Abstract
Selenium (Se) is beneficial for plant growth under different stressful conditions. In this study, we investigated the protective effects of Se supply from Cd-induced damages in tall fescue under Cd stress. Tall fescue seedlings (40 days old) were treated with Cd (30 mg/L, as CdSO4·8/3 H2O) and Se (0.1 mg/L, as Na2SeO3) individually and in combination using 1/2 Hoagland’s solution system for 7 days. Various physiological parameters, photosynthetic behaviors, and gene expressions were measured. The results showed that Cd-stressed plants displayed obvious toxicity symptoms such as leaf yellowing, decreasing plant height, and root length. Cd stress significantly increased the malondialdehyde (MDA) content and electrolyte leakage (EL), and remarkably reduced the chlorophyll and soluble protein content, antioxidant enzyme activities, and photosynthetic efficiency. Cd stress significantly inhibited the expression of two photosynthesis-related genes (psbB and psbC), but not psbA. In addition, it significantly inhibited the expression of antioxidant system-related genes such as ChlCu/ZnSOD, CytCu/ZnSOD, GPX, and pAPX, but significantly increased the expression of GR. However, Se improved the overall physiological and photosynthetic behaviors of Cd-stressed plants. Se significantly enhanced the chlorophyll and soluble protein content and CAT and SOD activities, but decreased MDA contents, EL, and Cd content and translocation in tall fescue under Cd stress. Furthermore, under Cd stress, Se increased the expression of psbA, psbB psbC, ChlCu/ZnSOD, CytCu/ZnSOD, GPx, and PAPx. The result suggests that Se alleviated the deleterious effects of Cd and improved Cd resistance in tall fescue through upregulating the antioxidant system, photosynthesis activities, and gene expressions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introducation
Cadmium (Cd) is the third major cause of pollution and the greatest hazard to the environment (Ismael et al. 2019). It has been reported that the soil Cd content has remarkably risen during the past decades (Chen et al. 2013b), and the area of Cd-contaminated arable land has increased to approximately 20 million hm2 (Liu et al. 2015a). Cd in soil is readily absorbed and accumulated by plants. A previous study has shown that Cd stress causes leaf chlorosis; decreases various cellular and metabolic functions such as photosynthesis and water and nutrient uptake; and results in plant growth inhibition and death (Yourtchi and Bayat 2013). Other adverse impacts of Cd in plants include DNA damage, protein dysfunction, and a decrease in the efficiency of the electron transport chain (Liu et al. 2013). In agricultural practice, some chemical reagents can be applied to suppress Cd uptake and alleviate Cd phytotoxic effect, which has been considered to be an economical and effective way for harmless crop production (Sun et al. 2016).
Selenium (Se) is a trace element which is essential for both human beings and animals (White and Brown 2010). At the same time, with its antioxidative property, Se is important for plant growth and development at low concentration (Djanaguiraman et al. 2005; Filek et al. 2008; Nawaz et al. 2015). It has been proved that exogenous Se plays a positive effect on various plant physiological processes (Ismael et al. 2018); therefore, it enhances plant biomass under either stressed or non-stressed environments (Chu et al. 2010; Malik et al. 2011). Se supply increased plant growth, chlorophyll content, photosynthetic performance, and nitrogen contents in tobacco (Liu et al. 2015b). Se application also improved the reproductive capacity of Brassica rapa by increasing seed production and seed viability (Lyons et al. 2009). Because of these merits, Se was often used as an exogenous protectant against diverse abiotic stresses in plants (Hartikainen et al. 2000; Feng et al. 2013). Numerous reports had indicated that Se application enhanced plant resistance against environmental stresses, such as salinity (Jiang et al. 2017), drought (Nawaz et al. 2014, 2015), high temperature (Djanaguiraman et al. 2010), water deficit (Andrade et al. 2018), and ultraviolet-B (Yao et al. 2013). Nowadays, the utilization of Se for the mitigation of the impact of heavy metals especially Cd toxicity is increasing.
Previous investigations have demonstrated that Se could decrease Cd levels and enhance photosynthesis performance in plants exposed to Cd stress, and therefore promote plant growth. For instance, it was found that Se supplementation could remarkably restrain Cd content in lettuce (Lactuca sativa) (He et al. 2004), roots of Pfaffia glomerata plants (Pereira et al. 2018), pepper (Capsicum annuum L.) fruits (Mozafariyan et al. 2014), and rice (Oryza sativa) shoots or stems (Wan et al. 2016; Gao et al. 2018), and alleviates the Cd-induced growth damage (Sun et al. 2013). Under Cd stress, Se application considerably enhanced the fresh weight of roots in cucumber (Cucumis sativus L.) (Hawrylak-Nowak et al. 2014), and the inhibition of plant height, root length, and biomass by Cd could be mitigated after treatment with 3 μM selenite (Sun et al. 2016). Se is important in the control of Cd toxicity in seaweed (Gracilaria dura) (Kumar et al. 2012). It is reported that 50 μM Se pretreatment improved Cd tolerance in rapeseed plants by enhancing the antioxidant defense and methylglyoxal detoxification system (Hasanuzzaman et al. 2012). In addition, under Cd stress, low Se concentration could also protect membrane lipids and recover the envelope membrane structure in rape (Brassica napus L.) (Filek et al. 2010). However, the mechanism of Se action in protecting plants subjected to Cd stress has not been completely illuminated. Previous reports suggested that the mitigation of Cd toxicity by Se might be associated with reduced Cd uptake, decreased ROS production, and a higher nutrient balance in plants (Zhu et al. 2009; Feng et al. 2013). Reports showed that Se application alleviated Cd-induced ROS damage by enhancing enzymatic (Saidi et al. 2014; Filek et al. 2008) and non-enzymatic (Schiavon et al. 2013) antioxidants. Furthermore, Se could induce the production of phytochelatins, which hinder Cd transport from the roots to shoots (Hawrylak-Nowak et al. 2014). At the same time, under Cd stress, Se was involved in the regulation of recovery of the photosynthetic system and reconstruction of cell membranes and chloroplasts (Feng et al. 2013; Gao et al. 2018).
Tall fescue (Festuca arundinacea Schreb) is an important cool-season grass species that is widely utilized in temperate regions, either for turf or forage. As a perennial grass, tall fescue possesses fast reproduction and outstanding resistance to various abiotic stresses. In addition, it was observed that tall fescue tolerated and accumulated certain heavy metals including Pb, Cd, and so on (Begonia et al. 2005; Xu and Wang 2013). Meanwhile, the perfect Cd tolerance of tall fescue was reported (Xu and Wang 2014), and its great prospects in future phytoremediation was suggested. However, the protective effects of Se application from Cd-induced damage in tall fescue are not investigated under Cd stress. Therefore, the aim of this study was to evaluate the effects of Se application on various physiological processes and related gene expressions in tall fescue under Cd stress. Cd, Se, chlorophyll, soluble protein and MDA content, electrolyte leakage, antioxidant enzyme activities, chlorophyll a fluorescence, and gene expressions were measured. The result indicated that Se is effective in improving Cd tolerance in tall fescue through regulating various physiological characteristics and gene expressions.
Materials and methods
Plant material and Cd and Se treatments
The seeds of tall fescue (commercial cultivar “Houndog 5”) were sowed in plastic pots (7.5 cm in diameter and 8.5 cm depth) containing pearl stone and vermiculite (1:1 v:v). After germination, the seedlings were maintained in a greenhouse with 14 h photoperiod (300 μmol photons m−2 s−1) and 24/22 °C (day/night) temperature for 40 days. The seedlings were watered daily to the pots’ capacity level and fertilized with 1/2-strength Hoagland’s solution (1 mM NH4H2PO4, 5 mM KNO3, 5 mM Ca(NO3)2·4H2O, 2 mM MgSO4·7H2O, 2.86 mg/L·H3BO3, 0.22 mg/L ZnSO4·7H2O, 0.08 mg/L CuSO4·5H2O, 1.81 mg/L MnCl2·4H2O, 0.1 mg/L H2MoO4, 0.08 mM Fe-EDTA) once a week. The height of the plants was kept at 12 cm above the medium surface through clipping. For accommodation, the seedlings were then transplanted into conical flasks filled with 1/2 Hoagland solution, and grown for a week. On the 7th day of accommodation, Cd (as CdSO4·8/3 H2O) and Se (as Na2SeO3) were added to the solutions to form 4 treatment regimes: (1) control; 1/2 Hoagland’s solution (CK); (2) Se, CK + 0.1 mg/L Se; (3) Cd, CK + 30 mg/L Cd; and (4) Cd + Se, CK + 30 mg/L Cd + 0.1 mg/L Se. Each treatment was performed with three replications, and the solution was renewed every other day. Following 7 days of treatment, samples were collected to determine various physiological responses and gene expressions.
Determination of Cd and Se content
After 7 days of treatment, the plant leaves and roots were sampled separately. To remove the possible ion contamination from the root surface, roots were rinsed with 20 mM Na2-EDTA solution for 20 min and washed with deionized water. All shoot and root samples were then dried at 70 °C to the constant weight, and subsequently milled and digested with 2 N HCl. Cd and Se content were then measured using inductively coupled plasma mass spectrometry (ICP-MS, 7700ce, Agilent Technologies, Santa Clara, CA, USA) (Wan et al. 2016).
Chlorophyll content determination
Chlorophyll content was determined following the method of Hiscox and Israelstam (1979). About 0.1 g of fresh leaves was cut into small pieces and transferred into a 15-mL tube containing 10 mL dimethylsulfoxide (DMSO), and the tubes were then kept in the dark for 48 h. Accordingly, the absorbance of the chlorophyll extract was measured at 663 and 645 nm using a spectrophotometer (UV-2600, UNICO Instruments Co., Ltd., Shanghai, China). The chlorophyll content (Chl a, Chl b, and Chl total) was calculated following the formula of Hiscox and Israelstam (1979).
Electrolyte leakage measurement
The electrolyte leakage (EL) level was measured using the method described by Hu et al. (2018). In brief, about 0.15 g fresh leaves was cut into small pieces and transferred into a 50-mL centrifuge tube containing 25 mL deionized water. The initial conductivity (Ci) was measured using a conductance meter (JENCO-3173, Jenco Instruments, Inc., San Diego, CA, USA) after the tubes were shaken for 24 h at room temperature. The tubes were then autoclaved for 15 min at 121 °C to release all the electrolytes, and the maximum conductivity (Cmax) was measured after the tubes cooled down to room temperature. The following formula was used to calculate the relative EL:
Relative EL% = Ci/Cmax*100%.
Determination of enzyme activities, soluble protein, and MDA content
Crude enzyme extract was prepared by homogenizing 0.3 g fresh leaves with 4 mL cooled potassium phosphate buffer (0.15 M, pH 7.0) using a pre-chilled mortar and pestle. The homogenate was then transferred into a 10-mL tube, and centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was collected and used for determination of soluble protein and malondialdehyde (MDA) content, catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) activity.
Soluble protein content was measured following the method of Bradford (1976), and the MDA contents were determined according to the method of a previous report (Fan et al. 2015b). The activities of SOD and POD were measured according to the method described by Fan et al. (2015b), and CAT activity was determined by the method of Chance and Maehly (1955).
Chlorophyll a fluorescence transient analysis and the JIP test
Chlorophyll a (Chl a) fluorescence transient was examined using a pulse-amplitude modulation fluorometer (PAM 2500, Heinz Walz GmbH) with high time resolution (10 μs). Each treatment was measured at least five times using different leaves. After 30 min of leaves dark accommodation, measurement was performed under a saturating light intensity of 2000 μmol photons m−2 s−1. The Chl a fluorescence emission triggered by the strong light pulses was measured and digitized between 10 μs and 300 ms according to Korres et al. (2003). Subsequently, the OJIP transient analysis was performed using the JIP-test following the method of Chen et al. (2013a).
According to Strasser et al. (2004), the JIP-test is a multi-parametric analysis of the OJIP transient. The chlorophyll fluorescence kinetics curve comprises the changing process from initial fluorescence intensity (FO) to maximal fluorescence intensity (FP). When illuminated by high-intensity actinic light, the dark-accommodated plants and other photosynthetic organisms will display a rise of OJIP curves. A typical JIP-test usually contains 4 phrases: O-J (0.05–5 ms), J-P (5–50 ms), and I-P (50–1000 ms). The chlorophyll fluorescence kinetics curve reflects the intensity of stress effects on photosynthesis function. In this study, chlorophyll a fluorescence transient analysis was performed using the JIP-test according to the method described by Chen et al. (2013a). The fluorescence intensities at 50 μs, 2 ms (J step), and 30 ms (I step) were designated as FO, FJ, and FI, respectively, and the maximal fluorescence (P step) was denoted as FP. Selected JIP-test parameters were calculated using the method described by Ni et al. (2012).
Real-time quantitative PCR analysis
The expression levels of three photosynthesis-related genes (psbA, psbB, and psbC) and five antioxidant system-related genes (ChlCu/ZnSOD, CytCu/ZnSOD, GPx, PAPx, and GR) were analyzed using quantitative PCR (Q-PCR). Total RNA was isolated from leaf samples using Trizol reagent (Invitrogen, USA). About 2 μg of total RNA was used for the first strand cDNA synthesis using a cDNA synthesis kit (Fermentas, Canada). Gene-specific primers for Q-PCR were listed in Table S1. The Q-PCR procedure was conducted according to our previous method (Li et al. 2017).
Statistical analysis
Three biological replicates were used in all the experiments. Results were expressed as mean ± standard deviation. Statistical analysis was carried out by one-way ANOVA with the Statistical Package for the Social Sciences (SPSS ver.11.5), Origin 7.5, and Excel 2003 for Windows. The statistical effects of treatments were evaluated by Duncan’s multiple range tests at a significance level of p ≤ 0.05.
Results
Effect of Se on Cd content
Under Cd stress, tall fescue plants showed obvious toxicity symptoms. Cd-treated plants showed yellow or withered leaves and a decrease in plant height and root length as compared to the control (Table S2). However, Se application mitigated Cd-induced toxic effects (Fig. S1; Table S2). The Cd contents were under the detection limit in both CK and Se treatments, either in shoots or in roots. Under Cd treatment, shoots and roots showed a high level of Cd (0.375 and 4.041 mg g−1), respectively. However, Se supplementation significantly decreased the Cd contents by about 31% and 29% in shoots and roots, respectively, when compared to Cd treatment alone (Table 1). In addition, our results showed that Cd treatment significantly decreased the Se level in shoots to under the detection limit, but increased remarkably the accumulation of Se in the roots of tall fescue (Table 1).
Effect of Se on EL and MDA content
As shown in Fig. 1, Cd stress significantly increased both MDA and relative EL in tall fescue leaves. Under Cd stress, EL and MDA content were about 63% and 166% higher than that of the CK, respectively. However, Se supplementation significantly reduced the MDA content by 52% and relative EL value by about 29% in tall fescue leaves, when compared to Cd treatment alone. At the same time, Se treatment alone had no effect on both MDA content and relative EL value under CK.
The effect of different concentrations of Se and Cd on the level of electrolyte leakage (EL) (a) and the content of MDA (b) in tall fescue leaves. CK was normal condition. Cd regime was treated with 30 mg/L Cd. Se regime was treated with 0.1 mg/L Se under normal condition. Cd + Se group was treated with combination of 0.1 mg/L Se and 30 mg/L Cd. Results are presented as means ± SE (n = 3). Different letters represent significant differences (p ≤ 0.05) in MDA or EL level in tall fescue between the treatments
Effect of Se on chlorophyll and soluble protein content
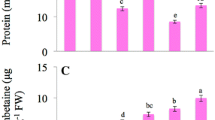
In regard to chlorophyll content, there was no obvious change between plants treated with Se alone and the control. Cd stress significantly decreased Chl a, Chl b, and total Chl content by about 23%, 27%, and 23%, respectively, when compared with the CK. However, the Cd-induced decline in chlorophyll content was remarkably recovered with Se application. Under Cd stress, Se treatment restored Chl b content to the control level, and surprisingly increased Chl a and total Chl content higher than that of CK (Fig. 2a).
The effect of different treatments on chlorophyll a/b (a), total chlorophyll (b), and soluble protein (c) content in tall fescue leaves. CK was normal condition. Cd regime was treated with 30 mg/L Cd. Se regime was treated with 0.1 mg/L Se under normal condition. Cd + Se group was treated with combination of 0.1 mg/L Se and 30 mg/L Cd. Data presented are the means ± SE (n = 3). Different uppercase or lowercase letters indicate significant difference at p ≤ 0.05 between the treatments
In addition, Cd stress significantly decreased soluble protein content as compared to the CK (p ≤ 0.05) (Fig. 2b), while application of Se promoted the accumulation of soluble protein in tall fescue under Cd stress. The soluble protein content in the plants under Se + Cd treatment was significantly higher than that of Cd stress alone (p ≤ 0.05), and reached to the control level. However, there was no significant difference between the Se and CK treatment groups.
Effect of Se on antioxidant enzyme activities
The antioxidant enzyme activities in tall fescue were varied in different treatment regimes (Fig. 3). Cd stress significantly decreased the SOD activity by about 10%, but Se supplementation greatly enhanced the SOD activity by nearly 30% when compared to the CK. Under Cd stress, Se-supplemented plants showed significantly higher SOD activity than that of CK, and was comparable to only Se-treated plants.
The effect of different concentrations of Se and Cd on activity of antioxidant enzymes SOD (a), CAT (b), and POD (c) in tall fescue leaves. CK was normal condition. Cd regime was treated with 30 mg/L Cd. Se regime was treated with 0.1 mg/L Se under normal condition. Cd + Se group was treated with combination of 0.1 mg/L Se and 30 mg/L Cd. Data presented are the means ± SE (n = 3). Different letters indicate significant difference at p ≤ 0.05 among the treatments
Similar to SOD, CAT activity was sharply increased by approximately twofold with Se application alone (p ≤ 0.05), but was significantly reduced by Cd treatment alone when compared with the CK. Furthermore, as compared to the Cd stress, Se treatment improved the CAT activity by 40%, which was almost equal to CK. Unlike SOD or CAT, the POD activity was significantly increased with Se application and Cd stress individually by about 46% and 137%, respectively, when compared to the CK. However, under Cd stress, Se decreased the activity of POD by 15% in tall fescue leaves, but much higher than CK and Se treatment groups.
Effect of Se on the expression of photosynthesis and antioxidant enzyme-related genes
The expression of three genes related to the photosynthetic system and five genes related to antioxidant enzymes were investigated in tall fescue under Cd stress. The result showed that Cd or Se alone had no effect on the expression of psbA in tall fescue compared to the CK. However, under Cd stress, Se significantly enhanced the gene transcription level of psbA as compared to the CK (Fig. 4a). Individually, both Cd and Se significantly downregulated the expression of psbB in tall fescue leaves, but Cd-treated plants supplemented with Se showed higher psbB transcription level when compared to the CK. In addition, under Cd stress, exogenous Se significantly enhanced the abundance of psbB compared to the Cd stress alone. Similar to psbB, the expression of psbC was significantly inhibited by both Cd and Se treatment individually, and the reduction of the transcription level was sharper in Cd-treated plants. Just like psbB, psbC was also significantly upregulated by Se application in Cd-treated plants when compared to Cd stress alone, but its transcription level was lower than that of the CK and Se treatment groups.
The transcriptional profiles of genes associated with PSII genes as well as antioxidant enzyme in tall fescue leaves after Se treatment under Cd stress. Relative expression level of psbA, psbB, and psbC (a); relative expression level of ChlCu/ZnSOD and CytCu/ZnSOD (b); relative expression level of GR, GPX, and pAPX (c). CK was normal condition. Cd regime was treated with 30 mg/L Cd. Se regime was treated with 0.1 mg/L Se under normal condition. Cd + Se group was treated with combination of 0.1 mg/L Se and 30 mg/L Cd. Values are given as means ± SE of three independent experiments. Different letters indicate statistical difference significance at p ≤ 0.05 among the treatments
The expression of ChlCu/ZnSOD was significantly upregulated in Se-supplemented tall fescue plants, but sharply inhibited in Cd-treated plants compared to the CK (Fig. 4b). Se supplementation enhanced the expression of ChlCu/ZnSOD in tall fescue plants exposed to Cd stress, but its transcription level was much lower than that of the CK. Similarly, the abundance of CytCu/ZnSOD was significantly decreased by Cd treatment, but the transcription level increased almost to CK level with the addition of Se under Cd stress. However, Se alone had no effect on the expression of this gene (Fig. 4b).
GPX and pAPX shared a very similar expression profile. Both genes were significantly downregulated by Cd treatment as compared to the CK (Fig. 4c). However, Se application enhanced their expression levels to a certain extent in plants under Cd stress when compared to Cd stress alone. Meanwhile, there was no difference in the expression levels of the two genes between Se and CK regimes. Unlike the above genes, Cd stress significantly increased the transcription level of GR. By contrast, Se treatment alone or combined with Cd did not apparently affect GR expression (Fig. 4c).
Effect of Se on the OJIP transient curve
Se treatment alone had no effect on the OJIP transient curve of tall fescue, but Cd stress alone obviously decreased the OJIP curve as compared to the CK, which was partially ameliorated by Se supplementation (Fig. 5).
The OJIP transient curves of tall fescue leaves under different concentrations of Se and Cd. After a 30-min dark adaption period, the OJIP transients of tall fescue leaves were induced by a red light of 3000 μmol photons m−2 s−1 provided by PAM 2500 through an array of light-emitting diodes. CK (in black) was normal condition. Cd regime (in red) was treated with 30 mg/L Cd. Se regime (in pink) was treated with 0.1 mg/L Se under normal condition. Cd + Se group (in blue) was treated with combination of 0.1 mg/L Se and 30 mg/L Cd
Furthermore, the JIP-test was performed to analyze the parameters of the OJIP transient curve, and used to quantify the photosynthesis of tall fescue. The basic parameters including FO, FK, FJ, FI, FP, and MO were extracted and listed in Table 2. Cd stress significantly enhanced the FO, FK, and MO with or without Se supplementation. Meanwhile, Se application alone increased the FK and MO, but reduced the FO. There were no obvious differences in the FJ and FI between Cd and CK regimes. However, Se application apparently increased FJ and FI, especially in Cd-stressed tall fescue plants compared to CK and Cd alone regimes. Unlike those above parameters, Cd-stressed plants showed a lower FP, compared to the CK, but were restored to the normal level with Se supplementation.
Cd stress alone significantly increased the value of specific energy fluxes such as ABS/RC and TP0/RC, but remarkably decreased with Se supplementation. However, under both CK and Se application alone, there was no obvious difference in the ABS/RC and TP0/RC. On the other hand, ET0/RC and RE0/R were both significantly reduced by Cd and Se application, either alone or in combination, compared to the CK.
Parameters related to quantum yield and efficiencies contained δRo, фEo, φPo (namely FV/FM), and ƴRC. Cd stress alone significantly decreased φPo. However, there was no obvious difference in φPo between Se alone and CK. Meanwhile, ƴRC showed similar trends with φPo. Compared to the CK, фEo was dramatically declined in all the other three treatments. Similarly, both Cd and Se treatment alone decreased the value of δRo. However, no distinct difference was observed in this parameter between CK and Cd + Se regime.
Both PItotal and PIABS are important performance indices which demonstrate the overall PSII activity. In this study, both parameters were decreased in Cd regimes when compared to the CK. However, the values were slightly higher in the Se-supplemented regime compared to the Cd stress alone. Se alone did not affect these two parameters.
Discussion
In the present study, Se supplementation significantly decreased the Cd uptake in both roots and shoots of tall fescue plants, while improving their growth. This is the first study to provide information about the beneficial role of exogenous Se in reducing Cd uptake or translocation, and enhancement of photosynthesis performance and antioxidant activity against Cd toxicity in tall fescue plants.
In our study, Cd stress significantly reduced the chlorophyll content of tall fescue leaves. Similar results were reported in sunflower (Helianthus annuus) and pepper (Mozafariyan et al. 2014; Saidi et al. 2014) under Cd stress. The reduction of chlorophyll concentration might be associated with lipid damage in the chloroplast membranes and/or the inhibition of chlorophyll biosynthesis (Feng et al. 2013; Hawrylak-Nowak et al. 2014).
In this study, Se application enhanced the chlorophyll content of tall fescue leaves under Cd treatment. In agreement with our results, the appropriate dosage of Se also significantly increased the concentration of photosynthetic pigments in Cd-stressed plants, such as spinach (Spinacia oleraceae L.), broccoli (Brassica oleracea), cucumber (Cucumis sativus L.), seaweeds (Gracilaria dura), and pepper (Capsicum annuum L.) (Pedrero et al. 2008; Kumar et al. 2012; Safaryazdi et al. 2012; Hawrylak-Nowak et al. 2014; Mozafariyan et al. 2014). Moreover, pre-soaking of seeds with Se could alleviate Cd-induced pigment loss in sunflower plants exposed to Cd (Saidi et al. 2014). It has been indicated that in salt-stressed sorrel (Rumex patientia × R. tianshanicus) seedlings, a suitable dosage of Se could promote the integrity of membrane systems and cellular organelles, including chloroplasts and mitochondria in the leaf mesophyll cells (Kong et al. 2005). Therefore, under Cd stress, the enhanced chlorophyll content by Se might be also attributed to its benefits in protecting the membrane system of chloroplasts.
MDA is the oxidized product of membrane lipids, and its content is generally considered as an indicator of lipid peroxidation and oxidative stress level. It has been reported that low doses of exogenous Se can reduce lipid peroxidation (Djanaguiraman et al. 2005). In this study, the MDA content was significantly increased under Cd treatment in tall fescue leaves. However, under Cd treatment, Se supplementation significantly decreased the MDA content (Filek et al. 2008). Similar to our result, many studies have indicated that optimal exogenous Se could decline the MDA concentration in diverse plant species under various stresses (Pukacka et al. 2011; Seppänen et al. 2003; Iqbal et al. 2015). The decrease in lipid peroxidation by Se might be attributed to its positive effects on plants’ antioxidant capacity (Feng et al. 2013). In addition to the induction of lipid peroxidation, membrane dysfunctioning was also generally observed in olive trees (Olea europaea L.) exposed to drought stress (Sofo et al. 2004). In this study, Cd treatment increased the EL of tall fescue, and the result was in accordance with the results obtained in mustard (Brassica juncea), rapeseed (Brassica napus cv. BINA sharisha 3), Cassia italica, and tomato (Solanum lycopersicum L.) (Hasanuzzaman et al. 2012; Asgher et al. 2014; Hashem et al. 2016; Alyemeni et al. 2018). However, just like MDA, the EL level was also reduced by Se application in tall fescue exposed to Cd stress. A similar result was reported in tomato (Alyemeni et al. 2018). The result indicated that Se supplementation is beneficial for the maintenance of structural and functional integrity of membranes, and consequently prevents electrolyte leakage in plants. Furthermore, Filek et al. (2008) suggested that the greater membrane stability in Se-supplemented plants might be associated with the increased production of unsaturated fatty acid.
In response to oxidative stress, plants have developed an enzymatic antioxidant system, which regulates oxidative stress through the catalytic reduction of ROS by antioxidant enzymes such as SOD, CAT, POD, etc. (Feng et al. 2013). In the present study, Cd stress significantly reduced the activities of both SOD and CAT in tall fescue leaves. Similar to our result, the activities of both SOD and CAT were significantly reduced by Cd in mung beans (Phaseolus vulgaris) (Somashekaraiah et al. 1992). In addition, a reduction of SOD activity was also observed in Helianthus annus and peas (Pisum sativum L.) under Cd stress (Gallego et al. 1996; Sandalio et al. 2001). The possible reason for decreased enzyme activities might be inactivation of enzymes by excess H2O2 and the binding of metal to the active center of the enzymes (Mishra et al. 2006). However, Se supplementation increased the activities of both enzymes under non-stressed and Cd-stressed conditions. Similar changes of CAT activity were reported in rapeseed seedlings when treated with Cd alone or Cd combined with Se (Hasanuzzaman et al. 2012). In addition, the reduction in activity of these antioxidant enzymes was reported in Brassica chinensis under Cd stress, but the reduction was not restored by Se supplementation (Yu et al. 2018). Unlike SOD and CAT, the activity of POD was enhanced by Cd and Se treatment, either individually or in combination when compared to the CK. Furthermore, Se increased the activity of antioxidant enzymes including SOD and CAT in tomato when exposed to Cd stress (Alyemeni et al. 2018), which was in accordance with our result. In B. napus, exogenous Se improved Cd tolerance through increasing both the activity of antioxidant enzymes and the content of non-enzymatic antioxidant components (Filek et al. 2008). Recently, it was also shown that Se increased the activities of SOD and guaiacol peroxidase in Pfaffia glomerata under Cd stress (Pereira et al. 2018). These investigations indicated that Cd may have complex effects on antioxidant enzymes depending on the difference in Cd concentration, plant species, and management methods. At the same time, an optimal dosage of Se was beneficial to enhance the antioxidant capacity, and therefore alleviates the Cd-induced oxidative stress in tall fescue as well as many other plants.
Photosynthesis is a sensitive process in tall fescue, and has been inhibited by diverse abiotic stresses including Cd treatment (Huang et al. 2017, 2018; Zhang et al. 2017; Hu et al. 2018). PSII is an important membrane structure in the photosynthesis system and is vulnerable to stress conditions (Chen et al. 2014; Huang et al. 2017). Here, the photosynthesis activities of tall fescue subjected to different treatments were evaluated by fluorescence transient curve. As shown in Table 2, Cd stress influenced the PSII function mainly by altering the FO, FK, FP, and MO. For example, FO was significantly enhanced by Cd stress, which was in agreement with previous studies (Huang et al. 2017, 2018; Zhuo et al. 2017). The possible reason might be the dissociation of light-harvesting complex II from the PSII complex and accumulation of inactive RCs of PSII (Yamane et al. 1997), or increased back electron transfer from QB to QA (Kouril et al. 2004). By contrast, FM was much lower in Cd-treated tall fescue. Similarly, Huang et al. (2018) reported the decrease of FM induced by chromium (Cr) treatment in tall fescue. However, exogenous Se recovered the inhibition or enhancement of Cd-induced changes in these parameters, which suggested that Se supplementation plays a positive role in protecting tall fescue PSII from the destruction by Cd stress. Previous studies also reported that exogenous regulators like nitric oxide and Spd alleviated the damage of PSII in tall fescue under various abiotic stresses (Huang et al. 2018; Zhang et al. 2017).
In addition, we analyzed Chl a fluorescence transient data using the JIP test (Yusuf et al. 2010; Chen et al. 2014). The parameters associated with quantum yields and efficiencies were evaluated including maximum quantum yield for primary photochemistry (фPo, also known as FV/FM), quantum yield of the electron transport flux from QA to QB (фEo), quantum yield for reduction of end electron acceptor at the PSI acceptor side (δRo), and the probability that a PSII Chl molecule functions as RC (ƳRC). It was observed that Cd treatment alone significantly declined the values of these parameters, and therefore inhibited the efficiency of electron transportation as well as the Chl molecule functions of PSII. This result was in agreement with that of Huang et al. (2017), who reported that different concentrations of Cd treatment decreased the FV/FM, фEo, and ƳRC in tall fescue. However, in Cd-treated tall fescue plants, the decline of δRo, FV/FM, and ƳRC was restored to the control level with Se supplementation. Recently, Alyemeni et al. (2018) reported that Se could enhance the value of FV/FM in tomato under Cd stress, which was consistent with our result.
It was reported that a decrease in PSII photochemical efficiency in lettuce (Lactuca sativa L.) under ozone might be partially attributed to the destruction of antennae pigments (Calatayud and Barreno 2004). In this study, specific energy fluxes were analyzed to examine the functional properties of PSII. The result indicated that Cd stress significantly increased the ABS/RC and TP0/RC, but Se supplementation recovered the two parameters almost to the normal level. By contrast, Cd stress decreased the ET0/RC and RE0/RC, but they were not recovered with Se supplementation. These changes were in accordance with that of Chl content in our study (Fig. 2a).
Both PIABS and PItotal are performance indices describing the overall behaviors of photosynthetic activities, which were usually used for assessing the photochemical activities of stressed plants (Fan et al. 2015a). In the present study, Cd significantly decreased the PITotal and PIABS values which was consistent with previous studies on plants under heavy metal stresses (Mathur et al. 2016; Huang et al. 2018). This decrease might be due to the ROS burst induced by Cd. However, the values of PIABS and PItotal were slightly higher in the Cd + Se regime when compared with the Cd stress alone. This result suggests that exogenous Se may have a positive effect on PItotal and PIABS under Cd stress conditions.
The expression of antioxidant enzymes was upregulated under Cd treatment in B. juncea, wheat (Triticum aestivum), sunflower (Helianthus annuus L.), tomato (Solanum lycopersicum L.), and ryegrass (Lolium perenne) (Hartikainen et al. 2000; Asgher et al. 2014; Abd-Allah et al. 2015; Khan et al. 2015; Alyemeni et al. 2018). Cd influenced the transcription of genes in tall fescue (Lou et al. 2017; Zhu et al. 2018). In the present study, the expression level of genes encoding antioxidant enzyme was analyzed under Cd, Se, and Se + Cd treatments (Fig. 4b, c). The results indicated that except GR, all the other genes were significantly downregulated by Cd stress alone, which was in disagreement with Lou et al. (2017) who reported that Cd induced the expression of antioxidant enzyme-related genes in tall fescue. This discrepancy might be attributed to the differences in cultivars, Cd dosages, treatment time, etc. However, in this study, the transcriptional inhibition of these genes was restored by Se supplementation at different extents. Meanwhile, the gene expression profiles of both ChlCu/ZnSOD and CytCu/ZnSOD were in accordance with SOD activity changes (Fig. 3).
In higher plants, psbC and psbB encode for CP43 and CP47 protein, respectively, which are the core antenna protein complexes with the composition of chlorophyll a located in the RC of PSII (Barber 2003), whereas psbA encodes for D1 protein, which is the most fundamental structure of PSII that can bind to cofactors and protect the structure of PSII RC (Kruse et al. 1997). A previous study has reported that these three genes were remarkably downregulated by heat stress in tall fescue. However, the expression of psbA and psbB were restored to a certain degree in the presence of Spd (Zhang et al. 2017). In this study, Q-PCR analysis indicated that Cd stress downregulated the transcription of these three genes, especially psbC and psbB (Fig. 4a), implying the possible adverse effects of Cd on PSII in tall fescue. By contrast, Se supplementation significantly enhanced the transcription level of the three genes, which might improve the behavior of RC to some extent. Subsequently, Se could partially alleviate the Cd-induced damage of RC and might be beneficial to the stability of PSII under Cd stress.
Conclusions
In summary, Cd stress had negative impacts on various physiological and gene expression behaviors of tall fescue, but Se application restored the effects of Cd by various complicated mechanisms. Optimal Se supplementation alleviated the Cd phytotoxicity in tall fescue through reducing the growth inhibition, decreasing Cd uptake, diminishing lipid peroxidation, enhancing the antioxidant enzyme activities and photosynthesis activity, and regulating the transcription of relative genes under Cd stress. This investigation provides important information about Se-mediated Cd resistance in tall fescue. The result would be helpful for further understanding the complex response mechanisms of Cd stress tolerance, and will be beneficial for exploring new strategies for Cd detoxification in grass species.
References
Abd-Allah EF, Hashem A, Alqarawi AA, Alwathnani HA (2015) Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak J Bot 47:785–795
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:459–469
Andrade FR, da Silva GN, Guimarães KC, Barreto HBF, de Souza KRD, Guilherme LRG, Faquin V, Reis ARD (2018) Selenium protects rice plants from water deficit stress. Ecotoxicol Environ Saf 164:562–570
Asgher M, Khan NA, Khan MIR, Fatma M, Masood A (2014) Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Saf 106:54–61
Barber J (2003) Photosystem II: the engine of life. Q Rev Biophys 36:71–89
Begonia MT, Begonia GB, Ighoavoda M, Gilliard D (2005) Lead accumulation by tall fescue (Festuca arundinacea Schreb.) grown on a lead-contaminated soil. Int J Environ Res Public Health 2:228–233
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Calatayud A, Barreno E (2004) Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol Biochem 42:549–555
Chance B, Maehly SK (1955) Assay of catalase and peroxidases. Methods Enzymol 2:64–75
Chen K, Fan J, Fu J (2013a) Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth Res 116:21–31
Chen WP, Lu SD, Peng C, Jiao WT, Wang ME (2013b) Accumulation of Cd in agricultural soil under long-term reclaimed water irrigation. Environ Pollut 178:294–299
Chen K, Sun X, Amombo E, Zhu Q, Zhao Z, Chen L, Xu Q, Fu J (2014) High correlation between thermotolerance and photosystem II activity in tall fescue. Photosynth Res 122:305–314
Chu J, Yao X, Zhang Z (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136:355–363
Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U (2005) Selenium—an antioxidative protectant in soybean during senescence. Plant Soil 272:77–86
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Fan J, Hu Z, Xie Y, Chan Z, Chen K, Amombo E et al (2015a) Alleviation of cold damage to photosystem II and metabolisms by melatonin in bermudagrass. Front Plant Sci 6:925
Fan J, Ren J, Zhu W, Amombo E, Fu J, Chen L (2015b) Antioxidant responses and gene expression in bermudagrass under cold stress. J Am Soc Hortic Sci 139:699–705
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Filek M, Gzyl-Marlcher B, Zembala M, Bednarska E, Laggner P, Kriechbaum M (2010) Effect of selenium on characteristics of rape chloroplast modified by cadmium. J Plant Physiol 167:28–33
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Gao M, Zhou J, Liu H, Zhang W, Hu Y, Liang J, Zhou J (2018) Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci Total Environ 631-632:1100–1108
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hashem A, Abd Allah EF, Alqarawi AA, Egamberdieva D (2016) Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci 23:39–47
Hawrylak-Nowak B, Dresler S, Wójcik M (2014) Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci Hortic 172:10–18
He PP, Lv XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hu L, Bi A, Hu Z, Amombo E, Li H, Fu J (2018) Antioxidant metabolism, photosystem ii, and fatty acid composition of two tall fescue genotypes with different heat tolerance under high temperature stress. Front Plant Sci 9:1242
Huang M, Zhu H, Zhang J, Tang D, Han X, Chen L et al (2017) Toxic effects of cadmium on tall fescue and different responses of the photosynthetic activities in the photosystem electron donor and acceptor sides. Sci Rep 7:14387
Huang M, Ai H, Xu X, Chen K, Niu H, Zhu H, Sun J, du D, Chen L (2018) Nitric oxide alleviates toxicity of hexavalent chromium on tall fescue and improves performance of photosystem II. Ecotoxicol Environ Saf 164:32–40
Iqbal M, Hussain I, Liaqat H, Ashraf MA, Rasheed R, Rehman AU (2015) Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol Biochem 94:95–103
Ismael MA, Elyamine AM, Zhao YY, Moussa MG, Rana MS, Afzal J et al (2018) Can selenium and molybdenum restrain cadmium toxicity to pollen grains in Brassica napus? Int J Mol Sci 19(8):E2163
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11(2):255–277
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45:155–163
Korres NE, Froud-Williams RJ, Moss SR (2003) Chlorophyll fluorescence technique as a rapid diagnostic test of the effects of the photosynthetic inhibitor chlorotoluron on two winter wheat cultivars. Ann Appl Biol 143:53–56
Kouril R, Lazár D, Ilík P, Skotnica J, Krchnák P, Naus J (2004) High-temperature induced chlorophyll fluorescence rise in plants at 40–50 °C: experimental and theoretical approach. Photosynth Res 81:49–66
Kruse O, Zheleva D, Barber J (1997) Stabilization of photosystem two dimers by phosphorylation: implication for the regulation of the turnover of D1 protein. FEBS Lett 408:276–280
Kumar M, Bijo AJ, Ravi S, Baghel CRK, Bhavanath JHA (2012) Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol Biochem 51:129–138
Li H, Hu T, Amombo E, Fu J (2017) Transcriptome profilings of two tall fescue (Festuca arundinacea) cultivars in response to lead (Pb) stress. BMC Genomics 18:145
Liu Y, Barber DS, Zhang P, Liu B (2013) Complex II of the mitochondrial respiratory chain is the key mediator of divalent manganese-induced hydrogen peroxide production in microglia. Toxicol Sci 132:298–306
Liu Y, Xiao T, Baveye PC, Zhu J, Ning Z, Li H (2015a) Potential health risk in areas with high naturally-occurring cadmium background in southwestern China. Ecotoxicol Environ Saf 112:122–131
Liu WX, Shang SH, Feng X, Zhang GP, Wu FB (2015b) Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ Toxicol Chem 34:92–99
Lou Y, Zhao P, Wang D, Amombo E, Sun X, Wang H et al (2017) Germination, physiological responses and gene expression of tall fescue (Festuca arundinacea Schreb.) growing under Pb and Cd. PLoS One 12:e0169495
Lyons GH, Genc Y, Soole K, Stangoulis JCR, Liu F, Graham RD (2009) Selenium increases seed production in Brassica. Plant Soil 318:73–80
Malik JA, Kumar S, Thakur P, Sharma S, Kaur N, Kaur R et al (2011) Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol Trace Elem Res 143:530–539
Mathur S, Kalaji HM, Jajoo A (2016) Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 54:185–192
Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65(6):1027–1039
Mozafariyan M, Shekari L, Hawrylak-Nowak B, Kamelmanesh MM (2014) Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol Trace Elem Res 160:97–107
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN (2014) Selenium (se) regulates seedling growth in wheat under drought stress. Adv Chem:1–7
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA (2015) Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem 175:350–357
Ni L, Acharya K, Hao X, Li S, Li Y, Li Y (2012) Effects of artemisinin on photosystem ii performance of Microcystis aeruginosa, by in vivo chlorophyll fluorescence. Bull Environ Contam Toxicol 89:1165–1169
Pedrero Z, Madrid Y, Hartikainen H, Cámara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56:266–271
Pereira AS, Dorneles AOS, Bernardy K, Sasso VM, Bernardy D, Possebom G, Rossato LV, Dressler VL, Tabaldi LA (2018) Selenium and silicon reduce cadmium uptake and mitigate cadmium toxicity in Pfaffia glomerata (Spreng.) Pedersen plants by activation antioxidant enzyme system. Environ Sci Pollut Res Int 25(19):18548–18558
Pukacka S, Ratajczak E, Kalemba E (2011) The protective role of selenium in recalcitrant Acer saccharium L. seeds subjected to desiccation. J Plant Physiol 168:220–225
Safaryazdi A, Lahouti M, Ganjeali A, Bayat H (2012) Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleraceae L.) plants. Not Sci Biol 4:95–100
Saidi I, Chtourou Y, Djebali W (2014) Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J Plant Physiol 171:85–91
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rio LA (2001) Cadmium induces changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Schiavon M, Dall'acqua S, Mietto A, Pilon-Smits EH, Sambo P, Masi A, Malagoli M (2013) Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J Agric Food Chem 61:10542–10554
Seppänen M, Turakainen M, Hartikainen H (2003) Selenium effects on oxidative stress in potato. Plant Sci 165:311–319
Sofo A, Dichio B, Xiloyannis C, Masia A (2004) Lipoxygenase activity and proline accumulation in leaves and roots of olive trees in response to drought stress. Physiol Plant 121:58–65
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorphyll degradation. Physiol Plant 85(1):85–89
Strasser RJ, Tsimillimichael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Govindjee J (ed) Advances in photosynthesis & respiration. Springer, Dordrecht, pp 321–362
Sun HY, Wang XY, Dai HX, Zhang GP, Wu FB (2013) Effect of exogenous glutathione and selenium on cadmium-induced changes in cadmium and mineral concentrations and antioxidative metabolism in maize seedlings. Asian J Chem 25:2970–2976
Sun HY, Dai HX, Wang XY, Wang GH (2016) Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotoxicol Environ Saf 133:114–126
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: influence of different forms of selenium. Ecotoxicol Environ Saf 133:127–134
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
Xu PX, Wang ZL (2013) Physiological mechanism of hypertolerance of cadmium in Kentucky bluegrass and tall fescue: chemical forms and tissue distribution. Environ Exp Bot 96:35–42
Xu PX, Wang ZL (2014) A comparison study in cadmium tolerance and accumulation in two cool-season Turfgrasses and Solanum nigrum L. Water Air Soil Pollut 225:1938
Yamane Y, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo level and reversible inhibition of photosystem II reaction center by high-temperature treatments in higher plants. Photosynth Res 52:57–64
Yao X, Jianzhou C, Xueli H, Binbin L, Jingmin L, Zhaowei Y (2013) Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol Environ Saf 92:320–326
Yourtchi MS, Bayat HR (2013) Effects of cadmium toxicity on growth, cadmium accumulation and macronutrient content of durum wheat (Dena CV.). Int J Agric Crop Sci 6:1099–1103
Yu Y, Yuan S, Zhuang J, Wan Y, Wang Q, Zhang J et al (2018) Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol Environ Saf 162:571–580
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee Sarin NB (2010) Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta 1797:1428–1438
Zhang L, Hu T, Amombo E, Wang G, Xie Y, Fu J (2017) The alleviation of heat damage to photosystem ii and enzymatic antioxidants by exogenous spermidine in tall fescue. Front Plant Sci 8:1747
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Zhu H, Ai H, Cao L, Sui R, Ye H, Du D et al (2018) Transcriptome analysis providing novel insights for Cd-resistant tall fescue responses to Cd stress. Ecotoxicol Environ Saf 160:349–356
Zhuo Y, Qiu S, Amombo E, Zhu Q, Tang D, Huang M et al (2017) Nitric oxide alleviates cadmium toxicity in tall fescue photosystem II on the electron donor side. Ecotoxicol Environ Saf 137:110–118
Funding
This research was funded by Poverty Alleviation through Agricultural Projects from the Agricultural Office of Chinese Academy of Sciences and the National Natural Science Foundation of China (Nos. 31672482).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Liu, X., Wassie, M. et al. Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ Sci Pollut Res 27, 9490–9502 (2020). https://doi.org/10.1007/s11356-019-06628-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06628-3