Abstract
Exposure to a high concentration of cadmium (Cd) is toxic to living organisms. The present study deals with the characterization of Cd resistant bacterial isolates, and analysis of the rhizoremediation of Cd using the metal resistant plant growth promoting (PGP) rhizobacteria applied through maize (Zea mays Sturt) rhizosphere. Cd resistant bacterial isolates were selected, analyzed for PGP attributes and applied in the rhizosphere of maize plants to study their effects on plant growth under metal stress, along with the Cd rhizoremediation potential. The bacterial isolates Serratia marcescens S2I7, S. marcescens BB-2B, Bacillus subtilis SR1 and Paenibacillus sp. S1I8 showed resistance to Cd and positive for PGP attributes, like phosphate-solubilization, production of indole acetic acid (IAA), and siderophore. The augmentation of the metal resistant bacteria caused 26% increase in the shoot length of the plants in Cd spiked soil. Under the stress of Cd, the activity of stress-responsive enzymes- glutathione S-transferase (GST), catalase (CAT), peroxidases (POD) of the treated plants were relatively higher. Augmentation of the bacterial isolates significantly reduced the activity of stress-responsive enzymes. The association of Z. mays-rhizobacteria played the most crucial role in the process of bioremediation, as it could remove up to 31% of Cd from soil after 30 days. The present study emphasizes the efficiency of plant–microbe interactions in the rhizosphere to remove metals from soil through the promotion of plant growth in contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of hazardous metals is one of the major global problem of the environment (Okolo et al. 2016; Thakare et al. 2021). Heavy metal pollution affects the soil system specifically agricultural soils due to its non-degradable feature as well as toxic characteristics for soil organisms and plants (Belyaeva et al. 2005; Huang et al. 2013; Bashir et al. 2018a) and it has severe threats to all living organisms present in the environment (Rehman et al. 2019). A high concentration of heavy metals has been reported to produce toxic effects on plants, reducing the growth, which ultimately may cause plant death (Singh et al. 2015; Rizwan et al. 2016). Cadmium (Cd) is a major metal found in the environment, which is extremely poisonous to humans, animals, plants, and microbes as it damages cell membranes, alters the particularity of enzymes, and destroys the structure of DNA (Olaniran et al. 2013). Cd is a toxic metal that can adversely alter the physiological processes of plants (Brunetti et al. 2011; Rizwan et al. 2016). In soils, the Cd is accumulated because of the coal combustion, regular use of phosphate fertilizers and pesticides (Xu et al. 2013). In the environment, the ionic form of cadmium (Cd2+) is usually present as oxide (CdO2), chloride (CdCl2), sulphate (CdSO4), or nitrite (Cd(NO3)2). Earlier studies have demonstrated the effect of Cd on different plants (Głowacka et al. 2019; Li et al. 2020; Piacentini et al. 2020). Plants were found to respond to such external stimuli through suitable measures to compensate the adverse effects of stress by altering the physiological, biochemical, and molecular response. It has been observed that exposure to heavy metals induces oxidative stress and produce reactive oxygen species (ROS) in plant tissue (Hernandez et al. 2012; Pena et al. 2012). To counter the oxidative stress, plants possess different enzyme systems and mechanisms to keep the routinely formed ROS at the physiological limit and thereby play an important role in the amelioration of abiotic stress (Mittler et al. 2004). Plants can also accumulate toxic metals as part of their mechanisms to ameliorate the stress effects.
The contaminations of heavy metals was found to affect the normal functioning of plants, including Zea mays (Ali et al. 2013). Z. mays is an important cereal crop and recently been recognized for its physiological responses to heavy metals, and hence used for present study (Romdhane et al. 2021). Since ancient times, human beings have relied heavily on the annual cereal plant Z. mays, which is a member of the family Gramineae, and one of the most extensively grown cereals in the world (Prasanna et al. 2001; Yaouba et al. 2012). Z. mays has fast growth rate, vast fibrous root systems with high shoot biomass production per hectare, and withstands adverse environmental conditions (Aladesanmi et al. 2019). In fact, recently Shahzad et al. (2021) also reported the use of Z. mays plant as a test crop to facilitate rhizoremediation the metal polluted soils. Although, it has also been reported that, high level of metal contamination causes negative metabolic, physiological, and morphological alterations in Z. mays plants (Rizvi and Khan, 2018). Hence a suitable environmental management approach is needed to control and remediate the contamination of these hazardous heavy metals (Hasnat et al. 2013).
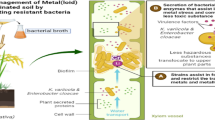
Microbe-assisted phytoremediation of contaminants is an eco-friendly approach that utilizes plant-microbes association for the treatment of pollutants (Rajkumari et al. 2021; Singha and Pandey, 2021). Microorganisms in the rhizosphere enhance the ability of plants to absorb or adsorb heavy metals (Bojorquez and Voltolina 2016) by reducing their mobility as well as increasing the bio-availability (Bashir et al. 2018b). The plant–microbe interactions enhance the activity of microbial community in the rhizosphere that leads to the development of effective rhizoremediation process (Kuiper et al. 2001). Microorganisms such as Bacillus subtilis and Serratia marcescens, possesing plant growth-promoting (PGP) attributes are most useful in polluted areas as they can increase the growth of plants, which ultimately helps in the phytoremediation process (Kotoky et al. 2017a, 2020). Therefore, microbes with the ability to enhance the growth of plants may play a crucial role in their survival in metal contaminated soil as well as assist the rhizoremediation of heavy metals. Considering these, the present study is aimed to check the effect of bioaugmentation of Cd resistant PGP rhizobacteria on the growth of Z. mays (maize) and their efficiency in the removal of Cd from the rhizosphere.
Materials and methods
Isolation of Cd resistant bacterial isolates
Five Cd-contaminated soil samples were collected from the rhizosphere of tea plants of Northwestern Cachar Tea Co. Ltd. and Silcoorie Tea Estate (24.7588° N, 92.7874° E), Assam, India to isolate bacteria. In earlier studies, these agro-ecosystems have been reported to have high concentrations of different heavy metals (Dey et al. 2014; Singh et al. 2018). The physico-chemical analysis of soil samples including soil type, pH, moisture content, organic carbon, available nitrogen and available potassium were estimated as per the standard methods (Osazee et al. 2013). From the collected soil samples, Cd resistant bacteria were isolated on Mueller Hinton agar media amended with 0.25 mM of CdSO4 by standard pour plate method (Saha et al. 2022). Moreover, bacterial isolates S. marcescens S2I7 (Kotoky et al. 2017b), B. subtilis SR1 (Kotoky et al. 2017a), Paenibacillus sp. S1I8 (Kotoky et al. 2019b) reported earlier to be resistant to Cd were also included in the present study. The maximum tolerable concentrations (MTC) of the isolates were determined by growing in gradually increasing concentrations of Cd (CdSO4 in agar plates) (0.25 mM, 0.5 mM, 0.75 mM, 1 mM, 1.25 mM, 1.5 mM, 1.75 mM, 2 mM, 2.25 mM, 2.5 mM, 2.75 mM, 3 mM, 3.25 mM, 3.5 mM, 3.75 mM, 4 mM). The MTC is the maximum concentration of Cd where visible colonies on agar media were observed. The non-pathogenic nature of S. marcescens was checked on the basis of virulence factors, such as DNase, gelatinase, hemolysin and chloroperoxidase activity by standard methods (Schulenburg and Ewbank 2004; Purkayastha et al. 2018; Diamandas et al. 2021) and were found to be absent in S. marcescens isolates. Also, the isolates were prodigiosin producer, which is an indicator of non-pathogenic S. marcescens.
Glutathione S-transferase (GST) activity of the isolates
The method of Habig et al. (1974) was used to determine GST activity of bacteria. The GST present in bacterial enzyme extract conjugates glutathione (GSH) and 1-chloro, 2, 4-dinitrobenzene (CDNB), which was used to measure the GST activity. The bacterial cultures were grown up to late log phase, and the extract of culture was used for analysis. The reaction was initiated by adding phosphate buffer and an equal amount of CDNB and GSH, and the optical density (OD) was measured at 340 nm. The assay mixture without the culture extract served as control. The GST activity was determined using the extinction coefficient (9.6 mM 1 cm 1) and reported as the n moles of CDNB conjugated per minute.
Effect of Cd on PGP attributes of bacterial isolates
The PGP attributes of isolates were determined quantitatively in presence 0.25 mM of Cd, as it was observed as the minimum tolerable concentration of Cd for all the selected isolates, to analyze the effect of presence of Cd, even at low concentrations, on PGP attributes (if any). For this, the bacteria were cultured in respective medium amended with 0.25 mM concentration of Cd. The experimental set without the Cd served as control. The siderophore release was qualitatively observed on Chrome Azurol S (CAS) agar medium, where the formation of orange halo zone around the colonies was indicative of siderophore production. CAS-shuttle assay was used for the quantitative estimation of siderophores. The culture supernatant was mixed with an equal volume of CAS reagent (10 mM HDTMA; 1 mM FeCl3 solution; 2 mM CAS solution) and absorbance was measured at 630 nm against a reference, having non-inoculated broth and CAS reagent. The activity was recorded in percentage of siderophore units calculated as
where Ar = absorbance of reference at 630 nm (un-inoculated media + CAS reagent) and As = absorbance of the sample (Singha and Pandey 2017).
The phosphate (P) solubilization assay of the isolates was determined, and quantified following standard protocols as reported by Kumar et al. (2001). The P solubilization assay was determined on Pikovskaya medium containing bromophenol blue (0.024 mg ml−1). After 48 h, P solubilizing bacteria were picked on the basis of size of clear zone around the colony. P-solubilization was quantified by growing the bacteria in National Botanical Research Institute's Plant growth (NBRIP) medium and measuring amount of free P in the culture supernatant, after 7 days, by using the vanado-molybdate colorimetric method.
Production of IAA was detected in nutrient broth inoculated with freshly grown cultures. The bacteria were grown at 30 °C at 120 rpm in an incubator shaker, and after 36 h, culture was centrifuged (10,000 rpm for 15 min). The supernatant was added with double volume of Salkowski reagent (2% 0.5 MFeCl3 in 35% perchloric acid) and orthophosphoric acid in a ratio of 10:20:0.2 and kept in dark, followed by measuring the OD at 530 nm (Kotoky and Pandey 2020).
Molecular characterization of the bacterial isolates
Selected isolates were identified based on 16S rDNA sequence analysis. The genomic DNA was extracted and 16S rDNA was amplified using the universal primers 27F (5'-AGAGTTTGATCMTGGCTCAG-3') and 1492R (5'-TACGGYTACCTTGTTACGACTT-3') under the conditions: initial denaturation at 94 °C for 4 min, denaturation at 94 °C for 30 s, annealing at 54 °C for 1 min, extension at 72 °C for 90 s and the final extension sequence at 72 °C for 10 min. Sequence homology was checked using the Basic Local Alignment Search Tool (BLASTn) facility (http://www.ncbi.nlm.nih.gov/blast) and MEGAX was utilized for the phylogenetic analysis (Kumar et al. 2018).
Pot trial with Zea mays
The pot trial study was carried out in three replications to examine the impact of bacterial augmentation on Z. mays Sturt growth under Cd stress, as well as the potential of bacteria and Z. mays association to remove Cd from the soil. The surface of Z. mays seeds was sterilized with 1% HgCl2. After the germination of seeds, they were immersed in a bacterial suspension of the respective isolates (BB-2B, S217, S1I8, SR1) (OD600 = 0.5–0.8) individually, for the purpose of treating seeds with bacteria (Zhou et al. 2014). Prior to sowing, the seeds were air-dried for one hour. Germinated seeds were then transferred to pots containing sterile soil with 100 mgkg−1 of Cd (CdSO4) and were grown in a greenhouse environment (photoperiod of 16 h light/8 h dark; temperature at 30 °C during the day and around 18 °C during the night). Four sets of treatments were experimented, one each for respective bacteria, applied with Z. mays plants in Cd contaminated soil, i.e. – Plant + Cd + S. marcescens BB-2B, Plant + Cd + S. marcescens S217, Plant + Cd + Paenibacillus sp. S1I8, Plant + Cd + B. subtilis SR1. The pots without Cd and bacterial inoculation, but only Z. mays plants, served as control. The morphological and physiological characteristics of plants were analyzed after 15 and 30 days of treatment. The shoot length, root length, and dry and fresh weights of the whole plants in each trial were noted for morphological analysis. A stress response parameter (GSH content) and the activity of stress responsive enzymes (GST, catalase, and peroxidase) were used to investigate the physiology of the plant. The pot trial experiment was carried out between June 2021 to September 2021.
Glutathione S-transferase (GST) activity of the plants
The GST activities of the plants were assessed after 15 and 30 days of the germination and Cd exposure. Fresh plant tissues were used to extract the crude enzymes for the GST activity using a mortar and pestle and phosphate buffer (pH 6.5, 0.1 M) (Habig et al. 1974). In order to produce the reaction mixture, the crude enzyme extract was added together with 1 mM CDNB, 1 mM GSH, and 0.1 M phosphate buffer in 1:1:1:29 proportions respectively. After every 15 s time interval, the OD was recorded at 340 nm using a spectrophotometer. In order to track the non-specific binding of the substrates, one control was also run along with the samples. This control comprised of the assay mixture without the enzyme extract. The extinction coefficient of the product generated (9.6 mM−1 cm−1) was used to determine the GST activity, which was then represented as the number of moles of CDNB conjugated tubes.
Glutathione content of the plants
The quantities of reduced glutathione in the plant tissue were measured after 15 and 30 days of the experiment (Moran et al. 1979). A tissue homogenate was made from the whole plant and 5 percent trichloroacetic acid (TCA) (5 ml g−1) and the solution was then centrifuged at 10,000 rpm for 10 min. After centrifugation, the supernatant was combined with freshly prepared DTNB or Ellman's reagent (5, 5′-dithiobis-(2-nitrobenzoic acid) solution) and 0.2 M sodium phosphate buffer (pH 8.0) at a ratio of 1:20:9. Reduced glutathione combines with DTNB to produce a yellow-colored product and after 5 min of incubation, glutathione intensity was measured at 412 nm using a spectrophotometer, and the data are expressed as n moles GSH g−1 sample.
Catalase (CAT) activity of plants under metal stress
After 15 and 30 days of the experiment, the plant CAT activities were measured. To prepare the enzyme extract for the CAT activity, plant tissues were removed and ground with phosphate buffer. After that, the CAT activities were determined spectrophotometrically (at 240 nm) in a 1:9:10 assay solution that contained an enzyme extract, 5.9 mM H2O2, and 50 mM phosphate buffer (pH 7.0). The test solution's absorbance was measured every 20 s, and a change of 0.01 units min−1 in absorbance was considered to be one unit of CAT activity (Hameed and Sheikh 2007).
Peroxidase (POD) activity of plants under metal stress
Similar to the CAT activity, POD activities were measured after 15 and 30 days of the experiment in an assay solution (2:4:5:1) that comprises guaiacol, H2O2, phosphate buffer (pH 7.0), and enzyme extract. Changes in the reaction solution's absorbance were measured at 470 nm every 20 s, and one unit of POD activity was defined as an absorbance change of 0.01 unit min−1 (Hameed and Sheikh 2007).
Removal of Cd from soil
After the plants had grown in the greenhouse for 30 days, the residual level of Cd in the soil was measured using atomic absorption spectroscopy (AAS). For this, soil (0.5 g) from the respective pots was dried, homogenized, and digested at 90 °C for 16 h with HNO3 (10 ml), HClO4 (5 ml), and HF (25 ml). AAS (Perkin Elmer. AAnalyst-700) was used to check for residual Cd in the remaining residue, which was subsequently dissolved in 10 ml of HCl (4 mol L−1) and diluted to 50 ml with deionized water (Zhang et al. 2010).
Statistical analyses
Softwares GraphPad Prism and Statistical Product and Service Solutions (SPSS) were used to conduct the statistical analysis. With IBM's SPSS Statistics Software version 19, numerical data were examined by analysis of variance (ANOVA), followed by a multiple comparison test (LSD), taking into account statistically significant differences in those with a p-value < 0.05 (Kotoky et al. 2019).
Results
Physiochemical properties of the soils and isolation of Cd resistant bacteria
It was found that the organic carbon content in the soil samples was significantly higher than the normal soil and ranged from 1.22 to 5.72%. The pH of the soil samples ranged from 3.13 to 8.39 and moisture content ranged from 3.1 to 9.13. The nitrogen content of the soil samples was 0.45 to 341.1 kg/ha. The potassium content of the soil samples was 26.24 kg/ha to 211.68 kg/ha and phosphorous of the soil sample was 0.31 to 124.14 kg/ha. In total 18 bacterial isolates were obtained and selected based on their ability to grow on Cd (0.25 mM) amended media. Further, the isolates were analyzed for maximum tolerable concentration of Cd by growing in increasing concentration of Cd. One of the isolates, BB-2B showed highest Cd tolerable concentration up to 2.75 mM and selected for further studies, whereas the isolates S. marcescens S2I7, B. subtilis SR1, and Paenibacillus sp. S1I8, previously isolated from petroleum-contaminated soil, were also able to grow in Cd concentration of more than 2.5 mM (Kotoky et al. 2019). For S. marcescens S2I7, the virulence factors, such as DNase, gelatinase, hemolysin, and chloroperoxidase activity were found to be negative and therefore, considered as non-pathogenic isolate.
GST activity of bacterial isolates
In the presence of Cd, the bacterial isolates' GST activity significantly changed. After the third day of incubation, GST activity rised, whereas GST activity of control (without Cd) remained steady throughout the incubation period. After 7 days of incubation, the isolate BB-2B's GST activity was considerably greater in the presence of Cd (Supplementary figure S1).
PGP attributes of bacterial isolates
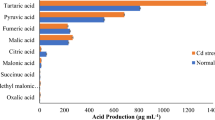
The Cd-resistant bacterial isolates were analyzed for PGP activities. CAS media was used for the analysis of siderophore production where the isolates S. marcescens BB-2B, S. marcescens S2I7, B. subtilis SR1, and Paenibacillus sp. S1I8 showed positive results for siderophore production, while S. marcescens S2I7 showed the highest siderophore production i.e. 54% siderophore unit (Fig. 1). Isolate S1I8 had the best solubilization activity for phosphate, and it was 170 g ml−1. Interestingly, the presence of Cd (0.25 mM) in the medium showed negative effects on the PGP attributes of all the isolates except SR1, where it showed no change in activity after growing for 7 days in presence of Cd (0.25 mM) (Fig. 1).The production of IAA by the isolates S. marcescens S2I7, B. subtilis SR1, Paenibacillus sp. S1I8, and S. marcescens BB-2B after 7 days of growth without Cd was quantified and was found to be 83.14 µg ml−1, 59.04 µg ml−1, 76.2 µg ml−1, and 96.17 µg ml−1 respectively. However, the presence of Cd led to a decrease in the production of IAA by up to 40% by the isolates (Fig. 1). In case of IAA production, after seven days of growth without Cd, the isolates S. marcescens S2I7, B. subtilis SR1, Paenibacillus sp. S1I8, and S. marcescens BB-2B produced 83.14 g ml−1, 59.04 g ml−1, 76.2 g ml−1, and 96.17 g ml−1 respectively. However, the presence of Cd led to a decrease in the production of IAA by up to 40% by the isolates (Fig. 1).
Molecular characterization of the isolates
The selected bacterial isolates were identified based on their 16S rDNA sequences. The sequences have been submitted to the NCBI database. Three of the isolates were identified and reported earlier also as B. subtilis SR1 (Kotoky et al. 2017a), S. marcescens S2I7 (Kotoky et al. 2017b), Paenibacillus sp. S1I8 (Kotoky et al. 2019b). Basic Local Alignment Search Tool (BLAST) analysis identified the isolate BB-2B as S. marcescens that showed 99% sequence similarity with the nearest homologue S. marcescens AG2102 and S. marcescens NBRC and the sequences of BB-2B have been submitted to the NCBI database under the accession number MF554655. The phylogenetic tree of the isolates was prepared with MEGAX taking sequence of type strains of same genus (Supplementary figure S2).
Effects of Cd on plant growth
The effects of Cd on the growth of Z. mays were observed after 30 days of growth in Cd spiked soil (100 mg kg−1). Plants were grown in pots containing Cd spiked soils and the root length, shoot length and dry and fresh weight of the whole plant were measured, where the non-spiked soil was kept as control. No significant effects of Cd were observed in the shoot length of the plants, but the yellowening of leaves at the tips was noted. However, the length of the roots was found to be reduced in presence of the Cd stress. Importantly, the plants augmented with the bacterial isolates showed better growth than the non-inoculated treatments (Table 1). The application of bacterial isolates led to an increase of shoot length by 16–26% in Cd spiked soil than non-inoculated treatments. Similarly, the root length of the plants was also found to be increased by 2–3% than non-inoculated treatments. The dry and fresh weight of the whole plant was also higher in bacterial inoculated treatments than in control (Table 1).
GST activity of plants under Cd stress
After 15 and 30 days of growth in Cd spiked soil, the plants' GST activities were assessed, where the non-spiked set was treated as control. It was found that in all samples, the GST activity was significantly greater after 15 days of Cd stress compared to 30 days of stress. The enzymes' activity in the presence of Cd was shown to be up to three times greater during the first 15 days compared to the activity at the end of the 30-day period. The difference in the activity of GST in the treated plants was not significant in comparison to non-treated samples after 30 days. Interestingly, the application of bacterial isolates had no significant effects on the activity of the GST enzyme of the plants (Fig. 2).
GST activity of the plant tissue in presence of Cd stress after 15 and 30 days of growth. Statistical analysis was performed by one-way ANOVA followed by post-hoc analysis using a pairwise T-test. Values with the same letters were significantly different (post hoc T-Test; abcde—p < 0.0001; f—p < 0.001). Values are the mean of five replicates and error bars indicate fixed values of error. Control Cd non-spiked soil, Cd Cadmium spiked soil, Cd + BB-2B/S2I7/SR1/S1I8 Cd spiked soil with S. marcescens BB-2B/S. marcescens S2I7/B. subtilis SR1/Paenibacillus sp. S1I8 augmented
Glutathione content of the plants to Cd stress
In the presence of Cd stress, the GSH content in the plant tissue were significantly increased. The majority of the treatments revealed that the GSH content was higher after 30 days than it was after 15 days. In compared to the control treatment set without bacterial inoculates, the quantity of GSH was observed to be considerably lower after the application of bacterial augmentation (Fig. 3). After 15 and 30 days, it was observed that, among all the bacterial treatments, the BB-2B treatment had a greater rate of GSH content than the other treatments.
Glutathione (GSH) content of the plants after treatment of Cd and bacterial inoculums. Statistical analysis was performed by one-way ANOVA followed by post-hoc analysis using a pairwise T-test. Values are mean of five replicates and are significantly different from the control (No Cadmium) ***p < 0.0001, but less significant after 30 days **p < 0.005 and error bars indicate fixed values of error
CAT activity of plants under Cd stress
After the plants had grown in a greenhouse for 15 and 30 days, their CAT activities were determined. The CAT activity was much greater in Cd spiked treatments over the first 15 days of growth. However, the stress impact was reduced and the activity of the catalase enzyme was shown to be lower in the presence of Cd-resistant bacterial isolates. After 15 and 30 days of incubation, CAT activity was significantly decreased in cases of BB-2B and S217 treatments, the highest reduction was observed in BB-2B treatment after 30 days of incubation. However, after 30 days of growth, there was no effect of Cd stress on plant tissue, and the activity of the enzyme was similar in all treatments (Fig. 4).
Catalase (CAT) activity of the plants grown in Cd spiked soil. Statistical analysis was performed by one-way ANOVA followed by post-hoc analysis using a pairwise T-test. Values with the same letters were significantly different (post hoc T-Test; abcdep < 0.0001; fp < 0.003) and error bars indicate fixed values of error
POD activity of plants under Cd stress
After 15 and 30 days of growth in Cd-spiked soil, POD activities of the treated plants were determined, and they were compared with the control, where no Cd was added. After 30 days of growth, the addition of Cd greatly increases the POD activity. Among all the bacterial treatments, after 15 and 30 days, the BB-2B and S217 treatments decreased the POD activity with greater rate in comparison to other treatments, which indicates that the activity of POD in the plant tissue was decreased by the inoculation of Cd-resistant bacterial isolates (Fig. 5).
Peroxidase (POD) activity of the plants after grown in Cd spiked soil. Statistical analysis was performed by one-way ANOVA followed by post-hoc analysis using a pairwise T-test. Values are mean of five replicates and the marks are significantly different from the control (No Cadmium): ***p < 0.0001 and error bars indicate fixed values of error
Removal of Cd from soil
After 30 days of growth of the plants, the residual amount of Cd in the soil was estimated through AAS analysis. It was found that the association of plant–microbe has great efficiency in the removal of Cd from the soil. The application of metal-resistant bacterial isolates could enhance the remediation by 23–31%. The association of Z. mays–B. subtilis SR1 and Z. mays–S. marcescens BB-2B were found to be the most efficient combination for the removal of Cd as it showed the highest removal percentage i.e. 31% and 28% respectively (Fig. 6).
Discussion
The quality of soil has great impact on human health through food consumption. Soil is a non-renewable resource and affects the yield and production of cultivated crop, where the presence of hazardous contaminants like metals affect the soil characteristics (Toth et al. 2016). The rapid urbanization, industrialization and excess agricultural practices have led to releasing of tremendous amount of metal pollutants in the agricultural fields (Romdhane et al. 2021). In this study, the application of bacterial isolates in the rhizopshere of Z. mays plant was aimed to ameliorate the stress-responsive effects in metal contaminated soil. Therefore, Cd-resistant rhizobacteria were isolated from tea rhizopshere of Cd contaminated for application using Z. mays to ameliorate the stress-responsive effects in metal contaminated soil. In total, 18 bacterial isolates were obtained from the tea garden soil and it was found that the isolate S. marcescens BB-2B was found to be the best among all the isolates based on the Cd resistance and PGP attributes. The isolate S. marcescens BB-2B showed highest Cd tolerable concentration up to 2.75 mM, whereas the isolates S. marcescens S2I7, B. subtilis SR1, and Paenibacillus sp. S1I8 previously isolated from petroleum-contaminated soil also could grow in Cd concentration of more than 2.5 mM (Kotoky et al. 2019). Earlier also microorganisms have been reported to have multiple genes that confer resistance to heavy metals. Previous study of the genomic features of the isolate B. subtilis SR1 reported the presence of functional genes (czcD and cadA) for resistance to cadmium, zinc, and cobalt (Kotoky and Pandey 2021). Similarly, the genomic characteristics of the isolate S. marcescens S2I7 was found to have genes for high resistance to Cd (czcD; cobalt-zinc cadmium resistance protein) as well as other metals like arsenic (arsA, arsB, arsC), cobalt, copper (CRD; copper resistance protein D), nickel, zinc (Cd/Zn/Copper/Silver efflux P-type ATPase), etc. (Kotoky and Pandey 2020). The isolates S2I7 and BB-2B have prodigiosin producing ability and versatile characters of resistance to Cd and promoting the growth of plants. The prodigiosin producing ability of given S. marcescens isolate is considered as indication of its non-pathogenic nature (Kotoky and Pandey, 2020). Similar to this, Khan et al. (2017) showed that S. marcescens RSC-14, a non-pathogenic bacteria from the roots of the Solanum nigrum plant, a Cd-hyperaccumulator, was extremely resistant to Cd and could reduce the heavy-metal stress in the plant.
It has been reported that the remediation of metals by microorganisms occurs mainly via bioaccumulation and/or through adsorption (Igiri et al. 2018). Patel et al. (2022) reported that 32 bacterial isolates from toxic element-contaminated rhizospheric soils that were collected from Daman Ganga riverside (Vapi, Gujarat, India) and tested for Cd tolerance; it was reported that 50% of the bacterial isolates were tolerant to Cd. Similarly, Pal and Sengupta (2019) reported that 20 Cd resistant bacterial isolates from rhizospheric soil samples. Becerra-Castro et al. (2011) reported several bacterias isolates from metal-polluted soils that were tolerant to Cd, lead, and zinc and could remediate metal from polluted sites. Raja et al. (2009) also reported 300 bacterial isolates capable to grow in heavy metal amended medium, where several isolates showed a high degree of resistance to heavy metals (600 mg/l) having the potential to be used for bioremediation. Due to its effective eco-friendly nature, bioremediation of metals using microorganisms and plants is a more potent technique. During remediation, heavy metals cannot be degraded easily, but can be changed from one form or oxidation state to another less dangerous form that is more readily volatilized and less bioavailable (Chibuike and Obiora 2014).
The PGP attributes along with the resistance to heavy metals establish that the isolates have potential for rhizoremediation of metal-polluted soil. The Cd resistant bacterial isolates showed significant results in PGP attributes, which is considered essential for promoting plant growth to enhance the rhizoremediation of heavy metals. In this study, it was found that the Cd-resistant bacterial isolates showed positive results for PGP attributes under the stress of 0.25 mM of Cd. Out of 18 isolates, S. marcescens BB-2B, S. marcescens S2I7, B. subtilis SR1, and Paenibacillus sp. S1I8 showed positive results for siderophore production, whereas the isolate S1I8 had the best phosphate solubilization activity, while the isolates S. marcescens S2I7, B. subtilis SR1, Paenibacillus sp. S1I8, and S. marcescens BB-2B showed positive results for IAA production. Patel et al. (2022) reported that under the stress conditions, Cd resistant bacterial isolate Curtobacterium oceanosedimentum DG-20 showed positive results for PGP attributes such as phosphate solubilization, siderophore production, and IAA production (116.33 ± 2.08 μg/mL). Similarly, Pal and Sengupta (2019) reported that Cd resistant bacterial isolates Lysinibacillus varians KUBM17 and Pseudomonas putida KUBM18 showed significant positive results for PGP attributes. The results of previous studies also demonstrated that metal resistant bacteria with PGP attributes improve the vegetative growth parameters of rapeseed (Sheng and Xia 2006), castor oil plant (Rajkumar and Freitas 2009), and mustard (Ma et al. 2009) in metal contaminated soil. In this study, it was found that the application of bacterial isolates led to an increase in plant growth parameters such as shoot length, root length, dry and fresh weights of the whole plant in comparison to control and the most significant plant growth parameters were observed in BB-2B and S2I7 treatments. In a recent study, Patel et al. (2022) determined the effect of Cd resistant bacterial isolate C. oceanosedimentum DG-20 in promoting the growth of Capsicum frutescens under the stress of Cd. Authors reported that the bacterial isolate C. oceanosedimentum DG-20 could significantly promote the shoot length, root length, dry and fresh weights of the whole plants in Cd stress conditions in comparison to control. Similarly, Heshmatpure and Rad (2012) also reported a significant increase (5–7 cm) in root length of plants grown from seeds presoaked with metal resistant bacterial isolate AO22, compared to those from control seeds under the stress of Cd (50–450 mg/l). Different studies have suggested that the PGP rhizobacteria can be used to reduce plant stress in the process of phytoremediation (Reed and Glick 2005). Bacillus sp. has been observed to enhance the accumulation of Cd and Ni in hyperaccumulators B. juncea and B. napus plants (Sheng and Xia 2006; Zaidi et al. 2006). However, another study reported a reduction in the accumulation of metals in tomato plants but increased plant growth in stress conditions when it was inoculated with Methylobacterium oryzae and Burkholderia spp (Madhaiyan et al. 2007). Therefore, it suggests that PGP rhizobacteria are mainly involved in the reduction of stress in plants, and the types of PGP rhizobacteria and the plants used in the experiment governs the process of phytoremediation of contaminated soil. The beneficial effects shown by inoculation of Cd-resistant bacteria indicate that metal-resistant PGP rhizobacteria possesses the potential to improve rhizoremediation efficiency of Cd contaminated soils.
Exposure to a higher concentration of toxic metals to the plants may cause inhibition of many enzymes (Van Assche and Clijsters 1990). The change in the activity of enzymes serves as an index of metal sensitivity or tolerance in different groups of plants (Li et al. 2006). The high concentrations of heavy metals induced free radical production, which may damage the tissues of host plant (Foyer et al. 1997). These oxidative stresses activate antioxidative defense mechanisms in plants including the production of enzymatic antioxidants like superoxide dismutase (SOD), POD, and CAT and non-enzymatic components glutathione, carotenoids and ascorbate, etc. (Caregnato et al. 2008). The exposure of heavy metals to plant tissue also disturbs metabolic pathways, resulting in the increased formation of free radicals and reactive oxygen species. In this study, after 15 and 30 days under Cd stress and non stressed conditions, the plant showed a difference in the activity of stress-responsive enzymes GST, CAT, POD as well as GSH content (of the treated and non treated plants). The augmentation of the bacterial isolates significantly reduced the activity of stress-responsive enzymes. For GSH content, the BB-2B treatment had a greater rate of GSH content than the other treatments, whereas CAT activity was significantly decreased in BB-2B and S217 treated treatments and for the POD activity the BB-2B and S217 treatments decreased the POD activity in comparison to other treatments. However, the activity of GST enzymes did not get affected by the inoculation of bacterial isolates, although the content of GSH was found to be less upon augmentation with bacterial isolates. Singha and Pandey (2017) demonstrated the effects of abiotic stress on GST activity and the GSH content of plants, and the GST activity in root tissues increased with a successive increase of pyrene concentrations. Marrs (1996) also reported that the plants with herbicide exposure have higher GST levels, which were mainly activated for detoxification of herbicides via GSH conjugation. Other studies also reported the roles of GST in enhancing stress tolerance such as drought, high salt, low temperature, and oxidative stresses in plants (Diao et al. 2011). Similarly, the activity of POD, and CAT (in the leaves of plants) also changes in response to Cd in a concentration-dependent manner (Irfan et al. 2013).
In the present study it was found that the residual amount of Cd showed reduction by 23–31% from the soil after 30 days of the experiment. The most efficient combination for the removal of Cd were observed in Z. mays- B. subtilis SR1 and Z. mays- S. marcescens BB-2B treatments, where it was observed that the removal percentage were 31% and 28% respectively. A recent study also showed that the inoculation of plant Miscanthus floridulus with the bacteria Klebsiella michiganensis TS8 significantly reduced (49.2%) Cd from the polluted soils after 60 days of the experiment in comparison to control (Liu et al. 2021). Similarly, Kotoky et al. (2019a) reported efficient removal of Cd from the contaminated soil by S. marcescens S2I7, when applied through rhizosphere of Oryza sativa. Plants of different species have different capabilities to remove or accumulate Cd. As Cd has low affinities with soil ligands, it can be easily extracted by roots and further transported to other tissues. However, many factors like soil pH, temperature, and concentration may play a crucial role in the remediation process.
Conclusion
The plants growing in metal contaminated soils showed a reduction in growth due to the abiotic stress that leads to change in the physiological and biochemical activities (of plants). Although some metals are required by plants in very small amounts, higher concentrations exert stress (on the plants) retarding their growth and development. We found that the application of metal resistant bacterial isolates enhances the growth of plants in Cd-contaminated soil and also led to rhizoremediation of Cd contaminated soil. The application of metal-resistant PGP bacteria thereby could promote the growth of the Z. mays plant by reducing the abiotic stress and the approach has great potential in remediation as well as for improvement of production of maize crop, in contaminated soils.
Data availability
The 16 s rDNA sequences of the isolates have been submitted to the National Centre for Biotechnology Information (NCBI) genebank database under the accession numbers MF992191 (B. subtilis SR1), KX602663 (Paenibacillus sp. S1I8), MF554655 (S. marcescens BB-2B), and KY744360 (S. marcescens S2I7).
References
Aladesanmi OT, Oroboade JG, Osisiogu CP, Osewole AO (2019) Bioaccumulation factor of selected heavy metals in Zea mays. J Health Pollut 9:24
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—Concepts and applications. Chemosphere 91:869–881
Bashir S, Hussain Q, Akmal M, Riaz M, Hu H, Ijaz SS, Iqbal M, Abro S, Mehmood S, Ahmad M (2018a) Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J Soils Sedim 18:874–886
Bashir S, Hussain Q, Shaaban M, Hu H (2018b) Efficiency and surface characterization of different plant-derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 211:632–639
Becerra-Castro C, Prieto-Fernandez A, Alvarez-Lopez V, Monterroso C, Cabello-Conejo MI, Acea MJ, Kidd PS (2011) Nickel solubilizing capacity and characterization of rhizobacteria isolated from hyperaccumulating and non-hyperaccumulating subspecies of Alyssum serpyllifolium. Int J Phytoremediat 1:229–244
Belyaeva ON, Haynes RJ, Birukova OA (2005) Barley yield and soil microbial and enzyme activities as affected by contamination of two soils with lead, zinc or copper. Biol Fertil Soils 41(2):85–94
Bojorquez C, Voltolina D (2016) Removal of cadmium and lead by adapted strains of Pseudomonas aeruginosa and Enterobacter cloacae. Rev Int Contam Ambie 32:407–412
Brunetti G, Farrag K, Rovira PS, Nigro F, Senesi N (2011) Greenhouse and field studies on Cr, Cu, Pb and Zn phytoextraction by Brassica napus from contaminated soils in the Apulia region, Southern Italy. Geoderma 160(3–4):517–523
Caregnato FF, Koller CE, MacFarlane GR, Moreira JCF (2008) The glutathione antioxidant system as a biomarker suite for the assessment of heavy metal exposure and effect in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Pollut Bull 56:1119–1127
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2:12
Dey S, Mazumder PB, Paul SB (2014) Accumulation of Cu at different concentration in tea plant (Camellia sinensis(L) OKuntze). IOSR J Agric Vet Sci 7(4):39–43
Diamandas A, Razon MR, Ramirez-Arcos S, Brassinga AKC (2021) The virulence of S marcescens strains isolated from contaminated blood roducts is divergent in the C. elegans infection model. Front Genet 12:667062. https://doi.org/10.3389/fgene.2021.667062
Diao G, Wang Y, Wang C, Yang C (2011) Cloning and Functional Characterization of a Novel Glutathione S-Transferase Gene from Limonium bicolor. Plant Mol Biol Rep 29:77–87
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signalling. Physiol Plant 100:241–254
Głowacka K, Źróbek-Sokolnik A, Okorski A, Najdzion J (2019) The effect of cadmium on the activity of stress-related enzymes and the ultrastructure of pea roots. Plants (basel, Switzerland) 8(10):413. https://doi.org/10.3390/plants8100413
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hameed A, Sheikh MA (2007) Changes in catalase, peroxidase activities and soluble proteins in wheat leaves on thiourea and H2O2 treatments. Biosci Res 4(1):21–27
Hasnat A, Rahman I, Pasha M (2013) Assessment of environmental impact for tannery industries in Bangladesh. Int J Environ Sci Dev 4(2):217–220
Hernandez LE, Villasante CO, Montero-Palmero MB, Escobar C, Carpena RO (2012) Heavy metal perception in a microscale environment: a model system using high doses of pollutants. In: Gupta DK, Sandalio LM (eds) Metal toxicity in plants: perception, signaling and remediation. Springer-Verlag, Berlin, pp 23–37. https://doi.org/10.1007/978-3-642-22081-4_2
Heshmatpure N, Rad MY (2012) The effect of PGPR (Plant-Growth-Promoting Rhizobacteria) on phytoremediation of cadmiums by canola, (Brassica napus L.) cultivars of Hyola. Ann Biol Res 2:5624–5630
Huang L, Pu X, Pan JF, Wang B (2013) Heavy metal pollution status in surface sediments of Swan Lake lagoon and Rongcheng Bay in the northern Yellow Sea. Chemosphere 93(9):1957–1964
Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018) Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. Hindawi J Toxicol 16:2
Irfan M, Ahmad A, Hayat S (2013) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21:125–131
Khan AR, Park GS, Asaf S, Hong SJ, Jung BK, Shin JH (2017) Complete genome analysis of Serratia marcescens RSC-14: a plant growth-promoting bacterium that alleviates cadmium stress in host plants. PLoS ONE 12(2):0171534. https://doi.org/10.1371/journal.pone.0171534
Kotoky R, Pandey P (2019) Rhizosphere mediated biodegradation of benzo(A)pyrene by surfactin producing soil bacilli applied through Melia azadirachta rhizosphere. Int J Phytorem. https://doi.org/10.1080/15226514.2019.1663486
Kotoky R, Pandey P (2020) Rhizosphere assisted biodegradation of benzo(a)pyrene by cadmium resistant plant-probiotic Serratia marcescens S2I7, and its genomic traits. Sci Rep 10:5279. https://doi.org/10.1038/s41598-020-62285-4
Kotoky R, Pandey P (2021) The genomic attributes of Cd-resistant, hydrocarbonoclastic Bacillus subtilis SR1 for rhizodegradation of benzo(a)pyrene under co-contaminated conditions. Genomics 113(1):613–623. https://doi.org/10.1016/j.ygeno.2020.09.0577
Kotoky R, Singha LP, Pandey P (2017a) Draft genome sequence of polyaromatic hydrocarbon-degrading bacterium Bacillus subtilis SR1, which has plant growth-promoting attributes. Genome Announc 2:1–2
Kotoky R, Singha LP, Pandey P (2017b) Draft Genome Sequence of Heavy Metal- Resistant Soil Bacterium Serratia marcescens S2I7, Which Has the Ability To Degrade Polyaromatic Hydrocarbons. Genome Announc 2:1–2
Kotoky R, Nath S, Maheshwari DK, Pandey P (2019) Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environ Sustain 2:135–144
Kuiper I, Bloemberg GV, Lugtenberg BJJ (2001) Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-degrading bacteria. Mol Plant-Microbe Interact 14:1197–1205
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kumar V, Behl RK, Narula N (2001) Establishment of phosphate-solubilizing strains of azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions. Microbiol Res 156 (1): 87–93 Retrieved http://www.sciencedirect.com/science/article/pii/S0944501304700142.
Li M, Hu CW, Zhu Q, Chen L, Kong ZM, Liu ZL (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the micro-alga Pavlova viridis (Prymnesiophyceae). Chemosphere 62:65–572
Li H, Liu X, Wassie M et al (2020) Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ Sci Pollut Res 27:9490–9502. https://doi.org/10.1007/s11356-019-06628-3
Liu S, Liu H, Chen R, Ma Y, Yang B, Chen Z, Liang Y, Fang J, Xiao Y (2021) Role of two plant growth-promoting bacteria in remediating cadmium—contaminated soil combined with Miscanthus floridulus (Lab). Plants 10:912. https://doi.org/10.3390/plants10050912
Ma Y, Rajkumar M, Freitas H (2009) Inoculation of plant growth promoting bacterium Achromobacter xylosoxidansstrain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J Environ Manage 90:831–837
Madhaiyan M, Poonguzhali S, Torgmin SA (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Moran MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathionereductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Okolo VN, Olowolafe EA, Akawu I, Okoduwa SIR (2016) Effects of industrial effluents 581 on soil resource in challawa industrial area. J Glob Ecol Environ 5(1):10
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14(5):10197–10228
Osazee OJ, Obayagbona ON, Daniel EO (2013) Microbiological and physicochemical analyses of top soils obtained from four municipal waste dumpsites in Benin City, Nigeria. Int J Microbiol Mycol 1(1):23–30
Pal AK, Sengupta C (2019) Isolation of cadmium and lead tolerant plant growth promoting rhizobacteria: Lysinibacillusvarians and Pseudomonasputida from Indian agricultural soil. Soil Sedim Contam 28(7):601–629. https://doi.org/10.1080/15320383.2019.1637398
Patel M, Patel K, Al-Keridis LA, Alshammari N, Badraoui R, Elasbali AM, Al-Soud WA, Hassan MI, Yadav DK, Adnan M (2022) Cadmium-tolerant plant growth-promoting bacteria Curtobacterium oceanosedimentum improves growth attributes and strengthens antioxidant system in Chili (Capsicum frutescens). Sustainability 14:4335. https://doi.org/10.3390/su14074335
Pena LB, Barcia RA, Azpilicueta CE, Mendez AA, Gallego SM (2012) Oxidative post translational modifications of proteins related to cell cycle are involved in cadmium toxicity in wheat seedlings. Plant Sci 196:1–7. https://doi.org/10.1016/j.plantsci.2012.07.008
Piacentini D, Corpas FJ, D’Angeli S, Altamura MM, Falasca G (2020) Cadmium and arsenic-induced-stress differentially modulates Arabidopsis root architecture, peroxisome distribution, enzymatic activities and their nitric oxide content. Plant Physiol Biochem 148:312–323. https://doi.org/10.1016/j.plaphy.2020.01.026
Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001) Quality protein maize. Curr Sci 81(10):1308–1319
Purkayastha GD, Mangar P, Saha A, Saha D (2018) Evaluation of the biocontrol efficacy of a Serratia marcescens strain indigenous to tea rhizosphere for the management of root rot disease in tea. PLoS ONE 13(2):e0191761. https://doi.org/10.1371/journal.pone.0191761
Raja C, Selvam GS, Omine K (2009) Isolation, identification and characterization of heavy metal resistant bacteria from sewage. International joint symposium on Geodisaster prevention and geo-environment in Asia JS-Fukuoka, 205–209.
Rajkumar M, Ma Y, Freitas H (2008) Characterization of metal-resistant plant growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J Basic Microbiol 48:1–9
Rajkumari J, Choudhury Y, Bhattacharjee K, Pandey P (2021) Rhizodegradation of pyrene by a non-pathogenic klebsiella pneumoniae isolate applied with tagetes erecta l and changes in the rhizobacterial community. Front Microbiol 12:593023. https://doi.org/10.3389/fmicb.2021.593023
Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51(12):1061–1069
Rehman M, Liua L, Bashirb S, Saleema MH, Chena C, Penga D, Siddiquec KHM (2019) Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper contaminated soil. Plant Physiol Biochem 138:121–129
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879
Romdhane L, Panozzo A, Radhouane L, Dal Cortivo C, Barion G, Vamerali T (2021) Characteristics and metal uptake of maize (Zea mays L.) under xtreme soil contamination. Agronomy 11:178. https://doi.org/10.3390/agronomy11010178
Saha J, Sarkar M, Mandal P, Pal A (2022) Comparative study of heavy metal uptake and analysis of plant growth promotion potential of multiple heavy metal-resistant bacteria isolated from arable land. Curr Microbiol 79:7
Schulenburg H, Ewbank JJ (2004) Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol Biol 2004(4):49. https://doi.org/10.1186/1471-2148-4-49
Shahzad A, Qin M, Elahie M, Naeem M, Bashir T, Yasmin H, Younas M, Areeb A, Irfan M, Billah M, Shakoor A, Zulfiqar S (2021) Bacillus pumilus induced tolerance of Maize (Zea mays L.) against Cadmium (Cd) stress. Sci Rep 11:17196. https://doi.org/10.1038/s41598-021-96786-7
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Singh VP, Singh S, Kumar J, Prasad SM (2015) Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 252:1217–1229. https://doi.org/10.1007/s00709-014-0753-6
Singh HN, Jayashree R, Narayan SL (2018) Heavy metal occurrence in the soil of high input tea agroecosystem of Southern Assam, Northeast India, Vegetos. Int J Plant Res 31(3):47–54. https://doi.org/10.5958/2229-4473.2018.00072.1
Singha P, Pandey P (2017) Glutathione and glutathione-S-transferase activity in Jatropha curcas in association with pyrene degrader Pseudomonas aeruginosa PDB1 in rhizosphere, for alleviation of stress induced by polyaromatic hydrocarbon for effective rhizoremediation L. Ecol Eng 102:422–432
Singha LP, Pandey P (2021) Rhizosphere assisted bioengineering approaches for the mitigation of etroleum hydrocarbons contamination in soil. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2021.1888066
Thakare M, Sarma H, Datar S, Roy A, Pawar P, Gupta K, Pandit S, Prasad R (2021) Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr Res Biotechnol 3:84–98
Toth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Van Assche F, Clijsters H (1990) Effects of metals on enzyme in plants. Plant Cell Environ 13:195–206
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368
Yaouba A, Tatsadjieu N, Jazet DP, Mbofung CM (2012) Inhibition of fungal development in maize grains under storage condition by essential oils. Int J Biosci 2(6):41–48
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64(6):991–997
Zhang L, Chang X, Li Z, He Q (2010) Selective solid-phase extraction using oxidized activated carbon modified with triethylenetetramine for preconcentration of metal ions. J Mol Struct 964:58–62
Zhou L, Zhang L, He Y, Liu F, Li M, Wang Z, Ji G (2014) Isolation and characterization of bacterial isolates for biological control of clubroot on Chinese cabbage. Eur J Plant Pathol 140:159–168. https://doi.org/10.1007/s10658-014-0451-4
Acknowledgements
BB and PP acknowledge the Department of Biotechnology (DBT), Govt. of India for financial support, RK acknowledges the Department of Science and Technology (DST), Govt. of India for DST-INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhuyan, B., Kotoky, R., Maheshwari, D.K. et al. Rhizoremediation of Cd-contaminated soil using Zea mays Sturt, with heavy metal resistant rhizobacteria that alleviate Cd-induced stress in plant. Environmental Sustainability 5, 375–387 (2022). https://doi.org/10.1007/s42398-022-00241-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-022-00241-w