Abstract
Ocean represents an unusual diversity of life. The largest proportion of microbial diversity has been found in the oceanic and terrestrial subsurface respectively. Marine habitats are inhabited by several microbial populations adapted to these ecosystems. Among these populations, bacteria are one of the important and dominant inhabitants of such environments. Marine bacteria themselves or their products such as enzymes, exopolymers, pigments, antimicrobial compounds, and biosurfactants represent a wide range of applications in food, textile, and pharmaceutical industries as well as in many environmental processes. This review aims to present the exopolysaccharide production from marine bacteria and its possible biosynthesis along with recovery of these polymers using various methods. Finally, the applications of these polymers, particularly in the field of bioremediation, are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 71% of the Earth’s surface is covered with oceans having an average depth of 3.8 km and an average pressure of 38 MPa (van Eldik and Hubbard 1996). Temperatures at deep-sea surfaces and the upper surfaces of the sea are different. The different conditions that prevail in the marine ecosystems are responsible for the existence of various extreme habitats, such as salt lakes, marine salterns, deep-sea, volcanic and hydrothermal marine areas as well as in the sea ice in Polar Regions, where organisms grow in such extreme conditions and flourish (Casillo et al. 2018). The marine organisms have developed unusual metabolic processes and defensive mechanisms for their survival under such extreme conditions, which might have resulted in the ability to produce novel bioactive compounds in comparison to other natural habitats (Chi and Fang 2005). Marine microorganisms produce many organic substances and one such product is exopolysaccharide (EPS) (Abreu and Taga 2016). There has been a growing interest in the isolation and identification of new marine microorganisms capable of producing polysaccharides. These polymers participate in the maintenance of marine environments by contributing to several processes like sedimentation, particle formation, cycling of dissolved metals and dissolved organic carbon (Verdugo 2012).
Although microbial cells require up to 70% of the total energy for EPS production, but once formed it benefits the microbes in multiple ways. EPS helps the organisms to grow and survive under adverse environmental conditions (Poli et al. 2010). Apart from these, EPS play a vital role in nutrient uptake, aggregation, adhesion to surfaces, and biofilms formation (Dave et al. 2016; Shukla and Dave 2018). EPS possess active and ionisable functional groups and non-carbohydrate substituents like amine, sulfhydryl, carboxyl, hydroxyl, phosphate, and sulphate groups that are responsible for the negative charge of the polymer. Due to this property, various heavy metals can bind to EPS by ion exchange, complexation, and entrapment like mechanisms (Gupta and Diwan 2017). Loaec et al. (1998) and Wuertz et al. (2000) have described the role of EPS producing heavy metal resistant isolates from deep-sea hydrothermal vents and purified EPS for metals and toxic substances binding ability.
Now a days the focus is to isolate novel marine microorganisms for the production of EPS with diverse properties. Different genera of marine bacteria such as Alteromonas, Bacillus, Cobetia, Colwellia, Geobacillus, Halomonas, Hyphomonas, Idiomarina, Pseudoalteromonas, Pseudomonas, Polaribacter, Rhodococcus, Shewanella, Vibrio, Exiguobacterium, Kocuria, Pontibacter, Planococcus, Marinobacter have been reported as EPS producers (Le Costaouec et al. 2012; Kumar et al. 2004; Lelchat et al. 2015; Carillo et al. 2015; Arena et al. 2009; Bouchotroch et al. 2000; Arias et al. 2003; Quintero et al. 2001; Martínez-Cánovas et al. 2004; Saravanan and Jayachandran 2008; Wu et al. 2016a, b; Sun et al. 2015; Urai et al. 2006; Vinogradov et al. 2005; Bramhachari and Dubey 2006; Upadhyay et al. 2016). Genera of halophilic archaea Thermococcus, Sulfolobus, Haloarcula, and Haloferax are also reported for EPS production by Poli et al. (2011).
The increasing commercial importance of marine microbial EPS has stimulated the efforts in the development of rapid and efficient techniques for their recovery and purification. The presence of microbial cells, medium ingredients in the fermentation broth, as well as its high viscosity, often causes problems in the recovery of EPS (Yang et al. 1998; Kumar et al. 2007). The steps involved in EPS recovery and purification are the removal of microbial cells and protein followed by precipitation, dialysis and lyophilisation of EPS (Smith and Pace 1982; Laroche and Michaud 2010; Castillo et al. 2015). Elucidations of chemical compositions and structures of EPS are necessary to establish their structure-function relationship. But the precise characterization of the EPS is a challenge to researchers because of its structural complexity (Chowdhury et al. 2011). Although the chief components of EPS are carbohydrates, it is difficult to derive their unique monomer linkage patterns and biochemical properties (Jiao et al. 2010). Thus acid hydrolysis, Fourier-transform infrared spectroscopy (FTIR), high-performance liquid chromatography (HPLC), methylation analysis, gas chromatography with mass spectroscopy (GC–MS), and 1H- and 13C-NMR (one and two dimensions) have been used for chemical characterisation of EPS (Liang and Wang 2015). The development of purification techniques and sophisticated analytical approaches permit researchers to provide an insight into primary structures and conformation of polysaccharides, which are important to acquire information about complete polymeric structures.
This review highlights the EPS production ability of various marine bacteria, chemical structure and biosynthetic mechanisms of EPS and their applications. The content also explores distinct strategies of metal remediation through marine bacteria and their EPS. To understand the mechanism behind metal ion uptake through EPS, it is essential to know its chemical structure and properties. The properties and structural aspects can be studied from purified EPS. Thus, the extraction, purification and recovery of EPS by various methods has also been illustrated.
EPS production by marine bacteria
In case of domain Bacteria, several species of Gram-positive bacteria such as Bacillus, Lactobacillus, Streptococcus, Diplococcus, Leuconostoc and Gram-negative bacteria such as Pseudomonas, Xanthomonas, Enterobacter, Azotobacter, Klebsiella, and Rhizobium are reported for EPS production (Sutherland 1972; Jekins and Hall 1997). Various marine bacteria are also reported for EPS production and some examples are depicted in Table 1.
Although extensive research has been carried out in this field, further investigations will result in identification of novel EPS producing organisms, new products with novel characteristics within the marine environment of the deep-sea hydrothermal vents, the Arctic and the Antarctic regions.
Chemical structure of EPS
EPS are chemical compounds that are synthesized as secondary metabolites by different microorganisms and are secreted as slime or jelly-like material outside the cell-wall. Polysaccharide chains of EPS vary from 103 to 108 kDa and contain sub-unit configurations that may also have species-specificity (Sutherland 1985). EPS are organic macromolecules and are formed by polymerization of simple building blocks of monosaccharides, uronic acids, amino sugars linked by glycosidic bonds, amino acids linked by peptide bonds, nucleic acids, phospholipids and humic substances. In a polymer they are arranged as repeating units (Frølund et al. 1996; Dignac et al. 1998; D’Abzac et al. 2010). EPS carry organic substituents such as acetyl, succinyl, or pyruvyl group or inorganic substituent such as sulphates. EPS are homo- or heterogeneous compounds having mono-, di-, and oligosaccharides along with some non-carbohydrate substituents (Sutherland 1985; Whitfield 1988). The bond angles of polysaccharides govern the relative orientation of adjacent sugar residues in a chain and determine the shape of EPS. In solution, EPS may have single-, double- or triple-helical conformation, which makes polysaccharides semi-rigid. Their helix is stabilized by an intermolecular hydrogen bond. Poor intramolecular interactions between polymer segments lead to aggregation and finally precipitation or gelation of EPS in organic solvents (Jekins and Hall 1997). Polysaccharides mostly possess negative charge, but occasionally they also have neutral and positive charges depending upon the constituents of the repeated units (Neu and Poralla 1990). The diversity of bacterial polysaccharides is eight folds higher in comparison to plant polysaccharides (Jekins and Hall 1997). Bacterial polysaccharides have constant and reproducible physicochemical properties, desired degree of purity and more diverse structural properties as compared to plant polysaccharides. In contrast to plants, cellulose produced by bacteria has more purity and a wider range of applications because of the absence of lignin and hemicellulose. Moreover, the bacterial cellulose provides high absorbent capacity and tensile strength as compared to the cellulose obtained from plants (Kambourova et al. 2015). In traditional applications, some bacterial EPS like xanthan gum or gellan gum can directly replace polysaccharides obtained from plants such as guar-gum or pectin, as well as carrageenan or alginate produced by algae, because of improved physical properties of bacterial EPS (Fialho et al. 2008; Rehm 2010). An EPS produced by Pseudomonas elodea was identified as gellan and it was recommended as a food additive by Japan, the United States and Europe (Prameela et al. 2018). It was categorized as high acyl (HA) and low acyl (LA) gellan depending upon the degree of acylation (Fallourd and Viscione 2009). Gellan gum forms a gel at low concentrations and it is highly biocompatible and biodegradable. A mixture of xanthan and gellan has been used instead of alginate to encapsulate the probiotic cells as they showed high resistance toward acidic conditions (Sun and Griffiths 2000). Due to these distinct physical properties, they have been used largely.
Classification of EPS
Bacterial polysaccharides normally include lipopolysaccharides (LPS), peptidoglycans, teichoic acids (TA) capsular polysaccharides (CPS), and EPS. Mostly the CPSs are made up of repeating oligosaccharide units and are heteropolysaccharides in nature. LPSs contain repeating units of core oligosaccharide, and an acylated disaccharide (lipid A). Peptidoglycans are made up of repeating disaccharide units called N-acetylglucosamine (NAG) and N-acetyl muramic acid (NAM). TA possesses mono- or oligosaccharides, or ester-bound amino acids with polyolphosphates and glycosylpolyolphosphates fragments. EPS are categorized as homo- and hetero-polysaccharides based on their chemical composition (De Vuyst and De Vin 2007). Figure 1 shows various classes of bacterial polysaccharides.
Homopolysaccharides are made up of only one type of sugar molecule and are categorized based upon their chemical composition such as type of linkage and monomeric units present in EPS. Various examples of homopolysaccharides are α-d-glucans, β-d-glucans, fructans and polygalactan, etc. (Ruas-Madiedo et al. 2002). Heteropolysaccharides possess repeating units of more than one type of sugars such as d-glucose, d-galactose, l-rhamnose, N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc) or glucuronic acid (GlcA). Moreover, some EPS also contain the non-carbohydrate substituents such as phosphate, sulphate, glycerol and acetyl. Homo- and heteropolysaccharide have a different chemical structure and linkages. The site of their synthesis and synthetic enzymes are also different. They possess mainly β-1,4- or β-1,3 linkages which are highly rigid and more flexible α-1,2 or α-1,6 linkages (Nwodo et al. 2012). Further detail of their synthesis is given in the next part.
Biosynthesis of EPS
Several studies have been carried out to understand the EPS biosynthesis mechanisms in bacteria. Pathway of EPS biosynthesis differs from genus to genus and is an energy-dependent process. The production and utility of substrate molecule also differ based on the organisms and types of EPS produced. In most cases, EPS are synthesized at the cell membrane and then exported from the cell. The only exceptions are homo-polysaccharides, which are synthesized extracellularly. The activated precursor monosaccharide is transferred from the substrate to growing polysaccharide by various enzymes. The sugars of polysaccharides are then assembled in a particular linkage pattern such as α and β. In the cyclic structure of monosaccharide, when the –OH is situated below the plane of the ring then α linkage occurs and when the –OH is placed above the plane then β-linkage forms. Janczarek (2015) has reported the production of succinoglucan and galactoglucan by S. meliloti. Succinoglucan represented the repeating units of d-glucose and d-galactose which were joined by β-1,3; β-1,4 and β-1,6 linkages. Whereas galactoglucans contained the same sugar units, which are joined by α-1,3 and β-1,3 linkages.
In intracellular EPS biosynthesis, the substrate entered the bacterial cell first and then it is catabolised by periplasmic oxidation or intracellular phosphorylation (Freitas et al. 2011). Biosynthesis of EPS mainly involves glycosyltransferases, which link sugars from intracellular nucleotide sugars to a lipid carrier molecule. The availability of sugar nucleotides affects greatly the biosynthesis of certain EPS namely alginate, gellan, etc. Biosynthesis of other EPS such as levan, alternan, reuteran, mutan and dextran is catalyzed extracellularly by levansucrase, alternansucrase, reuteransucrase, and mutansucrase and dextransucrase, respectively, from sucrose (Whitfield 1988; Boels et al. 2001; Patel et al. 2010).
The intracellular mechanism requires charged and energy-rich precursor monosaccharide in the form of nucleotide diphosphate/monophosphate sugar (NDP/NMP-sugar) for the synthesis of a biomolecule as simple sugar molecules cannot carry out the synthesis. The synthesis is carried out by phosphorylated sugars normally in the form of sugar-1P but rarely in the form of sugar-2P or sugar- 6P. The synthesis of sugar molecules follows an independent pathway. Then, these sugar molecules from activated NDP/NMP-sugar moieties get transferred to undecaprenyl phosphate (C55-P) with the help of enzyme glycosyltransferase (Sutherland 2001). In the assembly and transport of Gram-negative bacterial EPS the lipid intermediate pathway plays an important role, it is also reported that sometime it has also been utilized by Gram-positive bacteria. In Gram-positive bacteria, there is translocation of backbone chain to the cell surface after assembly of repeating moieties at the lipid carrier. Published literature provides evidence for the involvement of ATP-binding cassette (ABC) transporter-dependent pathway, Wzx/Wzy-dependent pathway and synthase-dependent pathway for the biosynthesis of EPS in marine bacteria (Cuthbertson et al. 2010; Ates 2015; Sara Pereira et al. 2015; Parkar et al. 2017). Gram-negative bacteria usually follow either ABC transporter-dependent or Wzx–Wzy dependent pathway but Pseudomonas aeruginosa follows the synthase-dependent pathway. The overall process of polymerization, chain length control, detachment from lipid and finally in the export of EPS is catalyzed by several enzymes.
The Wzx/Wzy-dependent pathway takes place in the cytoplasm and several membrane proteins play an important role in the synthesis of EPS. In this pathway, many distinct repeating units which are generally attached to a undecaprenol diphosphate anchor at the inner membrane are assembled by numerous glycosyltransferases and translocated across the cytoplasmic membrane by a Wzx protein known as flippase. Before they will be exported to the cell surface, their polymerization occurs at the periplasmic space by Wzy protein, and Wzz protein controls the length of the repeating units. Polymerized repeat units have been transported from the periplasm to the cell surface. This transportation is dependent upon the additional proteins allotted to the polysaccharide co-polymerase (PCP) and the outer membrane polysaccharide export families. The completely synthesized and exported EPS either secrete as slime or it gets attached to the cell surface as capsular polysaccharide material. EPS assembled by the Wzx/Wzy pathway contain a variety of sugars and thus they are classified as heteropolymers. The genes for the flippase (Wzx) and the polymerase (Wzy) are located within the extracellular polysaccharide operons in all strains which use this pathway (Schmid et al. 2015).
Synthase-dependent pathway not only secrets a complete polymer strand outside the membrane and the cell wall but it is often operated for the assembly of homopolymers. This pathway is independent of flippase. The process of polymerization and translocation are performed by single synthase protein (Islam and Lam 2014).
The capsular polysaccharide (CPS) biosynthesis is carried out by the ABC transporter dependent pathway. This pathway is also dependent on phosphoglycosyl transferases, which are relatively similar to that of the Wzx/Wzy-dependent pathway. The involvement of single phosphoglycosyl transferase containing operon in biosynthesis results in homopolymers and heteropolymers products, when multiple phosphoglycosyl transferases are used. The process occurs due to the ABC transporter proteins (which are present across the inner membrane), periplasmatic proteins of the polysaccharide co-polymerase (PCP) and proteins of outer membrane polysaccharide export (OPX) families (Willis and Whitfield 2013; Gupta and Diwan 2017; Parkar et al. 2017).
Recovery and purification of EPS
Pre-treatment
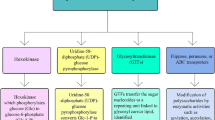
The processing and recovery costs of EPS are fairly less when it is extracted directly from the fermentation broth without any pre-treatments. The resultant product has a higher viscosity than that obtained from powder product and it can be dissolved easily. But in such products, impurities of microbial cells, medium constituents and colour are present. The presence of bacterial cells and colour impurities are usually undesirable from the application point of view (Smith and Pace 1982; Sutherland 1983). The main aim of any recovery process is the removal/lysis of microbial cells and precipitation of EPS from the fermentation broth. Purification of EPS is carried out either in the broth itself or after recovery (from the broth) to improve some aspects of the quality and performance of EPS for a given application (Leroy and De Vuyst 2016). Various steps involved in EPS recovery and purification are summarized in Fig. 2.
Cell removal
The first step in EPS downstream process is the removal of microbial cells from the fermentation broth. EPS might occur as either capsular or slime polysaccharides. The slime polysaccharides are loosely bound to the cell surface and can be extracted by centrifugation or by ultra-centrifugation. The speed and duration of the centrifugation depend upon the viscosity of fermentation broth (Morin 1998; Mende et al. 2013; Notararigo et al. 2013; Kreyenschulte et al. 2014). The capsular polysaccharides are firmly attached to the cell, thus various chemical and physical pre-treatments (alkaline pre-treatments with sodium hydroxide or heat treatments) are needed before centrifugation (Notararigo et al. 2013). The fermentation broth can be filtered, centrifuged or ultracentrifuged before polysaccharide recovery to remove cells and associated material at laboratory level studies (Stredansky et al. 1999). But at an industrial scale, the above mentioned procedures cannot be used effectively due to the large volume and highly viscous nature of the broth. This demands the development of alternative methods for cell removal. Several mechanical, chemical and thermal treatments have been established to lyse, deactivate or remove cells from the broth. Different chemical treatments when performed at high pH generally affect the structural properties of the product whereas enzymatic treatments increase the cost of downstream processes. Mostly the physical treatments such as pasteurization or sterilization are used to kill the microbial cells (Smith and Pace 1982; Garcia-Ochoa et al. 1993; Mende et al. 2013; Notararigo et al. 2013). Thermal treatments decrease the viscosity of broth, inactivate some of the enzymes and also kill the microbial cells and enhance EPS detachment from the cells. Capsular polysaccharides can also be extracted using autoclaving and various alkali treatments. Several other methods like boiling of fermentation broth for 15 min in water, heating at 60 °C in a mixture of phenol and water or in saline solution and sonication have also been used (Smith and Pace 1982; Freitas et al. 2011; Kreyenschulte et al. 2014; Leroy and De Vuyst 2016). Mechanical methods make use of centrifugation, ultracentrifugation, and filtration of fermentation broth. Stredansky et al. (1999) used activated charcoal to remove cells, colour impurities and odour from fermentation broth.
Deproteinization
The presence of protein with polysaccharide directly affects the purity of EPS hence it is required to perform deproteinization. Protein removal from the fermentation broth or crude EPS can be carried out using various chemicals such as trichloroacetic acid (TCA) and enzymes like proteases. TCA treatment results in co-precipitation of about 50% of the EPS with the medium proteins along with other impurities. It has been suggested to wash the TCA precipitate at least once to improve EPS recovery (Rimada and Abraham 2003). Various treatments used for the deproteinization of EPS are illustrated in Table 2.
Precipitation of EPS from the fermentation broth
After performing pre-treatment steps described above, recovery of EPS can be carried out using various organic solvents, salts and acids. The lower alcohols (methanol, ethanol, isopropanol) and acetone can be added to the fermentation broth to decrease the solubility of EPS and also to remove some impurities. Colo et al. (1997) have reported the use of ultrafiltration of fermented broth for better precipitation of EPS.
Some anionic polysaccharides in solution may be precipitated using acids. The anionic polysaccharides get precipitated upon the action of protons present in acid. The addition of salts in sufficient concentration also causes precipitation or complex coacervation due to the binding of the cations of the added salt to the ionized groups of EPS. This leads to charge reversal when all the available anionic groups are bound to cations. Polyvalent cationic salts (calcium, aluminium, and quaternary ammonium salts) are more effective in precipitation of EPS as compared to monovalent salts like sodium and potassium chloride (Pace and Righelato 1981). Solvents and salt in combination promote precipitation by decreasing the water affinity of the polymer and by increasing its affinity to bind cations. Thus, EPS precipitates wita-Ochoah lesser amounts of solvents (Garci et al. 1993). Various treatments used for EPS precipitation are showed in Table 3.
Partial purification of EPS
EPS precipitation treatments mostly follow the procedure of dialysis and lyophilisation. Number of researchers (Górska-Frączek et al. 2013; Marcial et al. 2013; Notararigo et al. 2013; Donnarumma et al. 2014; Ismail and Nampoothiri 2014; Shao et al. 2014; Fontana et al. 2015; Upadhyay 2017; Vaishnav 2017) have suggested to follow the steps like dissolution of EPS in deionized water, dialysis against water for 2–4 days at 4 °C, optional treatment with activated charcoal to decolourize, washing with anhydrous ethanol, acetone, and ether and finally lyophilisation of EPS. The dialysis of EPS is mainly important to remove small molecules such as salts and other impurities that are present in the material. Dialysis membranes with a molecular weight of 6000–8000 Da are suggested for dialysis, as EPS fractions of low molecular mass may otherwise be lost in the dialysis water and finally the content of dialysis bag is lyophilized (Rimada and Abraham 2003).
The lyophilized EPS can be treated further for the removal of proteins and other impurities. It can be done by using 80% v/v ethanol solution with 0.1% v/v formic acid to solubilize the protein followed by washing with 96% v/v ethanol (Tuinier et al. 1999; Ruas-Madiedo and de los Reyes-Gavilán 2005). The dissolution of lyophilized EPS in 0.3 M NaOH followed by centrifugation can also help to eliminate extra contaminants (Notararigo et al. 2013). EPS purification steps generally include size exclusion chromatography, ion-exchange chromatography or preparative SDS-PAGE (Notararigo et al. 2013; Zhang et al. 2013; Shao et al. 2014; Fontana et al. 2015).
When the ultimate aim is to quantify EPS production, some of these purification steps are less important as they affect the final yield of the product. For example, when protein removal is conducted using different chemicals and enzymes, these might react with EPS components and lower down the yield (Rimada and Abraham 2003). Purification method becomes an important criterion when EPS characterization is considered. At that time purity of EPS becomes the first concern as compared to its yield. If the product is pure then it will be less difficult to elucidate its structure. Therefore, the use of complex and non-complex media should be decided from the beginning of the experiment, as the EPS produced using complex media require extensive pretreatment procedures whereas the EPS recovered from non-complex media require simple deproteinization method before the centrifugation step.
Applications of EPS from marine microorganisms
In 1960s microbial polymers were explored for their applications in various fields, and since then there has been a remarkable increase for their commercial applications. Microbial polysaccharides are mainly used in the field of agriculture, pharmaceutical, food, textile, detergent, paper, paint, and petroleum industries. They are also used in processes like bioremediation, as a tool in drug delivery and cancer therapy, as well as for formulation of various culture media (Quesada et al. 1993; Dave et al. 2016; Vaishnav et al. 2016). Technological advancement has led to the discovery of the utility of microbial biopolymers to man (Nwodo et al. 2012). Microbial polymers help the organisms for attachment, biofilm formations, stabilization, aggregation and to maintain structural integrity (Lorenz et al. 1988). The EPS interacts with water molecules and change the rheological properties by increasing stability, thus can be used in the formulation of many pharmaceutical and cosmetic products. Most of the toothpaste making industries utilizes EPS as a binding and thickening agent (Tabibloghmany and Ehsandoost 2014).
Recently the applications of EPS from marine bacteria are increasing in various fields as they offer a great diversity of polysaccharides. Marine bacteria produce EPS having unique composition and properties. These bacterial polymers are reported to have anti-oxidant, antitumor, anti-microbial and immune-modulatory properties (Mohamed et al. 2018). The non-toxic EPS of marine microorganisms has been used for several medical applications such as in wound dressing and in drug delivery (Sutherland 1998; Otero and Vincenzini 2003; Rehm 2010; Laurienzo 2010). The spirulan produced by Arthrospira platensis and EPS from Spirulina has been used in the treatment of pulmonary metastasis and as an anti-inflammatory agent in many drugs (Wu et al. 2016a, b). Vibrio diabolicus, a marine bacterium has been reported for the production of “Hyalurift” polysaccharides having properties that are similar to hyaluronic acid and known for its restoration of bone integrity (Nwodo et al. 2012; Onesti et al. 2013). Romano et al. 2007) and de Morais et al. 2010) have suggested the use of microbial polysaccharides for bone integrity. A marine Pseudomonas sp. is reported to produce sulphated polysaccharide B-1, which showed cytotoxic activity against human cancer cell lines (Matsuda et al. 2003). EPS secreted by Bacillus licheniformis and Geobacillus thermodenitrificans has been used as an immune-modulatory agent for therapeutic purposes (Arena 2004). An acidic EPS released by Alteromonas sp. strain 1545 has interesting rheological properties and may be used as a thickening agent (Talmont et al. 1991); EPS secreted by A. madeodii sub sp. fijiensisbiovar has found application in cosmetics (patent number 94907582-4). The EPS secreted by Hahella chejuensis gen. nov., sp. nov., has emulsifying properties (Lee et al. 2001); polymer produced by Cyanothece sp. ATCC 51142 has the capability of gel formation and use in food industries (Shah et al. 2000). Kumar et al. (2007) isolated Planococcus maitriensis, which produced an EPS having biosurfactant properties. Microbial EPS are extensively used for Microbial Enhanced Oil Recovery (MEOR) and transport of polyaromatic and aliphatic hydrocarbons. The marine Pseudomonas sp. strain S9 was found to produce EPS in nutrient availability as well as in nutrient starvation conditions (Wrangstadh et al. 1990). An EPS capable of binding heavy metals was produced by the Alteromonas strain 1644 isolated from Alvinellidae collected from the East Pacific Rise (Bozzi et al. 1996). The Pseudoalteromonas strain SM9913 was isolated from deep-sea sediments in the Gulf of the Yellow Sea (China). EPS of this strain showed flocculating and biosorptive capacity (Qin et al. 2007; Li et al. 2008). Muralidharan and Jayachandran 2003) described the physicochemical properties of bioadhesives produced by marine biofouling bacterium, Vibrio alginolyticus. The EPS of Artic marine bacterium Polaribacter sp. SM1127 showed antioxidant activity, moisture-retention ability and protective property on human dermal fibroblasts (HDFs) at low temperature. EPS has also promoted the skin wound healing and prevented the frostbite injury in Rat Skin (Sun et al. 2020).
Heavy metal remediation using marine bacteria and their EPS
Biosorption is one of the mechanisms through which organisms remove or accumulate heavy metals. It is a rapid and passive process of metal uptake for which the cells need not be in a live state. Biosorption is a physicochemical process, which includes various mechanisms such as adsorption, absorption, intracellular or extracellular accumulation, redox reaction, ion exchange, surface complexation and precipitation (Gadd 2010). Agricultural waste such as rice straw, wheat straw, soya bean straw, coconut husks, waste tea, waste coffee powders, dried plant leaves, wool, cork biomass, and cottonseed hulls are used for metal removal. Sewage, sludge and microbial cells such as bacteria, fungi and algae have been also used for their metal- binding capacity under various conditions (Abbas et al. 2014). Microbial EPS have the ability to bind with anion and cations, resulting in a candidate of choice for the bioremediation process (Saikia et al. 2013). In some remediation processes EPS modified by chemical processes such as acetylation, methylation, phosphorylation, and sulfonylation are used (Desbrieres et al. 2018). Acetylation of EPS decides the selectivity of metal-binding (Sutherland 1983). The metal binding property of the EPS plays a significant role for metal remediation from the wastewater (Choi and Yun 2006).
The reports of Gupta and Diwan (2017) demonstrated almost 85–95% of zinc, copper and chromium removal using consortium developed from activated sludge. They also reported that many Gram-negative bacterial consortia could remove 75–78% of zinc, lead, chromium, nickel, copper, cadmium, and cobalt within two hours. Immobilized EPS of Chryseomonas and Paenibacillus polymyxa showed the removal of cadmium, cobalt, copper, and lead (Ozdemir et al. 2005; Acosta et al. 2005). Dead cell-bound EPS of Bacillus cereus, Bacillus pumilus, Pentoea agglomerans showed 85.5–89% of chromium removal (Sultan et al. 2012). EPS of Acidithiobacillus ferrooxidans helps the organisms to bind with the mineral and thus extract metals from the sulphide ores (Yu et al. 2011). Salehizadeh and Shojaosadati (2003) reported the biosorption of copper (74.9%), lead (98.3%) and zinc (61.8%) by the EPS of Bacillus firmus. The EPS produced by Azotobacter chroococcum XU1 showed the sorption of lead (40.48%) and mercury (47.87%) (Rasulov et al. 2013). The EPS of Ensifer meliloti, showed 89, 85 and 66% of lead, nickel and zinc ion reduction respectively (Lakzian et al. 2008).
Various marine bacteria are also reported for their metal removal ability. The specific structure and high uronic acid content impart an enhanced anionic property to marine bacterial EPS which may be responsible for metal removal. EPS of Marinobacter sp. showed sorption of metals like lead and copper (Bhaskar and Bhosle 2006). EPS from marine Enterobacter cloacae demonstrated the sorption of cadmium (65%), copper (20%) and hexavalent chromium (75%) (Iyer et al. 2004, 2005). Halomonas sp. associated with marine micro-alga was also reported to chelate metals such as calcium, aluminium, iron, and magnesium (Gutierrez et al. 2012). The EPS secreted by the Pseudoalteromonas sp. SM9913 showed the adsorption of Fe2+ (85.00 %), Zn2+ (58.15 %), Cu2+ (52.77 %), Co2+ (48.88 %), Mg2+ (30.69 %), Mn2+ (25.67 %) and Cr6+ (5.15 %) (Qin et al. 2007). Details regarding the EPS producing organisms and their metal removal efficiency are enlisted in Table 4.
Future prospects
The marine biopolymers contribute only a small portion to the current polymer market. Mainly the high production costs of the EPS affect the profit margin at market level. The high production costs are mainly due to the use of expensive and specific nutrients in the preparation of fermentation media; this generally contributes about 30% of the cost for the fermentation process. To make the processes cost effective, cheaper alternative substrates such as cane molasses, sugarcane bagasse, corn steep liquor, fruit peels, potato peels etc. should be used for the large scale production. Some biopolymers like xanthan, curdlan, dextran, gellan have been produced by solid state fermentation using raw substrates like spent malt grains, vegetable and fruit wastes, citrus peels, olive mill waste water etc. But it requires lots of efforts to scale-up the process from lab level to industrial level for the production of a commercial product using cheaper or solid substrates (Poli et al. 2011; Casillo et al. 2018).
Although marine microbial EPS have been studied in recent times for their various industrial applications, there have been only a few reports highlighting their production and recovery. More detailed research in this field is needed to understand the properties of EPS in depth. To achieve the higher EPS yields, the marine bacterial strains can be improved using genetic engineering (use of mutagenic strains, gene manipulations) and also EPS having specific properties and structures can be produced using the same.
The present methods that are used for structural determination of EPS are labor-intensive and tedious. So suitable modifications can be incorporated in the existing protocols or novel strategies can be developed to make the process simpler. Moreover, the EPS extraction methods can be suitably modified in a cost-effective manner which would significantly lower down the overall cost of the downstream processes.
Conclusions
The review intends to provide information on EPS producing marine bacteria, their unique properties, purification methods and applications in various fields. This also provided an insight of novel marine biopolymers of applied interest. There are several methods for recovery, extraction and purification of EPS, but they need to be considered critically depending upon the source of production and biochemical nature of the EPS. All the purification and recovery methods are having one or the other limitation and no universal extraction method is available due to wide variety of EPS specially from marine bacteria. Thus for the potential biotechnological and industrial applications of these polymers, further developments in the methods used for their recovery and purification are needed. Marine bacterial EPS can be a good source for metal remediation, MEOR as well as in the field of medicine thus can play role in maintaining environmental sustainability.
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3(4):74–102
Abreu NA, Taga ME (2016) Decoding molecular interactions in microbial communities. FEMS Microbiol Rev 40:648–663
Acosta MP, Valdman E, Leite SG, Battaglini F, Ruzal SM (2005) Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J Microbiol Biotechnol 21(6–7):1157–1163
Ahmed Z, Wang Y, Anjum N, Ahmad A, Khan ST (2013) Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir—Part II. Food Hydrocoll 30(1):343–350
Arena A (2004) Exopolysaccharides from marine thermophilic bacilli induce a Th1 cytokine profile in human PBMC. Clin Microbiol Infect 10:366
Arena A, Gugliandolo C, Stassi G, Pavone B, Iannello D, Bisignano G, Maugeri TL (2009) An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: antiviral activity on immunocompetent cells. Immunol Lett 123(2):132–137
Arias S, Del Moral A, Ferrer MR, Tallon R, Quesada E, Bejar V (2003) Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 7(4):319–326
Asker MS, El Sayed OH, Mahmoud MG, Yahya SM, Mohamed SS, Selim MS, El Awady MS, Abdelnasser SM, Elsoud MM (2018) Production of exopolysaccharides from novel marine bacteria and anticancer activity against hepatocellular carcinoma cells (HepG2). Bull Natl Res Cent 42(1):30
Ates O (2015) Systems biology of microbial exopolysaccharides production. Front Bioeng Biotechnol 3:200. https://doi.org/10.3389/fbioe.2015.00200
Aullybux AA, Puchooa D, Bahorun T, Jeewon R (2019) Phylogenetics and antibacterial properties of exopolysaccharides from marine bacteria isolated from Mauritius seawater. Ann Microbiol 69:957–972. doi:https://doi.org/10.1007/s13213-019-01487-2
Bacosa HP, Kamalanathan M, Chiu MH, Tsai SM, Sun L, Labonté JM, Schwehr KA, Hala D, Santschi PH, Chin WC, Quigg A (2018) Extracellular polymeric substances (EPS) producing and oil degrading bacteria isolated from the northern Gulf of Mexico. PloS One 13(12):e0208406
Bajaj IB, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45(4):341
Béjar V, Llamas I, Calvo C, Quesada E (1998) Characterization of exopolysaccharides produced by 19 halophilic strains of the species Halomonas eurihalina. J Biotechnol 61(2):135–141
Bhaskar PV, Bhosle NB (2006) Bacterial extracellular polymeric substance (EPS): a carrier of heavy metals in the marine food-chain. Environ Int 32(2):191–198
Boels IC, van Kranenburg R, Hugenholtz J, Kleerebezem M, de Vos WM (2001) Sugar catabolism and its impact on the biosynthesis and engineering of exopolysaccharide production in lactic acid bacteria. Int Dairy J 11(9):723–732
Bouchotroch S, Quesada E, Izquierdo I, Rodriguez M, Béjar V (2000) Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J Ind Microbiol Biotechnol 24(6):374–378
Bouchotroch S, Quesada E, del Moral A, Llamas I, Béjar V (2001) Halomonas maura sp. nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol 51(5):1625–1632
Bozzi L, Milas M, Rinaudo M (1996) Characterization and solution properties of a new exopolysaccharide excreted by the bacterium Alteromonas sp. strain 1644. Int J Biol Macromol 18:9–17
Bramhachari PV, Dubey SK (2006) Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett Appl Microb 43(5):571–577
Bramhachari PV, Kavi Kishor PB, Ramadevi R, Kumar R, Rao BR, Dubeyj SK (2007) Isolation and characterization of mucous exopolysaccharide (EPS) produced by Vibrio furnissii strain VB0S3. J Microbiol Biotechnol 17:44
Carillo S, Casillo A, Pieretti G, Parrilli E, Sannino F, Bayer-Giraldi M, Cosconati S, Novellino E, Ewert M, Deming JW, Lanzetta R (2015) A unique capsular polysaccharide structure from the psychrophilic marine bacterium Colwellia psychrerythraea 34H that mimics antifreeze (glyco) proteins. J Am Chem Soc 137(1):179–189
Caruso C, Rizzo C, Mangano S, Poli A, Di Donato P, Nicolaus B, Di Marco G, Michaud L, Giudice AL (2018) Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ Sci Pollut Res 25(5):4667–4677. doi:https://doi.org/10.1007/s11356-017-0851-z
Caruso C, Rizzo C, Mangano S, Poli A, Di Donato P, Nicolaus B, Finore I, Di Marco G, Michaud L, Giudice AL (2019) Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct Sci 31(2):69–79
Casillo A, Lanzetta R, Parrilli M, Corsaro M (2018) Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Mar Drugs 16(2):69
Castillo NA, Valdez AL, Fariña JI (2015) Microbial production of scleroglucan and downstream processing. Front Microbiol 6:1106
Chi Z, Fang Y (2005) Exopolysaccharides from marine bacteria. J Ocean U China (English Edition) 4(1):67–74
Choi SB, Yun YS (2006) Biosorption of cadmium by various types of dried sludge: an equilibrium study and investigation of mechanisms. J Hazard Mater 138(2):378–383
Chowdhury SR, Manna S, Saha P, Basak RK, Sen R, Roy D, Adhikari B (2011) Composition analysis and material characterization of an emulsifying extracellular polysaccharide (EPS) produced by Bacillus megaterium RB-05: a hydrodynamic sediment‐attached isolate of freshwater origin. J Appl Microbiol 111(6):1381–1393
Cuthbertson L, Kos V, Whitfield C (2010) ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev 74(3):341–362. https://doi.org/10.1128/MMBR.00009-10
D’Abzac P, Bordas F, Van Hullebusch E, Lens PN, Guibaud G (2010) Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: comparison of chemical and physical extraction protocols. Appl Microbiol Biotechnol 85(5):1589–1599
Dave SR, Vaishnav AM, Upadhyay KH, Tipre DR (2016) Microbial exopolysaccharide—an inevitable product for living beings and environment. J Bacteriol Mycol 2(4):00034
de Morais MG, Stillings C, Dersch R, Rudisile M, Pranke P, Costa JAV, Wendorff J (2010) Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour Technol 101(8):2872–2876
De Vuyst L, De Vin F (2007) Exopolysaccharides from lactic acid bacteria. In: Kamerling JP (ed) Comprehensive glycoscience: from chemistry to systems biology. Elsevier, Oxford, pp 477–519
Desbrieres J, Peptu CA, Savin CL, Popa M (2018) Chemically modified polysaccharides with applications in nanomedicine. In: Popa V, Wolf I (ed) Biomass as renewable raw material to obtain bioproducts of high-tech value. Elseveir, pp 351–399
Deschatre M, Ghillebaert F, Guezennec J, Simon-Colin C (2015) Study of biosorption of copper and silver by marine bacterial exopolysaccharides. WIT Trans Ecol Environ 196:549–559
Dignac MF, Urbain V, Rybacki D, Bruchet A, Snidaro D, Scribe P (1998) Chemical description of extracellular polymers: implication on activated sludge floc structure. Water Sci Technol 38(8–9):45–53
Dong S, Yang J, Zhang XY, Shi M, Song XY, Chen XL, Zhang YZ (2012) Cultivable alginate lyase-excreting bacteria associated with the arctic brown alga Laminaria. Mar Drugs 10:2481–2491
Donnarumma G, Molinaro A, Cimini D, De Castro C, Valli V, De Gregorio V, De Rosa M, Schiraldi C (2014) Lactobacillus crispatus L1: high cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol 14(1):137
Enikeev R (2012) Development of a new method for determination of exopolysaccharide quantity in fermented milk products and its application in technology of kefir production. Food Chem 134(4):2437–2441
Fallourd MJ, Viscione L (2009) Ingredient selection for stabilisation and texture optimisation of functional beverages and the inclusion of dietary fibre. In: Paquin P (ed) Functional and speciality beverage technology. Woodhead Publishing Limited and CRC Press LLC, pp 3–38
Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K, Sá-Correia I (2008) Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol 79(6):889
Fontana C, Li S, Yang Z, Widmalm G (2015) Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr Res 402:87–94
Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29(8):388–398
Frølund B, Palmgren R, Keiding K, Nielsen PH (1996) Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res 30(8):1749–1758
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiol 156(3):609–643
Galindo E, Albiter V (1996) High-yield recovery of xanthan by precipitation with isopropyl alcohol in a stirred tank. Biotechnol Prog 12:540–547
Garcia-Ochoa F, Casas JA, Mohedano AF (1993) Precipitation of xanthan gum. Sep Sci Technol 28:1303ą13
Górska-Frączek S, Sandström C, Kenne L, Paściak M, Brzozowska E, Strus M, Heczko P, Gamian A (2013) The structure and immunoreactivity of exopolysaccharide isolated from Lactobacillus johnsonii strain 151. Carbohydr Res 378:148–153
Gupta P, Diwan B (2017) Bacterial Exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71
Gutierrez T, Shimmield T, Haidon C, Black K, Green DH (2008) Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl Environ Microbiol 74(15):4867–4876
Gutierrez T, Biller DV, Shimmield T, Green DH (2012) Metal binding properties of the EPS produced by Halomonas sp. TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals 25(6):1185–1194
Islam ST, Lam JS (2014) Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can J Microbiol 60(11):697–716
Ismail B, Nampoothiri KM (2014) Molecular characterization of an exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510 and its efficacy to improve the texture of starchy food. J Food Sci Technol 51(12):4012–4018
Iyer A, Mody K, Jha B (2004) Accumulation of hexavalent chromium by an exopolysaccharide producing marine Enterobacter cloaceae. Mar Pollut Bull 49(11–12):974–977
Iyer A, Mody K, Jha B (2005) Biosorption of heavy metals by a marine bacterium. Mar Pollut Bull 50(3):340–343
Janczarek M (2015) Exopolysaccharide production in rhizobia is regulated by environmental factors. In: de Bruijn FJ (ed) Biological nitrogen fixation. Willey, New York, pp 365–380
Jekins RO, Hall JF (1997) Production and applications of microbial exopolysaccharides. In: Currel B, Mieras Van Dem RC (eds) Biotechnological innovations in chemical synthesis. Butterworth, Heinemann, Oxford, pp 193–230
Jiao Y, Cody GD, Harding AK, Wilmes P, Schrenk M, Wheeler KE, Banfield JF, Thelen MP (2010) Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol 76(9):2916–2922
Kambourova M, Oner ET, Poli A (2015) Exopolysaccharides from prokaryotic microorganisms—promising sources for white biotechnology processes. In: Pandey A, Höfer R, Taherzadeh M, Madhavan Nampoothiri K, Larroche C (eds) Industrial biorefineries and white biotechnology. Elsevier, pp 523–554
Kennedy JF, Bradshaw IJ (1984) Production, properties and applications of xanthan. Prog Ind Microbiol 19:319–371
Kim IH, Choi JH, Joo JO, Kim YK, Choi JW, Oh BK (2015) Development of a microbe-zeolite carrier for the effective elimination of heavy metals from seawater. J Microbiol Biotechnol 25(9):1542–1546
Kreyenschulte D, Krull R, Margaritis A (2014) Recent advances in microbial biopolymer production and purification. Crit Rev Biotechnol 34(1):1–5
Kumar CG, Joo HS, Choi JW, Koo YM, Chang CS (2004) Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Micro Tech 34(7):673–681
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides: A perception. J Basic Microbiol 47:103–117
Lakzian A, Berenji AR, Karimi E, Razavi S (2008) Adsorption capability of lead, nickel and zinc by exopolysaccharide and dried cell of Ensifer meliloti. Asian J Chem 20(8):6075
Laroche C, Michaud C (2010) Microbial polysaccharides. In: Larroche C, Pandey A, Dussap CG (eds) Comprehensive food fermentation and biotechnology, Chapter 8. Asiatech Publisher, Inc., New Delhi
Laurienzo P (2010) Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs 8(9):2435–2465
Le Costaouec T, Cérantola S, Ropartz D, Ratiskol J, Sinquin C, Colliec-Jouault S, Boisset C (2012) Structural data on a bacterial exopolysaccharide produced by a deep-sea Alteromonas macleodii strain. Carbohyd Polym 90(1):49–59
Lee HK, Chun J, Moon EY, Ko SH, Lee DS, Lee HS, Bae KS (2001) Hahella chejuensis gen. nov., sp. nov., an extracellular-polysaccharide-producing marine bacterium. Int J Syst Evol Microbiol 51(2):661–666
Lelchat F, Cérantola S, Brandily C, Colliec-Jouault S, Baudoux AC, Ojima T, Boisset C (2015) The marine bacteria Cobetia marina DSMZ 4741 synthesizes an unexpected K-antigen-like exopolysaccharide. Carbohydr Polym 124:347–356
Leroy F, De Vuyst L (2016) Advances in production and simplified methods for recovery and quantification of exopolysaccharides for applications in food and health. J Dairy Sci 99(4):3229–3238
Li WW, Zhou WZ, Zhang YZ, Wang J, Zhu XB (2008) Flocculation behavior and mechanism of an exopolysaccharide from the deep-sea psychrophilic bacterium Pseudoalteromonas sp. SM9913. Bioresour Technol 99(15):6893–6899
Liang TW, Wang SL (2015) Recent advances in exopolysaccharides from Paenibacillus spp: production, isolation, structure, and bioactivities. Mar drugs 13(4):1847–1863
Lim DJ, Kim JD, Kim MY, Yoo SH, Kong JY (2007) Physicochemical properties of the exopolysaccharides produced by marine bacterium Zoogloea sp. KCCM10036. J Microbiol Biotechnol 17(6):979–984
Llamas I, Mata JA, Tallon R, Bressollier P, Urdaci MC, Quesada E, Béjar V (2010) Characterization of the exopolysaccharide produced by Salipiger mucosus A3T, a halophilic species belonging to the Alphaproteobacteria, isolated on the Spanish mediterranean seaboard. Mar drugs 8(8):2240–51
Lo YM, Yang ST, Min DB (1997) Ultrafiltration broth: process and economic analyses. J Food Eng 31:219–236
Loaec M, Olier R, Guezennec J (1998) Chelating properties of bacterial exopolysaccharides from deep-sea hydrothermal vents. Carbohydr Polym 35(1–2):65–70
Lorenz MG, Aardema BW, Wackernagel W (1988) Highly efficient genetic transformation of Bacillus subtilis attached to sand grains. Microbiol 134(1):107–112
Marcial GJ. Messing B, Menchicchi FM, Goycoolea G, Faller G, Font de Valdez G, Hensel A (2013) Effects of polysaccharide isolated from Streptococcus thermophilus CRL1190 on human gastric epithelial cells. Int J Biol Macromol 62:217–224
Margaritis A, Pace GW (1985) Microbial polysaccharides. In: Blanch HW, Drew S, Wang DI (eds) Comprehensive biotechnology. Pergamon Press, Oxford, pp 1005–1044
Martínez-Cánovas MJ, Quesada E, Llamas I, Bejar V (2004) Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol 54(3):733–737
Marx JG, Carpenter SD, Deming JW (2009) Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can J Microbiol 55:63–72
Mata JA, Béjar V, Llamas I, Arias S, Bressollier P, Tallon R, Urdaci MC, Quesada E (2006) Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Res Microbiol 157(9):827–835
Mata JA, Béjar V, Bressollier P, Tallon R, Urdaci MC, Quesada E, Llamas I (2008) Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family Alteromonadaceae. J Appl Microbiol 105(2):521–528
Matsuda M, Yamori T, Naitoh M, Okutani K (2003) Structural revision of sulfated polysaccharide B-1 isolated from a marine Pseudomonas species and its cytotoxic activity against human cancer cell lines. Mar Biotechnol (NY) 5:13–19
Mende S, Peter M, Bartels K, Rohm H, Jaros D (2013) Addition of purified exopolysaccharide isolates from S. thermophilus to milk and their impact on the rheology of acid gels. Food Hydrocoll 32(1):178–185
Mohamed SS, Amer SK, Selim MS, Rifaat HM (2018) Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egypt J Basic Appl Sci 5(3):204–209
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb (II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manag 247:121–134
Morin A (1998) Screening of polysaccharide-producing microorganisms, factors influencing the production and recovery of microbial polysaccharides. In: Dumitriu S (ed) Polysaccharides---structural diversity and functional versatility. Marcel Dekker Inc. Publication, New York, pp 275–296
Muralidharan J, Jayachandran S (2003) Physicochemical analyses of the exopolysaccharides produced by a marine biofouling bacterium, Vibrio alginolyticus. Process Biochem 38(6):841–847
Neu TR, Poralla K (1990) Emulsifying agents from bacteria isolated during screening for cells with hydrophobic surfaces. Appl Microbiol Biotechnol 32:521–525
Notararigo S, Nácher-Vázquez M, Ibarburu I, Werning ML, de Palencia PF, Dueñas MT, Aznar R, López P, Prieto A (2013) Comparative analysis of production and purification of homo-and hetero-polysaccharides produced by lactic acid bacteria. Carbohydr Polym 93(1):57–64
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13(11):14002–14015
Onesti MG, Fioramonti P, Carella S, Fino P, Sorvillo V, Scuderi N (2013) A new association between hyaluronic acid and collagenase in wound repair: an open study. Eur Rev Med Pharmacol Sci 17(2):210–216
Otero A, Vincenzini M (2003) Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J Biotechnol 102(2):143–152
Ozdemir G, Ceyhan N, Manav E (2005) Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour Technol 96(15):1677–1682
Pace GW, Righeloto RC (1981) Production of extracellular microbial polysaccharides. Adv Biochem Eng 15:41–70
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15(1):86–102
Park JH, Ahn HJ, Kim SG, Chung CH (2013) Dextran-like exopolysaccharide-producing Leuconostoc and Weissella from Kimchi and its ingredients. Food Sci Biotechnol 22(4):1047–1053
Parkar D, Jadhav R, Pimpliskar M (2017) Marine bacterial extracellular polysaccharides: A review. J Coast Life Med 5(1):29–35
Patel AK, Michaud P, Singhania RR, Soccol CR, Pandey A (2010) Polysaccharides from probiotics: new developments as food additives. Food Technol Biotechnol 48(4):451–463
Poli A, Schiano Moriello V, Esposito E, Lama L, Gambacorta A, Nicolaus B (2004) Exopolysaccharide production by a new Halomonas strain CRSS isolated from saline lake Cape Russell in Antarctica growing on complex and defined media. Biotechnol Lett 26:1635–1638
Poli A, Anzelmo G, Nicolaus B (2010) Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar drugs 8(6):1779–1802
Poli A, Di Donato P, Abbamondi GR, Nicolaus B (2011) Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011:1–13
Prameela K, Mohan CM, Ramakrishna C (2018) Biopolymers for food design: consumer-friendly natural ingredients. In: Grumezescu AM, Holban AM (eds) Biopolymers for food design. Academic Press, UK pp 1–32
Qin G, Zhu L, Chen X, Wang PG, Zhang Y (2007) Structural characterization and ecological roles of a novel exopolysaccharide from the deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913. Microbiol 153:1566–1572
Quesada E, Béjar V, Calvo C (1993) Exopolysaccharide production by Volcaniella eurihalina. Experientia 49(12):1037–1041
Quintero EJ, Langille SE, Weiner RM (2001) The polar polysaccharide capsule of Hyphomonas adhaerens MHS-3 has a strong affinity for gold. J Ind Microbiol Biotechnol 27(1):1–4
Rasulov BA, Yili A, Aisa HA (2013) Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J Environ Prot 4(09):989
Rehm BH (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8(8):578–592
Rimada PS, Abraham AG (2003) Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Le Lait 83(1):79–87
Rodríguez-Tirado V, Green-Ruiz C, Gómez-Gil B (2012) Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: kinetic and equilibrium studies. Chem Eng J 181:352–359
Romano I, Poli A, Finore I, Huertas FJ, Gambacorta A, Pelliccione S, Nicolaus B (2007) Haloterrigena hispanica sp. nov., an extremely halophilic archaeon from Fuente de Piedra, southern Spain. Int J Syst Evol Microbiol 57(7):1499–1503
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12(2–3):163–171
Ruas-Madiedo P, de los Reyes-Gavilán CG (2005) Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Dairy Sci 88:843–856
Sahana TG, Rekha PD (2019) A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int J Biol Macromol 131:10–18
Saikia U, Bharanidharan R, Vendhan E, Yadav S, Siva Shankar S (2013) A brief review on the science, mechanism and environmental constraints of microbial enhanced oil recovery (MEOR). Int J Chem Technol Res 5(3):1205–1212
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37(17):4231–4235
Sara Pereira R, Mota C, Vieira J, Vieira P (2015) Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci Rep 5:14835
Saravanan P, Jayachandran S (2008) Preliminary characterization of exopolysaccharides produced by a marine biofilm-forming bacterium Pseudoalteromonas ruthenica (SBT 033). Lett Appl Microbiol 46(1):1–6
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:496
Shah V, Ray A, Garg N, Madamwar D (2000) Characterization of the extracellular polysaccharide produced by a marine cyanobacterium, Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr Microbiol 40(4):274–278
Shang N, Xu R, Li P (2013) Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr Polym 91(1):128–134
Shao LI, Wu Z, Zhang H, Chen W, Ai L, Guo B (2014) Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr Polym 107:51–56
Shukla PJ, Dave BP (2018) Screening and molecular identification of potential exopolysaccharides (EPS) producing marine bacteria from the Bhavnagar coast, Gujarat. Int J Pharm Sci Res 9(7):2973–2981
Smith IH, Pace GW (1982) Recovery of microbial polysaccharides. J Chem Technol Biotechnol 32(1):119–129
Stredansky M, Conti E, Bertocchi C, Navarini L, Matulova M, Zanetti F (1999) Fed-batch production and simple isolation of succinoglycan from Agrobacterium tumefaciens. Biotechnolo tech 13(1):7–10
Sultan S, Mubashar K, Faisal M (2012) Uptake of toxic Cr (VI) by biomass of exopolysaccharides producing bacterial strains. Afr J Microbiol Res 6(13):3329–3336
Sun W, Griffiths MW (2000) Survival of Bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan–xanthan beads. Int J Food Microbiol 61(1):17–25
Sun ML, Zhao F, Shi M, Zhang XY, Zhou BC, Zhang YZ, Chen XL (2015) Characterization and biotechnological potential analysis of a new exopolysaccharide from the Arctic marine bacterium Polaribacter sp. SM1127. Sci Rep UK 5:18435
Sun ML, Zhao F, Chen XL, Zhang XY, Zhang YZ, Song XY, Sun CY, Yang J (2020) Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Mar Drugs 18(1):48
Sutherland IW (1972) Bacterial exopolysaccharides. Adv Microb Physiol 8:143–213
Sutherland IW (1983) Extracellular polysaccharides. In: Rehm H, Reed G, Dellwag H (eds) Biotechnology: biomass, microorganisms for special applications, microbial products I, energy from renewable resources. Verlag Chemie Gmbh, Wienheim, pp 531–574
Sutherland IW (1985) Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol 39(1):243–270
Sutherland IW (1990) Biotechnology of Microbial exopolysaccharides. Cambridge University Press, Cambridge, pp 122–132
Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16(1):41–46
Sutherland IW (2001) Microbial polysaccharides from Gram-negative bacteria. Int Dairy J 11(9):663–674
Suzuki C, Kobayashi M, Kimoto-Nira H (2013) Novel exopolysaccharides produced by Lactococcus lactis subsp. lactis, and the diversity of epsE genes in the exopolysaccharide biosynthesis gene clusters. Biosci Biotechnol Biochem 77(10):2013–8
Tabibloghmany FS, Ehsandoost E (2014) An overview of healthy and functionality of exopolysaccharides produced by lactic acid bacteria in the dairy industry. Eur J Food Res Rev 4(2):63
Talmont F, Vincent P, Fontaine T, Guezennec J, Prieur D, Aymard P, Cuvelier G, Launay B, Fournet B (1991) Structural investigation of an acidic polysaccharide from a deep-sea hydrothermal vent marine bacterium. Food Hydrocoll 5(1–2):171–172
Tuinier R, Zoon P, Olieman C, Cohen-Stuart MA, Fleer GJ, de Kruif CG (1999) Isolation and physical characterization of an exocellular polysaccharide. Biopolymers 49:1–9
Upadhyay KH (2017) Isolation characterization and application of metal immobilizing marine bacteria (Ph.D. Thesis). Gujarat University, Gujarat, India
Upadhyay KH, Vaishnav AM, Tipre DR, Dave SR (2016) Diversity assessment and EPS production potential of cultivable bacteria from the samples of coastal site of Alang. J Microbiol Biotechnol Food Sci 6(1):661–666
Upadhyay KH, Vaishnav AM, Tipre DR, Patel BC, Dave SR (2017) Kinetics and mechanisms of mercury biosorption by an exopolysaccharide producing marine isolate Bacillus licheniformis. 3 Biotech 7(5):313
Urai M, Anzai H, Ogihara J, Iwabuchi N, Harayama S, Sunairi M, Nakajima M (2006) Structural analysis of an extracellular polysaccharide produced by Rhodococcus rhodochrous strain S-2. Carbohydr Res 341(6):766–775
Urai M, Anzai H, Ogihara J, Iwabuchi N, Harayama S, Sunairi M, Nakajima M (2007) Structural analysis of an acidic, fatty acid ester-bonded extracellular polysaccharide produced by a pristane-assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr Res 342:933–942
Vaishnav AM (2017) Bacterial exopolysaccharide production from fruits and potato waste (Ph.D. Thesis). Gujarat University, Gujarat, India
Vaishnav AM, Upadhyay KH, Tipre DR, Dave SR (2016) Characterization of potent exopolysaccharide producing bacteria isolated from fruit pulp and potato peels and enhancement in their exopolysaccharide production potential. J Microbiol Biotechnol Food Sci 6(3):874–877
van Eldik R, Hubbard CD (eds) (1996) Biomolecules under extreme conditions. In: Chemistry under extreme and non-classical conditions. Wiley, New York p 515–546
Verdugo P (2012) Marine microgels. Annu Rev Mar Sci 4:375–400
Vinogradov E, Nossova L, Korenevsky A, Beveridge TJ (2005) The structure of the capsular polysaccharide of Shewanella oneidensis strain MR-4. Carbohydr Res 340(10):1750–1753
Whitfield C (1988) Bacterial extracellular polysaccharides. Can J Microbiol 34(4):415–420
Willis LM, Whitfield C (2013) Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res 378:35–44
Wrangstadh M, Szewzyk U, Ostling J, Kjelleberg S (1990) Starvation-specific formation of a peripheral exopolysaccharide by a marine Pseudomonas sp., strain S9. Appl Environ Microbiol 56:2065–2072
Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K (2016). The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol 90(8):1817–1840
Wu S, Liu G, Jin W, Xiu P, Sun C (2016b) Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front Microb 7:102
Wuertz S, Müller E, Spaeth R, Pfleiderer P, Flemming HC (2000) Detection of heavy metals in bacterial biofilms and microbial flocs with the fluorescent complexing agent Newport Green. J Ind Microbiol Biotechnol 24(2):116–123
Yang ST, Lo YM, Chattopadhyay D (1998) Production of cell-free xanthan fermentation broth by cell adsorption on fibers. Biotechnol Progr 14(2):259–264
Yilmaz MT, Dertli E, Toker OS, Tatlisu NB, Sagdic O, Arici M (2015) Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: an optimization study based on fermentation kinetics. J Dairy Sci 98(3):1604–1624
Yu RL, Yang OU, Tan JX, Wu FD, Jing SU, Lei MI, Zhong DL (2011) Effect of EPS on adhesion of Acidithiobacillus ferrooxidans on chalcopyrite and pyrite mineral surfaces. Trans Nonferrous Metal Soc 21(2):407–412
Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X, Yang Z, Li S (2013) Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54:270–275
Zhang Z, Chen Y, Wang R, Cai R, Fu Y, Jiao N (2015) The fate of marine bacterial exopolysaccharide in natural marine microbial communities. Plos One 10:e0142690
Zhang Z, Cai R, Zhang W, Fu Y, Jiao N (2017) A novel exopolysaccharide with metal adsorption capacity produced by a marine bacterium Alteromonas sp. JL2810. Mar drugs 15(6):175
Acknowledgements
We are thankful to the Department of Science and Technology (DST), New Delhi, India for providing the INSPIRE Fellowship to Kinjal H. Upadhyay and University Grants Commission (UGC), New Delhi for Emeritus Professor fellowship award to Prof. Shailesh R. Dave.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We all the authors have no conflict of interest for publishing this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dave, S.R., Upadhyay, K.H., Vaishnav, A.M. et al. Exopolysaccharides from marine bacteria: production, recovery and applications. Environmental Sustainability 3, 139–154 (2020). https://doi.org/10.1007/s42398-020-00101-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-020-00101-5