dysbiosis contributes to the development of hypertension[J]. Microbiome, 2017,5 (1):14.
Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, et al. Association between polycystic ovary syndrome and gut microbiota[J]. PLoS One. 2016;11(4):e0153196.
Giamarellos-Bourboulis E,Tang J,Pyleris E,et al.Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome[J].Scand J Gastroenterol,2015,50: 1076–1087.
Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247.
Russler-Germain EV, Rengarajan S, Hsieh CS. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017;10(6):1375–86.
Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69.
Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20(5):524–30.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Kim Y-G, Sakamoto K, Seo S-U, et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science. 2017;356:315–9.
Steed AL, Christophi GP, Kaiko GE, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357(6350):498–502.
Singh A, Sarangi AN, Goel A, Srivastava R, Bhargava R, Gaur P, et al. Effect of administration of a probiotic preparation on gut microbiota and immune response in healthy women in India: an open-label, single-arm pilot study. BMC Gastroenterol. 2018;18(1):85.
Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–20.
Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–28.
Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501–18.
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206.
Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–3.
Baxter NT, Zackular JP, Chen GY, Schloss PD. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome. 2014;2:20.
Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–9.
Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Guthrie L, Gupta S, Daily J, Kelly L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes. 2017;3:27.
Paul B, Barnes S, Demark-Wahnefried W, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. 2015;Clin Epigenetics, 7:112.
Geurts L, Everard A, Van Hul M, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6:6495.
Blaut M1. Gut microbiota and energy balance: role in obesity. P Nutr Soc. 2015;74(3):227–34.
Ellekilde M, Krych L, Hansen CH, et al. Characterization of the gut microbiota in leptin deficient obese mice - correlation to inflammatory and diabetic parameters. Res Vet Sci. 2014;96(2):241–50.
Suarez-Zamorano N, Fabbiano S, Chevalier C, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–501.
Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26(5):801.
Zhang C, Zhao L. Strain-level dissection of the contribution of the gut microbiome to human metabolic disease. Genome Med. 2016;8(1):41.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–36.
Kumar H, Lund R, Laiho A, et al. Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. MBio. 2014;5(6):e02113–4.
Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–4.
Hullar MAJ, Fu BC. Diet, the gut microbiome and epigenetics. Cancer J. 2014;20(3):170–5.
Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, et al. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12(10):1656–67.
Westfall S, Lomis N, Prakash S. Longevity extension in Drosophila through gut-brain communication. Sci Rep. 2018;8(1):8362.
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–66.
Callaway E. “Young poo” makes aged fish live longer. Nature. 2017;544(7649):147.
Portal-Celhay C, Bradley ER, Blaser MJ. Control of gut microbiotal proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 2012;12:49.
Nguyen TP, Clarke CF. Folate status of gut microbiome affects Caenorhabditis elegans lifespan. BMC Biol. 2012;10:66.
Virk B, Correia G, Dixon DP, Feyst I, Jia J, Oberleitner N, et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012;10:67.
Li Q, Yan Q, Chen J, He Y, Wang J, Zhang H, et al. Molecular characterization of an ice nucleation protein variant (InaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int J Biol Sci. 2012;8(8):1097–108.
Ho CL, Tan HQ, Chua KJ, Kang A, Lim KH, Ling KL, et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng. 2018;2:27–37.
Frahm M, Felgner S, Kocijancic D, et al. Efficiency of conditionally attenuated Salmonella enterica serovarTyphimurium in acterium-mediated tumor therapy. MBio. 2015;6(2):e00254–15.
Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Zhong Xu Y, Loghin D, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11(11):941–7.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastaticmelanoma patients. Science. 2018;359:104–8.
Kaiser J. Gut microbes shape response to cancer immunotherapy. Science. 2017;358(6363):573.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6.
Kim JK, Choi MS, Jeong JJ, Lim SM, Kim IS, Yoo HH, et al. Effect of probiotics on pharmacokinetics of orally administered acetaminophen in mice. Drug Metab Dispo. 2018;46(2):122–30.
Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67(8):1445–53.
Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298.
Sohail MU, Shabbir MZ, Jörg M. Steiner, et al. Molecular analysis of the gut microbiome of diabetic rats supplemented with prebiotic, probiotic, and synbiotic foods. Int J Diabetes Dev C. 2017;37(4):419–25.
Park DY, Ahn YT, Huh CS, McGregor R, Choi MS. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J Gastroentero. 2013;19:274–83.
Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY, et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antivir Res. 2012;93(1):138–43.
Jung YJ, Lee YT, Ngo VL, Cho YH, Ko EJ, Hong SM, et al. Heat-killed Lactobacillus casei confers broad protection against infuenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci Rep. 2017;7(1):17360.
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–6.
Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, Mahillon J, et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr. 2015;34(3):501–7.
Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9(1):2655.
Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24(7):1070–80.
Korem T, Zeevi D, Suez J, et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic. Science. 2015;349(6252):1101–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
This article was conducted using published literature and it did not involve ethical issues and informed consent.
Additional information
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Wu, Z.A., Wang, H.X. A Systematic Review of the Interaction Between Gut Microbiota and Host Health from a Symbiotic Perspective. SN Compr. Clin. Med. 1, 224–235 (2019). https://doi.org/10.1007/s42399-018-0033-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-018-0033-4