Abstract
Fluoride has a significant impact on our surroundings. Various treatment methodologies are available to reduce the fluoride level in groundwater and surface water. Water containing fluoride is most likely to affect humans, plants, and animals in different ways, based on the allowable limits set by the countries. This way, an attempt has been made to review the different parameters related to fluoride occurrence, effect, and treatment. The aim of this work is to review the literature about the background of fluoride availability, the source of fluoride, highlight the potential effect of fluoride on the surrounding, and compile different treatment techniques for fluoride removal. This review will be helpful to the research scholars who work in the field of water and wastewater treatment from groundwater and surface water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is among the primary elements required for the survival of all life forms; it is abundant in nature and makes up around three-fourths of the earth's surface [1, 2]. As reported by the UNEP, a third of the world's population or 2.4 billion people, still lack access to safe drinking water. Water quality is essential for home, agricultural, and industrial uses [3, 4]. In developing nations with arid climates like the South of Algeria, groundwater is the only available source [5]. The chemical substances of water are one of the most significant factors that affect its utility for different areas all over the world, so all water is not suitable for drinking, which contributes to the issue of freshwater scarcity [6]. Even if industrialization has contributed to a decline in the quality of natural water, the majority of chemicals substance are of natural origin [7]. Due to complicated interactions between precipitants, soil, and subterranean geological strata, these chemical compounds end up in the water supply. As a result, the organic and inorganic components of the natural waterways are both natural and manmade [8]. The nature and concentrations of the compounds dissolved in water determine the chemical properties of water. Drinking water typically has a dissolved solids concentration of 50 to 500 mg/L, but arid locations can have a concentration of up to 1500 mg/L [9,10,11]. While some of the dissolved organic elements have been found to be carcinogenic, it happens frequently that two or more dissolved components mix to generate a compound with properties that are more harmful than the original component. Bicarbonates, sulfates, chlorides, magnesium nitrates, and calcium nitrate are the most common dissolved substances in drinking water, although sodium potassium concentrations are generally lower. Inorganic elements found in drinking water include iron, aluminum, and manganese, there are also many other trace metals and metalloids, some of which have toxicity-based restrictions [12].
Pollution levels can be found almost everywhere, and uncontaminated water sources are the exception rather than the standard. Early drinking water quality standards focused nearly entirely on bacteriological elements of water quality, with the bulk of chemical components primarily mentioned concerning aesthetic properties. However, in the last years, scientists have paid much attention to hazardous inorganic compounds like fluoride, cadmium, and arsenic etc. In addition, limits have been set for each of these substances to evaluate if the water is safe to drink.
The existence of fluoride in above permissible limit amounts is a serious issue to human as well as animal health through the food chain. Like other contaminants, fluoride pollution occur naturally or due to human activities like industry process, such as aluminum and iron smelting, electronics and steel manufacturing, fertilizer industry etc. [13, 14]. Numerous minerals, such as fluorspar in sedimentary rocks and cryolite in igneous rocks contain fluoride, which can leach out by rainwater to pollute surface and groundwater. Depending on the concentration and total amount consumed, fluoride can be either healthy or unhealthy for humans, plants as well as for animals. The World Health Organization (WHO) recommends the maximum acceptable concentration of fluoride present in drinking water between 0.5 and 1.5 mg/L. When the fluoride content is less than 0.5 mg/L, the relative risk of dental and skeletal disease rises. Long-term intake of high fluoride water over than 1.5 mg/L can result in the thyroid, kidney, joint stiffness, paralysis, dental and skeletal fluorosis [15, 16]. Fluoride level between 1.0 and 1.5 mg/L in water is advantageous, especially for young children, as they aid in the calcification of dental enamel. Fluoride level in drinking water that exceeds WHO recommendations is an issue in more than 20 nations worldwide, particularly in China, India, Pakistan, South Africa, Türkiye, Argentina, Mexico, and the Rift Valley countries in Africa [17]. Fluorosis, which is caused by long-term consumption of drinking water with high fluoride levels, affects millions of peoples globally in different ways.
Fluoride is often removed from water supplies through chemical precipitation, adsorption, ion exchange, coagulation, filtration, and other treatment methods. Even though they are both economically and practically feasible, the facilities are typically not present in small towns with staggered habitats. A low cost, simply designed, abundantly available materials, urgently are needed for small and dispersed habitats like in the Malwa Belt of Punjab, India [18]. In this regard, the process of adsorption using ceramic clay based is inexpensive, ecofriendly and widely accessible materials everywhere would be more acceptable, especially for small and rural areas [19]. Therefore, it is a relevant issue globally to review the different parameters related to fluoride occurrence, effect, and treatment. Recent literature reviews focused on the fluoride occurrence, the effect of fluoride on humans, and defluoridation methods [20,21,22], but there is limited work discussed on the effect of fluoride on the surrounding such as plants, animals, and soil.
The aim of this paper is to review the literatures about the background of fluoride availability, the source of fluoride, highlight the potential effect of fluoride on the surrounding, and compiling different treatment techniques for fluoride removal which are available today. This unit is the role of contributing to the development and advancement of engineering technology by applying current and appropriate approaches, outperforming smaller attempts for practical usage.
2 Source of Fluoride
Fluoride is the fluorine anion with the chemical formula (F⁃), fluorine element is widely dispersed throughout different geological environments, it is the 13th most prevalent element and constitutes between 0.06 and 0.09% by weight of the earth's crust [23]. Since fluorine is a strong oxidant, it occurs naturally as the fluoride ion (F⁃) which is reactive in the environment and found naturally through the groundwater and surface water [24,25,26,27].
2.1 Groundwater
All subterranean water found in saturated and unsaturated zones is referred to as groundwater (GW). The surface, rivers, and streams provide the water, and precipitation and snowfall replenish it. It is located at the water table, which is the depth below the surface [28]. The water table can be found between one meter and several hundred meters below the surface. GW infiltrate between the pore spaces of rocks and soil, cracks, and in a different geological formation. The hydraulic properties, the quantity as well as the shape of void space affect how GW infiltrates inside the rocks and soil. Although water can easily pass through some rocks and into the subterranean aquifer system, it usually seeps through fissures, cracks, and other rock bodies [29]. Aquifers, aquitards, and aquicludes are the three main categories of geological formation GW that in general govern the existence of GW resources [28]. An aquifer is a highly porous saturated layer of sand, gravel, conglomerate, or bedrock, which serves as a substantial GW resource, since it not only retains water, but which also discharges adequate amounts of it. The Aquitard is a partially saturated layer (clay) that allows water to flow through but does not supply enough readily available water when compared to the aquifer [30]. The aquiclude is an impermeable stratum, that generates a big amount of water due to its high porosity, but it does not give a considerable quantity of water. To reach surface water at low elevations, GW traverses flow routes with varied lengths from recharge zones to discharge zones. The process of reaching groundwater that may be hundreds of meters below the surface may interest someone. Naturally, water is driven to the surface in the form of a spring or by discharging into rivers or lakes. Nevertheless, usually sometimes, abstraction is the process of bringing water to the surface that requires drilling wells or boreholes. A borehole or underground pipe that extends until it hits an aquifer is called a well. In unconfined aquifers, a pump is necessary to elevate the water through a borehole and well. In artesian limited aquifers, the water is under pressure, which causes it to rise to the surface naturally.
GW pollution can occur naturally or as a result of human activity such as waste dumping, industry, and farming [20]. The three categories of characteristics of groundwater are physical, chemical, and biological [7]. Physical characteristics in water are determined by suspended solids, odor, color, flavor, taste, turbidity, and temperature. Chemical characteristics are both organic and inorganic; the properties of groundwater involved by assessing chemical oxygen demand (COD), acidity, pH, carbon dioxide, alkalinity, sulphur dioxide, sodium, calcium, magnesium, potassium, chloride, fluoride, bicarbonate, sulfate etc. Biological characteristics, various changes observed in water due to organisms, bacteria, coliform, virus, protozoa etc. High fluoride groundwater (GW) is usually caused by the dissolution of the fluorine compound. The groundwater contamination due to fluorite minerals dissolving are mentioned below [31]:
The F− precipitates as CaF2 since the concentration of Ca2+ is high.
Equations (1–4) depict geochemical mechanisms that precipitate Ca2+ while releasing Na+ and F− into the groundwater.
According to the study of fluoride sources, the occurrence of fluoride is due to anthropogenic activities and natural factors as depicted in (Fig. 1) [32]. Natural causes includes fluorine compounds combined with rock minerals such as quartz, granites, felsic, and alkaline volcanic have a big amount of fluoride [33]. Fluoride can enter surface water, permeate into the soil, and lastly infiltrate underground water through long term weathering, washing, and dissolving of fluorine compound minerals by rainwater. This causes a progressive increase in the fluoride level in groundwater [22, 32, 34]. Furthermore, the enrichment of fluoride is further increased by the ongoing evapotranspiration of GW, which causes high fluoride water to occur in the region. Anthropogenic activities include the mining of fluorine compound ores using outdated techniques and the discharge of high fluoride waste residue and wastewater from a variety of industries, which include metallurgical processes, glass and coke fabrication, semiconductor fabrication, manufacturing of phosphate fertilizer, and the photovoltaic industry. Fluoride is then leached and washed into GW by rainwater or surface water.
2.2 Surface Water
All water bodies found on the surface of the Earth, from tiny ponds to enormous oceans, refer to surface water (SW). It comprises both saltwater water bodies, mostly oceans and freshwater bodies like lakes, ponds, rivers, etc. Rainfall is the principal source of SW. Surface water resources are mostly dependent on local rainfall, and they may be lost by infiltration via streambeds, layers of moist soil, and fissures or fractures that interact with the GW sources and the underflow zone as an area where the two systems combine [35]. Surface water and groundwater interact in a variety of ways. If surface water goes toward the groundwater, it is named a losing stream, whereas water moving the other way is known as a gaining stream [36].
Surface water is often heavily contaminated with physical, chemical, and biological pollutants [37]. Surface water pollution can result from various physical, chemical, and biological pollutants as discussed. The fluorine component discharged by human activities like phosphate fertilizers can reach surface water by deposition and rainfall [38, 39]. Fluoride concentrations in most rivers are normally within the permitted level of 1.5 mg/L. Usually, the dissolution of fluorine compounds results in the formation of fluoride in surface water [40].
2.3 Fluoride Level in Various Locations Globally
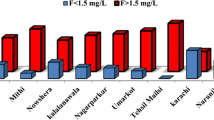
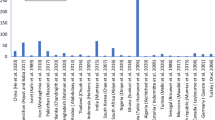
The occurrence of fluoride has been reported in numerous surface water and groundwater around the World. The countries such as India, Pakistan, Afghanistan, Brazil, South Africa and Malawi, fluoride concentration in groundwater is remarkably high as shown in (Table 1 a and b) [40]. The high concentration of fluoride in surface water occurs in Ethiopia and Pakistan [41, 42].
3 Effect of Fluoride
3.1 Effect on Human Health
Fluoride plays a significant role in the body construction of a human being by increasing the hardness of teeth and bones, the acceptable limit in drinking water is 1–1.5 mg/L as per WHO. If beyond the level consumed it may cause dental and skeletal fluorosis [22]. Globally an estimated 200 million people from 35 different countries suffer the dangerous effect of fluoride concentrations beyond the recommended limits in groundwater [68], among those countries India and China are the most affected by fluorosis [69]. The intake of fluoride contaminated water is a major health issue in 62 million Indians, including 6 million children [70]. In East African countries, 80 million people are facing the issue of fluorosis due to excessive drinking water with fluoride level higher than the permissible limit [71]. The severity increases as the fluoride level in the water rise as shown in (Table 2) [72, 73], and the occurrence varies from place to place [74, 75].
Dental fluorosis is an enamel developing abnormality brought on by repeatedly consuming water containing high levels of fluoride above the permissible limit. The danger of fluoride overexposure leading to dental fluorosis is very high in children of young ages while teeth and bone maturation are taking place because it happens most actively during tooth development. Dental fluorosis in its milder variants first appears unremarkable, with hardly perceptible white streaks on tooth enamel. However, the brown color spoils the aesthetic of teeth as it worsens. Teeth get tougher to clean when the tooth enamel gets more abrasive. Permanent fluorosis pits and spots on teeth tend to grow darker and nastier with time. Too much fluoride in bones causes skeletal fluorosis. Fluoride concentration in the bone rises because of structural changes that weaken the bone. This disease can present with torn ligaments, instability, muscle wasting, and neurological issues linked to spinal compression. Fluorosis happens when ionic compounds of hydroxyapatite Ca5 (PO4)3OH, the main component of human bones is replaced by fluoride ion to form fluorapatite Ca5 (PO4)3F [22].
Other effects of high concentrations of fluoride on human health are non-skeletal fluorosis due to the interaction of fluoride with other organs and soft tissue. The linked disorders due to high concentrations of fluoride are serious issue to human health such as anemia, hypertension, thyroid gland anomalies, and myosin filament leading to the weakness of the muscles [76]. In addition, too much fluoride can affect brain tissue as Alzheimer’s disease [17], and infertility by reducing the level of testosterone [77]. A study has shown that excessive fluoride exposure during pregnancy and in youth hampered the development of a child's IQ. Additionally, children exposed to fluoride during pregnancy had poorer IQ scores compared to children who were not exposed to it during pregnancy; therefore, the decline in IQ scores at greater levels of fluoride in drinking water in childhood does not come as a surprise [78]. The poisonous fluoride effect caused by consuming water with high a concentration of fluoride have two effects, acute poisoning and chronic poisoning (Fig. 2) [27]. Acute poisoning effect generally occurs occasionally, for short time, and sometimes can cause death. The symptoms depend on the concentration of fluoride, time, and age of humans. Over 80% of cases of acute poisoning effect reported, were in children less than 6 years old [79]. Chronic poisoning of fluoride is more known than acute poisoning. Its effects depend on time, concentration, humility, and nutrition of individual.
3.2 Effect on Animals
High concentration of fluoride is not harm only to human health but also it is a threat to animal health. The symptoms of fluoride effect on animals and human health tend to be the same means if animals consume water or plants with high concentrations of fluoride, dental and skeletal fluorosis appear [80]. The appearance of the symptoms of fluoride toxicity depends on concentrations, time, age, and health status of animals. Many research studies showed that fluoride also could affect the reproduction, thyroid hormones, and growth of animals [81] as presented in (Table 3). A male rat was exposed to water containing different concentrations of fluoride 2,4 and 6 ppm, after six months the fertility was decreased by 44% and the sperm motility was reduced by 19.94%, 31.65%, 42.53% respectively [81]. A study was performed in Rajasthan, India where the concentration of fluoride in fodder and water was 401–875 mg/kg and 0.271–2.46 mg/L respectively due to fume and gas released from the phosphate fertilizer plant available in this location. In 25 buffalos and 141 cattle examined, 72% of buffalo and 46.8% of cattle resulted from chronic fluorosis [82]. In another study done on the effect of fluoride consumed by pigs [83], a fluorine supplement was prepared with different fluoride concentrations 0, 50, 100, and 150 mg/kg. It resulted that after 12 weeks, the 96 pigs by consuming foods with 100 and 150 mg/kg fluoride concentration had low growth capacity by 5.29% and 8.60% respectively. Additionally the study done Miao et al. [84] in China, Hy-Line Gray hens were exposed to food with different F concentrations at 0, 400, 800, and 1200 mg/kg. After 49 days, it resulted that the concentration of 800 and 1200 mg/kg reduced the laying rate of Hy-Line Gray hens by 33.49% and 57.95%, and it show a decrease in eggs weight by 5.70% and 6.19% respectively compared to the control groups.
3.3 Effect on Plants
Plants get fluoride contaminants through diverse ways like air, soil, and water. The appearance of the symptoms due to fluoride poisoning depends on concentration, exposure time, temperature, and the maturity of the plant [87]. Plants may accumulate fluoride via stomata or by roots through soil diffusion, transported to the transpiration organs of plants then kept in leaves [88, 89]. Fluoride has a negative effect on plant leaves in some instances due to its high solubility. It has the potential to disrupt photosynthesis and other vital plant functions [90]. The earliest sign of fluoride harm in plant leaves is marginal, tip necrosis, and chlorosis. Nevertheless, the same symptom may also occur during dryness or stress, which may be the same as fluoride harm. In many plants, fluoride affects growth, germination, yield, and photosynthesis by inhibiting the membranes and stromal enzymes involved in carbon dioxide fixing, resulting in decreased chlorophyll amounts [91]. The poisoning of fluoride decreases shoots and root length because of the unstable nutrients of the seeds. A study conducted Lima et al. [92] by exposing the beans to different fluoride concentrations of 20 mg/L and 30 mg/L for 7 days, proved that the bean seedlings tolerated fluoride concentrations up to 20 mg/L and were sensitive when exposed to the concentration of 30 mg/L. It reduced the germination index and the root length of beans. A laboratory experiment was conducted to study the effect of fluoride on phytotoxicity and germination of rice by applying NaF solution of different concentrations of fluoride 5 mg/L, 10 mg/L, and 20 mg/L, after 28 days the result showed a gradual reduction of germination with rising in concentration of fluoride. At 5 mg/L, the reduction was between 1–7%, at 10 mg/L the reduction was 6–14% and at 20 mg/L was 12–19%. Fluoride phytotoxicity was seen in the rice seedlings after 28 days of exposure to a 20 mg/L of fluoride solution as inter-vein chlorosis and leaf-margin necrosis [93]. A significant decrease in the growth of corn and soybean is observed while exposed to a concentration of fluoride over 2 ppm, contrary sorghum may be resistant to the such an amount [94]. In addition, the experimental study showed that the yield of soybean decreased by 30% at a fluoride concentrations of 375 mg/L and above [95]. The phytotoxicity level in onion shoots calculated while exposed to different F concentration in soil 0, 100, 200, 400, 600, and 800 mg/kg. At 55 mg/L concentration of fluoride in soil, resulting in a 50% yield decrease [96]. Moreover, the F toxicity occurs from 400 mg/kg concentration in soil. There is no permissible limit concentration of fluoride in plants established by any pollution board. Table 4 summarizes the effect of high fluoride concentration exposed to plants.
3.4 Effect on Soil
The fluoride in the soil is present in the parent rock and its mode of dispersion reflects the mechanism of soil formation [23]. There are four types of soil (sandy, silt, clay, and loamy) classified mostly by their structure, proportions, various organic, and mineral components [97]. Most fluorine compounds found in soil are associated with clay and other minerals [98]. The fluoride level is typically much higher on the top soil layer than on the deeper soil layer [99]. The lowest concentration of fluoride appears in sandy soil in mostly wet condition, whereas the highest fluoride concentration is found in weathered mafic rock soil and heavy clay soil [88]. The inorganic component (Al, Ca) of the soil and pH are principally important for fluoride accumulation in soil. In general, soil minerals absorb fluoride between pH 6 and pH 7, which is considered an acidic pH. Silt, clay, and loamy soils all contain more fluoride than sandy soil accomplishes [98, 100].
According to a study done Wang et al. [101], black soil has a better potential to absorb fluoride than other soils including red soil and dark brown soil. This is because black soil contains clay particles, high iron, alumina as well as calcium and magnesia content, which has a high adsorption capacity. The results indicate that the adsorption of fluoride in soils reduces from wet to dry regions and from acid to alkaline soils. In the region where there is no natural phosphate and fluoride disposal, the organic soils are typically low. The fluoride level in soil parent rock range between 180 and 1000 μg/g [102]. High concentrations of fluoride occur in the soil where there is the use of phosphate fertilizers, and different industries releasing fluoride such as coal burning, aluminum smelting, glass industry, and in the area of hazardous waste disposal [103,104,105].
4 De-Fluoridation Technology
De-fluoridation is the process of reducing the level of fluoride ions in drinking water to the permissible level. De-fluoridation can be carried out either at the source of groundwater (boreholes, well) or at the point of use (domestic level). The choice of a particular de-fluoridation technique is influenced by several criteria, particularly in developing nations. These include the cost, efficiency, and simplicity of the procedure, the accessibility of materials, and the acceptability of the flavor and odor of the treated water. The development of different treatment technology to remove fluoride from groundwater has been a focus of study over the past few decades. The de-fluoridation techniques were divided into three categories: physical, biological, and advanced methods.
4.1 Physical Treatment Method
4.1.1 Coagulation-Flocculation method
Coagulation and flocculation are two procedures that are utilized in combination to eliminate suspended solids and other contaminants during the treatment of groundwater, surface water, and wastewater [106]. These methods help to destabilize particles by adding coagulants and flocculants to improve particles aggregation [107]. Coagulants are chemical compounds applied to neutralize the surface charge of suspended particles, allowing them to develop into bigger particles known as flocs. That means the coagulation method is the process used in water treatment to eliminate suspended solids by adding soluble metal cations in water to accumulate particulates and cause the particle to stick together to form insoluble aggregates (micro flocs). Flocculation is the process that occurs after coagulation as shown in (Fig. 3). During a gradual and gentle mixing process, tiny particles called microflocs are made to clump together into larger suspended aggregates known as macroflocs. This is achieved by adding flocculants and other commercial polymers to increase the size of the microflocs. Once the macroflocs are formed, they can be removed from the mixture through sedimentation and filtration.
Then these aggregates form flocculate which has a high sorbent surface for fluoride ions. The most used coagulant in this method is alum (Al2(SO4)3.14H2O). During coagulation, adsorption and coprecipitation are the main processes for de-fluoridation using alum Eq. (6) and (7) [108]. This aluminum salt reacts with hydroxide ions and gives the formation of Al(OH)3 flocs as shown in Eq. (5) [109].
Adsorption of fluoride ion on Al (OH)3
Co-precipitation:
A well-known illustration of the coagulation method is the Nalgonda technique established by NEERI in India. The following chemicals must be added in the correct order for this method to work (aluminum salt, Ca(OH)2, Ca(ClO)2) then quick mixing followed by sedimentation, filtration, as well as disinfection [13, 110]. The quantity of fluoride or alkalinity in the water being treated determines how much these chemicals should be added. Aluminum sulfate (alum) and aluminum chloride are the two types of aluminum salts most frequently employed in this process. To prevent excess concentrations of these contaminants in water over the acceptable range, the salt is chosen according to the level of sulfate and chloride contents in the water being treated. The Nalgonda technology is exceedingly adaptable and has been used to successfully treat water at both the domestic and community levels in different countries like India, Ethiopia, Kenya, and Tanzania [111]. However, it has been demonstrated that this technique requires a high dose of alum, and has issues with disposing of huge amounts of sludge [112].
The most factors that affect this process are the chemical composition of groundwater like pH, the concentration of contaminants, coagulant characteristics, and flocculants characteristics (Fig. 4). Considering the relationship of these factors is essential for the proper utilization of coagulation and flocculation techniques, as well as in the selection of coagulants [113, 114]. The pH of the groundwater has a considerable impact on de-fluoridation. If the alkalinity of the groundwater is high, the same amount of coagulant doesn’t have a big effect on fluoride removal minimal. To accomplish the best elimination of fluoride and other contaminants in groundwater via coagulation, the coagulant dose must be increased significantly [115]. On the contrary, when the water has high alkalinity, it is very important to add strong acid in water to balance the pH. Furthermore, the pH of the ideal coagulation method effect on different target contaminants for the specific groundwater is different, since the pH influences the existing state of the coagulant's hydrolysis process [116].
Several investigations have been done on the use of various metal salts as coagulants to remove fluoride from water with a pH adjustment. As presented in (Table 5), the results of the experiments showed that aluminum cations were more effective than iron cations [117]. Fluoride levels decrease significantly as aluminum content increases. The initial pH of the solution has a significant impact on the removal efficiency. It was shown that the pH range for initiating operations should be between 6 and 7. Moreover, other experimental findings indicate the removal effectiveness of fluoride by ferric chloride, aluminum sulfate, titanium sulfate, and titanium dioxide as a function of final solution pH [118]. The pH has a substantial impact on fluoride elimination from water. For aluminum sulfate, the optimal pH for de-fluoridation was about 6.5 which is in the range of other reported aluminum salts coagulant that optimum pH is between 6 and 9 [6, 119]. It should be highlighted that at a pH range of 3–5 titanium dioxide removed more fluoride than the other coagulant. The main problem with the coagulation and flocculation process in fluoride removal is that some toxic polyvalent ions, like Al3+, can remain in the water and constitute a danger to public health [21].
4.1.2 Precipitation Method
The precipitation method is the technology used to remove the ionic toxic elements from water, it is the transformation of substances dissolved in water into insoluble solid particulates during the treatment of water by chemical reaction [128]. The precipitation technique involves adding the right chemicals, known as precipitants, to react with the fluoride molecule dissolved in water and then produce solid particles or precipitates, which are insoluble salts [129]. The precipitate that has formed is able to be eliminated from the water using filtering or sedimentation. Additionally, metallic cations and other anions including cyanide, phosphate, and organic molecules can be eliminated using the precipitation method [130]. The chemical compounds utilized most frequently in the precipitation process are calcium salts like CaCO3, Ca (OH), and CaCl2. These substances can be used independently or together with magnesium salts and a coagulant aid. The mechanism of fluoride precipitation in water is represented by the equations below [109]. The lime reacts with soluble calcium bicarbonate to precipitate insoluble calcium carbonate as shown in Eq. (8):
Moreover, in the water containing fluoride, a portion of lime precipitates and eliminates insoluble fluorite, as shown by Eq. (9):
The de-fluoridation by lime is enhanced in the existence of dissolved Mg+ and precipitate as magnesium hydroxide as shown by Eq. (10):
The efficiency of the precipitation method is reliant on different influences, such as the concentration of fluoride present in water, temperature, the condition of the reaction (pH), the utilized precipitant, and the availability of other compounds that can prevent or stop the precipitation process. The most commonly utilized method is the precipitation by pH also known as hydroxide precipitation, where there is the formation of fluoride precipitate using precipitants such as caustic soda or lime [131]. An experimental study to remove fluoride in water with a precipitation method using calcite, showed that the fluoride concentration decreased rapidly with the decrease of pH from 300 mg/L to 8 mg/L [132], while the remaining fluoride ion concentration remained relatively constant as the reaction time increased. Fluoride cannot be successfully eliminated by adding water immediately under neutral circumstances because calcite is not soluble in water. To dissolve calcite and create Ca2+, which might precipitate fluoride ions, a specific quantity of acid is required. Magnesium salts were used in the experiment carried out to study the influence of various factors on chemical precipitation in wastewater treatment. It resulted that the fluoride removal efficiency improved with increasing pH of water and Mg:F molar ratio [133]. The primary drawback of the precipitation technology in water treatment is that they frequently alter the pH, flavor, or odor of the treated water by adding chemicals, and occasionally the water becomes unsafe to drink [112]. Other disadvantages of this technology is that it requires expensive chemicals and the development of abundant amounts of sludge. This technology is mostly used as a pretreatment while removing fluoride in high concentration. Table 6 presents the efficiency of various precipitants used in defluoridation and the optimum pH.
4.2 Biological Methods
The biological treatment process (BTP) also called secondary treatment or conventional method is mostly used to remove a wide range of contaminants both organic contaminants and suspended solids from wastewater using microorganisms such as algae, fungi, protozoa, yeast, and bacteria under the aerobic and anaerobic conditions where the contaminants are oxidized or degraded and converted to energy, the new cell which gradually settle down as sludge and can be eliminated by sedimentation [141]. The biological treatment technique achieves a large amount reduction in BOD and COD content [142, 143]. The biological treatment procedure (BTP) is less complicated and more affordable than other physicochemical methods [144]. In the biological treatment methods two terms aerobic and anaerobic conditions are very important. Aerobic means the existence of oxygen and anaerobic means the lack of oxygen. Both terms have a significant effect on the kind of microorganisms which are implicated in the breakdown or degradation of organic contaminants in a particular wastewater sample as well as the operating circumstances of the bioreactor. Thus, in aerobic conditions, aerobes microorganism use free oxygen to capture or absorb the contaminants converting them to CO2, biomass, and water. Contrary to anaerobic conditions, anaerobe microorganisms use free oxygen or molecules to capture the contaminants and convert them to CO2, biomass, and methane gas. In the BTP, the most popular used are conventionally activated sludge, trickling filters, and oxidation ponds.
4.2.1 Activated Sludge
A combination of dense microbial pathogens floating in the wastewater in the presence of oxygen is known as the activated sludge process (ASP). Considering sufficient nutrients and oxygen, it is possible for bacteria to proliferate and respire at higher rates. This results in the oxidation of the available contaminants into byproducts such as carbon dioxide, nitrate, sulfate, and phosphate. This is considered to be the most traditional and oldest biological treatment technology used to remove impurities from groundwater; it has an advantage over alternative techniques due to its low cost and lack of secondary pollutants [145]. The ASP has a efficiency removal of over 85% [146]. After initial treatment, which involves removing suspended contaminants, wastewater is often processed in a biological treatment system based on the activated sludge process (Fig. 5), which includes an aeration tank where organic matter or BOD present in water are degraded or decomposed by microorganisms under aeration, and a secondary clarifier or secondary sedimentation tank where the biological cell mass is separated from the effluent of the aeration tank and the settled sludge is recycled partly to the aeration tank and the remaining is wasted. Even though activation sludge gives good quality effluent, it has some drawbacks such as high operation cost, needs of large space for sludge disposal, and skilled supervisor.
Sometimes, activated sludge can be used in combination with membrane filtration in the process called membrane bioreactor [147]. The membrane bioreactors (MBR) process is a water treatment method using biological treatment technology, especially ASP and membrane filtration together to enable enhanced organic matter and suspended particles in wastewater [148, 149]. Membrane bioreactors are among the significant technology to treat water over activated sludge due to their high efficiency and fewer drawbacks compared to activation sludge [150]. The benefit of this technology is to remove biochemical oxygen demand and chemical oxygen demand in water during water treatment. The study on this technology has reduced because of the challenge of membrane fouling which decreases its performance and lifetime, this causes an increase in the cost of this whole process. In order to more effectively accomplish environmental sustainability, several wastewater treatment facilities for example in China and Egypt have switched from the traditional activated sludge method to the membrane bioreactors (MBRs) [151,152,153], because of it need small footprint and excellent effluent quality than activated sludge process [154]. Contrary Iorhemen et al. [148] and Hao et al. [155] recommend that MBR can’t replace activated sludge because of its high consummation of energy and operating costs.
4.2.1.1 Factor Affecting the Activated Sludge
Numerous factors can affect the performance of conventional activated sludge, such as the pH [156], temperature [157], rate of aeration [141], sludge retention time [158], hydraulic retention time [159], organic loading rate (OLR) [158], COD/BOD ratios [160], a considerable variation in pH can affect the rate of synthesis of enzymes required for the efficient conversion of selected contaminants found in wastewater. In terms of temperature, the rate of development of microorganisms is often associated with the temperature in aeration tank. Moreover, rising temperatures are often beneficial to development [156, 157]. It was observed that reducing the sludge, increases the efficiency removal which read to an apparent higher sludge retention time while the concentration of total suspended solids in the tank is maintained constant [158]. Similarly, if the hydraulic retention time is decreased, the organic loading rate will increase, and the volume of the tank needed to achieve the target removal performance will decrease. Nonetheless, greater HRTs typically lead to more effective elimination [161].
4.2.2 Trickling Filter
A trickling filter, also called a percolator is a bed in the form of a cylindrical area composed of materials such as crushed stone, bottles or filters that have been carefully built to form a slime layer or biofilm (~ 0.1 to 0.2 mm). The microorganisms flourish in the layer that covers the area. They balance the contaminants in the wastewater by adsorbing them, and this produces H2O and CO2 through aerobic metabolism, and which results in the reduction of BOD. The effluent is diffused on the media’s surface, then it is further cleaned when it percolates downward and comes into touch with the microorganisms' slime layer [162]. Since it can only remove less than 70% of the organic material from wastewater, the trickling filter (Fig. 6) is less efficient than activated sludge [141, 146]. When the layer thickens due to bacterial proliferation, oxygen cannot reach the medium surface; instead, anaerobic bacteria thrive there. The microorganisms at the surface become unable to adhere to the medium as the biological film thickens, and some of the biofilms peel off the filter in the sloughing process. The underdrain system collects the sloughed solids and moves them to a clarifier where they are separated from the wastewater. Generally, the drawbacks of trickling filter are vector odor problem, requires additional treatment, and regular operator attention.
4.2.2.1 Factor Affecting Trickling Filter Process
The effectiveness of trickling process was controlled by organic loading rate, hydraulic distribution to filter, recirculation of filter effluent, and hydraulic loading rate [163]. It was found that decreasing the organic loading rate and hydraulic distribution increased the effectiveness of contaminant removal; similarly, a distribution that is successfully designed and runs can boost the removal of contaminants [141]. Furthermore, the degrading performances of microorganisms in the reactor are affected by major changes in organic loading input and the type of contaminants.
4.2.3 Oxidation Ponds
Oxidation ponds or lagoons are others types of good alternatives to standard wastewater treatment technique used in various countries [164]. It is a technique for treating water that makes use of the interaction between sunlight, algae, and microbes. There are many other types of ponds utilized in the water treatment process, including facultative, anaerobic, and maturation ponds [165], however anaerobic and facultative ponds are the most often employed pond types for treating wastewater. Water can be transported between facultative ponds, anaerobic ponds, and finally maturation ponds by using them as a single series for each method or occasionally as numerous consecutive series [166]. It should be noted that they can be used both separately and combined; (Fig. 7) presents the typical configuration of oxidation ponds in series.
4.2.3.1 Anaerobic Ponds
Anaerobic ponds are profound pond 2–5 m deep that promote the development of microorganisms to decompose and stabilize the wastewater with high concentration of organic materials and suspended particles in the condition where there is no existence of oxygen [167]. Anaerobic ponds look like open tanks that decompose the organic materials found in the wastewater with the use of microorganisms to produce methane gas and carbon dioxide. This pond helps the development of microorganisms that will help to break down the organic materials available in the wastewater then release carbon dioxide and methane gas, and then the sludge released can be taken out by sedimentation [168]. The anaerobic pond can be used in effluent containing high levels of organic matter and BOD. This procedure is straightforward, inexpensive, and can reduce BOD by 60 to 85%, however, it takes up a larger space than previous approaches [169]. These ponds are typically built to accommodate an organic loading rate (OLR) of 3 T ha/day, 1.5 days of detention time, temperatures greater than 15 °C, and an ideal pH of no greater than 6.2 [170].
4.2.3.2 Facultative Ponds
Facultative ponds are the types of ponds with 1–2 m in deep used for the biological treatment of wastewater, they consist aerobic zone near the top and an anaerobic zone near the bottom [171]. The facultative ponds receive the effluent from anaerobic ponds, then the microorganism (bacteria) existing in the pond will decompose the organic material available in the effluent, which causes the release of CO2. This technique, established by employing solar energy, CO2 and inorganic chemicals produced by bacteria in water, which subsequently promote oxygen generation to the pond, is intended to remove BOD, COD, and other organic matter from water using algae [172]. It suggests that bacteria use the oxygen produced by algae to break down inorganic material, and bacteria also use algae to proliferate by releasing carbon dioxide. Similarly, vertical mixing of effluent can cause the production of more oxygen via the wind. Oxygen generation takes place up to the depth where light can reach. If the chemical oxygen demand and biochemical oxygen demand in the bottom layer exceed the supply, oxygen cannot be produced in the bottom levels. Another factor that will not allow the production of oxygen is the high depth of the pond, because the color in the pond will be dark, so the light will not be able to fully enter. The quantity of dissolved oxygen (DO) varies during the day due to the photosynthetic algal available in the pond. The pond will have a high quantity of DO during peak light exposure because of algae activities but lowest at night. The facultative ponds can reduce BOD in the range of 80–95% which means that combining the two ponds (anaerobic ponds and facultative ponds) will have overall removal of 95% [173]. This pond has a longer detention time than anaerobic ponds in the range of 2–3 weeks, and the organic loading rate can vary between 100 and 400 kg BOD, COD / ha/day. The hydraulic retention time and the surface of the facultative pond are the most factors affection this method [174].
4.2.3.3 Maturation Ponds
Ponds used for maturation are shallow ponds between 1 and 1.15 m deep that allow sunlight to penetrate to the pond's depth [170]. They often accept water from facultative ponds and are utilized in the water treatment process to obtain eliminate pathogens and any residual suspended materials. Considering they are the final stages of the sequence of ponds and the tertiary water treatment, they are also known as finishing ponds. As discussed that facultative ponds use algae to reduce organic matter, also maturation ponds use algae to decrease pathogen and organic matter [175]. These ponds can remove BOD between 60–80% and 90% of pathogens in water in 15 to 20 days. This shows that they have a good performance in pollutant removal. The primary drawbacks of maturation ponds are the odor issue, the massive amount of sludge deposition, especially in the winter, and the requirement for a broad operating area.
4.2.3.4 Factors Affecting Maturation Ponds
The efficient removal of contaminant using ponds are affected by hydraulic retention time, pH, temperature, contaminant load, light intensity, and the depth of the pond. The shallower the depth, which cause the pond remains aerobic due to the penetration of sunlight to the whole column of the water, which increases the algal and bacteria activities but it reduces the hydraulic retention time [176]. According to Dias and Von Sperling [177] the efficiency of anaerobic ponds also depend on climate.
Biological treatment methods are not used alone but adding this method with physical and chemical methods, is supposed to remove the fluoride to a certain level. The concept of these methods to reduce fluoride in wastewater is that if these methods reduce or decompose organic matter and BOD to a certain level in water means that fluoride also decreases because it is a fluorine ion which is an organic compound. Hence, some studies show the efficiency of biological methods in removing fluoride. Liu et al. [178] investigated the performance of a novel facultative anaerobic denitrifying cupriavidus sp. W12 which is capable of performing calcium precipitation caused by microbes to remove fluoride in wastewater, it was observed that under the anaerobic and aerobic conditions the removal efficiency of fluoride reached 87.52% and 50.17% respectively in 120 h. The anaerobic condition pH was 8.26 and 7.77 for the aerobic condition while the initial fluoride concentration was 2.64 mg/L. Similarly the study done by Ali et al. [179] observed a fluoride removal efficiency of 96.33% with an initial fluoride concentration of 3 mg/L at a pH of 6.5 from water in a quartz sand-filled biofilm reactor using acinetobacter sp. H12. It is likely that strain H12 can facilitate the occurrence of calcium precipitation that is driven by bacteria to remove fluoride. Moreover, compared to other studies, the denitrifying and mineralizing bacteria pseudomonas sp. WZ39 was able to eliminate the fluoride content (1.99 mg/L) in water with an efficiency of 87.49% at a pH of 7.62 after 60 h [180]. The effectiveness of de-fluoridation as determined by several research to remove fluoride from water and wastewater using biological techniques is presented in (Table 7).
4.3 Membrane Technology
Membrane technology has become a growing component in water filtration because of its technological, lower energy consumption, economic, and environmentally friendly benefits. A membrane acts like a barrier that allows certain materials to pass through while blocking others from achieving so. Upon applying pressure to the system, the permeation process begins. Membrane technology is mostly used to remediate saline and black water. Membranes are classified as organic, or inorganic based on their composition [187]. Most membranes that depend on pressure or force for the separation process are made from synthetic organic polymers such as polyethylene and polypropylene, those membranes include microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) membrane as well as reverse osmosis (RO). Contrarily inorganic membranes are made from ceramics, metals, and zeolites or silica. These membranes are frequently applied in industrial applications such as hydrogen separation because they are chemically and thermally stable.
4.3.1 Reverse Osmosis
Reverse osmosis (RO) is the most well-known pressure-driven membrane technology due to it being 99.5% effective at removing monovalent ions like Na+ and Cl− [188, 189]. RO is a water treatment technique that uses a semi-permeable membrane to remove ions, pollutants, and other particles from water. Osmosis is a naturally occurring process in which water spontaneously pass through a membrane or semi permeable barrier that allows some molecule like water to pass through but other molecules like the majority of salts are unable to pass easily to the membrane structure, the flow of liquids through the such membrane occurs naturally to try and even out the salt concentration between two solutions, that is the liquid flow from the less concentrated solution such as fresh water to a more concentrated solution such as seawater. By applying pressure to the concentrated solution, reverse osmosis (RO) occurs when the liquid flows in the direction (Fig. 8). In this instance, water molecules are pushed through the membrane in the opposite direction, from the salinity of the seawater side to the freshwater side. In addition to its low cost and positive effects on the environment, reverse osmosis (RO) has been used often in water treatment to remove dissolved solutes, ions, and other contaminants from drinking water. The feed pressure, flow rate, pH, RO membrane temperature, salt passage, and permeating flow rate are the variables impacting the performance of RO [190].
As discussed before MF, UF as well as NF are also pressure driven membrane technology. MF membrane has the highest pores size which often filters out big contaminants, different pathogens, oil water, and suspended particles such as turbidity and color. These membranes are porous, their size can range between 0.1 and 5 µm with an average operating pressure of 5 bar. UF has a small pore size compared to MF which allows it to filter out bacteria and different soluble molecules such as protein, protein, glucose, and fructose. The pore size of UF can range between 1 and 100 nm, and usually operate on an average pressure of 10 bar. Lastly, NF which has a small pore size in the range of 1–10 nm compared to MF and UF but higher than RO allows small particle (monovalent ions) to pass through the membrane [191]. Different membrane filtration procedures that can reject or remove particular contaminants are categorized depending on their pore size rejection in (Fig. 9).
In the past years RO and NF membranes have long dominated the field of membrane technology [134, 188]. Additionally, even though RO has a very high fluoride removal efficiency, NF is more practical for drinking water purification compared to RO. Extremely high pressure is necessary for RO, which also means that high power and expensive operation costs are necessary. The RO process also eliminates vital elements that are required in drinking water, necessitating further addition of minerals after treatment. Different studies are done using commercial membranes such as NF90 and RO-SG for RO and NF to remove fluoride, it shows that the efficiency of these methods exceed 90% [192, 193], it was observed that the efficiency of fluoride retention is influenced by the working condition like membrane pressure, pH, ionic strength and feed water concentration. Another study done using RO combined with other treatment methods such as precipitation, crystallization, and UF shows that the efficient removal of fluoride is up to 99.9% [194]. Additionally, a study was done [195] comparing the efficiency of NF and membrane distillation, show that NF is more efficient 10 times more than membrane distillation with 80% fluoride retention where the initial concentration of fluoride was 15 mg/L. The NF was achieved at 9 bars as the permeating flux at the beginning was 42 L (m2 h)−1 at 20 °C and the crossflow rate of 0.17 L s−1. Many works of the literature showed that MF and UF alone are not suitable to remove contaminants such as fluoride, the reason it is used in combination or following the coagulation-flocculation method or precipitation methods [196]. According to Guigui et al. [197] and Zevenhuizen et al. [198] coagulation process before membrane filtration increases the permeate quality, because it decreases membrane fouling. The studies done by Conceição et al. [123, 199] showed that coagulation-flocculation followed by MF and UF resulted in the efficiency fluoride removal of 80.4% and 83% respectively at 2 bar pressure corresponding to 0.98 mg/L which is the range of permissible established by WHO. Table 8 below presents the fluoride retention efficiency of membrane filtration reported in previous studies.
4.3.2 Electro-Dialysis
Electro-dialysis (ED) is a membrane technology assisted by electricity as a driving force that is commonly used in water treatment such as desalination to remove dissolved ions from water. This method use electricity to transfer ion from a solution with less concentration (dilute) to a solution with a high concentration through the permeable membrane [207]. As shown in (Fig. 10 (a)), two different types of ion exchange membranes are used throughout the ED phase; the first is permeable to anions but repels the cation, while the second enables the cations to flow through but repels the anions. Like that, there are two different sorts of solutions used in this process: concentrated and diluted. Anions are moved to the anode and cations are transferred to the cathode after electricity is supplied to the cross system, which causes the ions present in the dilute solution to migrate to the concentrated solution via an opposite charges membrane. The cation exchange membrane (CEM) then maintains the anion in a manner like how the anion exchange membrane (AEM) maintains the cations. As a result, the concentration of ions in the concentrated solution is raised while they are reduced in the permeate [208, 209]. The amount of applied power, the temperature, the configuration of the system, the length of ED, the water flow rate, the properties of the membrane, and the total dissolved solids in the feed water are the variables that can affect electro-dialysis performance [188, 210]. Every element has a perfect operating condition, but the precise value depends on the goal and the kind of contaminants present in the feed water matrix.
Many studies indicated that the ED method has the potential to remove the fluoride to a certain level, which summarized in (Table 9). The de-fluoridation mechanism of ED is explained as the transfer of fluoride ions as shown in (Fig. 10 (b)). Like other membrane technologies such as RO and NF, recent studies have relied solely on available commercial membranes and simply enhanced the factors affecting the performance of de-fluoridation. The mechanism of ED is the same as RO and NF, the difference is that ED uses electricity or sometime Donnan effect as the driving force while RO and NF use hydraulic pressure as the driving force. ED compared to RO and NF, has a high performance in removing fluoride, as well as cost effective related to low pre and post treatment needs [188]. A study done by Bhadja et al. [211] to access the performance of ED in de-fluoridation from water taken in Rajasthan, India, it resulted that ED is able to remove fluoride concentration up to the permissible limit for drinking water settled by the WHO and the Bureau of Indian Standards (BIS) from 14.4 to 1.45 mg/L which is approximate to 90% removal. Similarly Gmar et al. [212] showed the high effectiveness of ED to remove fluoride from water in their study. The initial concentration of fluoride (ICFs) was 2.65 and 4 mg/L from a water sample taken in Tunisia, the conventional ED resulted in removal efficiency of over 92% at 12 V applied potential and 90L/h flow rate. Contrarily ED was not able to remove fluoride up to the permissible limit for drinking water in water containing a high concentration of fluoride. In the experimental conducted by Bagastyo et al. [213] to evaluate the efficiency of electro-dialysis to remove high concentration of fluoride (9270 mg/L) from wastewater produced by the fertilizer industry, the highest de-fluoridation performance was up to 260 mg/L which is equivalent to 2.7%, and it was observed at 1 A of current and at surface membrane of 100 cm2. This poor fluoride reduction was caused by the phosphate ion transfer through the membrane. Enhancing the area and the microstructure of the membrane can be a good tactic in electro-dialysis to augment the ion selectivity [214]. Moreover, Peng et al. [215] showed that the efficiency of ED in removing fluoride available in the Brick tea infusion is between 10.70 and 66.93% by applying 20 V, it was observed that the removal rate of fluoride was influenced by the ICF in the tea infusion between 0.5 and 10 g/kg and the duration of ED between 1 to 15 min. Despite ED electricity needs, it had greater remaining fluoride contents in most of the studies. So, this membrane technology is more commonly used as a preliminary treatment to significantly minimize the concentration of fluoride for subsequent treatment.
4.4 Electrocoagulation
In order to remove suspended particles, heavy metals, anions, and other contaminants from water and wastewater using electrochemistry, the process is known as electrocoagulation (EC). The principles of electrochemistry, flotation, and aggregation are all combined in the proficient process known as electrochemistry (EC) [221]. The mechanism of this process is related to the chemical coagulation process, the difference is that EC use coagulant produced by electrode often aluminum and iron due to electricity [222]. Comparing EC to other existing water treatment methods, it is more environmentally friendly, and suitable because of its great efficiency removal of contaminants from water, minimal sludge discharge, no other extra chemical requirements, and reasonably the operation price is low [223].
Govindan et al. [224] have shown that the electrocoagulation technique is a reliable method to remove fluoride from water and wastewater using aluminum based anode electrode as showed efficiency removal of 99%. Generally, the EC process consists of an anode electrode and a cathode electrode flooded in an aqueous solution. By applying electric current to electrodes, the anode electrode is subjected to be oxidized and produces metal ions often Al3+ or Fe3+ ions at the same time the reduction reaction causes the cathode electrode to produce OH− ion and H2 [225]. The Al3+ which plays the role of an electro coagulant reacts with OH− to form Al (OH) 2+ and then converted lastly into solid Al(OH)3 which increases the floc formation through the destabilization of fluoride. Lastly, the floc can be removed by other methods such as sedimentation or filtration. The de-fluoridation mechanism of EC is illustrated in (Fig. 11). In the following corresponding Eqs. (11–14) [73, 226, 227].
However, the performance of EC in removing fluoride in water is influenced by the pH of the water, applied electricity, electrode constituents, electrode configuration, concentration, and the time of the process [228]. Using mild steel electrodes in an electrocoagulation process with electrode currents of 37.72 and 75.44 A/m2 to remove fluoride from prepared water with a fluoride concentration of 50 mg/L, Chandraker et al.'s study [222] found that the fluoride was removed at a rate of 85.4 and 89.6%, respectively, at pH 6. Similar to López et al. [229] showed that the de-fluoridation efficiency was 85.65% in removing the fluoride water of 5 mg/L, while the optimum condition was pH 6, 15 min operation time, and electric current of the current density of 4.5 mA/cm2. Combining electrocoagulation with other technology such as adsorption, after repeating this process six times, resulted in high fluoride removal efficiency of 87% compared to the use of EC alone and the use of low electric current. The optimum condition was a pH of 6.724, the electric current of 11.303 mA cm2, F concentration of 5 mg/L, and a process time of 1h20 min [230]. It can be concluded that coupling EC with others technologies will enhance the performance removal of fluoride and reduce the cost of the overall process.
4.5 Ion Exchange
Ion exchange technology is a cutting-edge water treatment method that removes pollutants from water by reversibly exchanging ions from ion exchange resin (IER) to a charge of a similar charge in an aqueous solution [210]. According to the ions to be exchanged, two types of IER can be used: the cation exchanger, which exchanges ions with positively charged ions, and the anion exchanger, which exchanges ions with negatively charged ions. The best known application of ion exchange technology in water treatment is the demineralization of hard water and water softening by the zeolite process [231]. For instance, a study was done to treat water by removing the totals hardness, Ca and Mg using natural zeolite resulted in the efficient removal of 81%, 80.2%, and 84.8% respectively [232]. Additionally, various studies revealed that ion exchange technology has the potential to remove fluoride from water using basic anion exchanger resin that, for instance, contains quaternary ammonium compounds in exchanging Cl or OH with F as mentioned in Eq. (15), where R and M denote an alkyl group and matrix, respectively. The resins may become exhausted during this process and lose their ability to exchange ions; at this point, the resins are regenerated using diluted NaCl and can then be utilized again. The de-fluoridation procedure using anion exchange resin is shown in (Fig. 12).
The factors affecting the performance of anion exchange technology in de-fluoridation are the contact time, the existence of others anions in the water, pH, and the temperature [233]. Singh et al. [234] investigated the efficiency of de-fluoridation from groundwater with zirconium-impregnated anion exchange resin (HAIX-Zr) and determined approximately 60% of fluoride was removed in 30 min at pH 6 and temperatures ranging from (10–40 °C). It was observed that by increasing pH the HAIX-Zr removal ability was reduced, but increasing the temperature increased its ability. Another study done by Rodríguez et al. [235] showed that ion exchange technology was able to remove fluoride from coke wastewater up to 57.8–89.3% and 72.0–92.1% by using two Al-doped exchange resins (TP260 and TP207) respectively at pH of 7.18. However, the alkyl group attached can influence the efficient removal of fluoride in ion exchange technology, and the existence of other ions such as sulfate and phosphate in water reduces the de-fluoridation performance of ion exchange since they induce ion competition [236].
4.6 Adsorption
In water treatment, the adsorption process is an advanced technology used to remove or reduce the harmful inorganic and organic impurities from water and wastewater to a certain level. This process is basically the adhesion of an adsorbate (contaminants) to the surface of the adsorbent [237]. Adsorbents are explained as porous materials that attract contaminants to their surface [238]. Generally, the adsorption process happens due to physisorption or physical adsorption as well as chemisorption or chemical adsorption [237]. Physisorption occurs when the contaminants are attached to the surface of the adsorbent by low intermolecular force also named Van Del Waals forces, contrarily to chemisorption which involves the formation of the chemical bond between the ions of the contaminants and the surface of adsorbents [239]. This process is always followed by filtration [238]. The adsorption process compared to other water treatment technology discussed above it is found to be more advantageous because it showed high efficiency in removing contaminants to the allowed amounts for drinking water, low cost of materials, environmentally friendly, easy to operate and can be even applied in domestic [22]. Another benefit of this process is that the adsorbents can be regenerated and reused again [240]. In recent years, the adsorption process has been recognized as one of the trendiest methods of study in de-fluoridation, and this technology can remove fluoride up to the permissible range established by the water pollution boards [241].
4.6.1 Mechanism of De-Fluoridation
De-fluoridation mechanism of adsorption necessitates physical and chemical reactions involving fluoride and the adsorbents, which can be explained as the adhesion of fluoride available in the water to the surface of the adsorbent, then the water and the adsorbents are separated by filtration [236]. The overall schematic de-fluoridation mechanism by adsorption process is presented in (Fig. 13) and can be confirmed using different analysis techniques such as XRD, FTIR, and XPS [242]. Generally, by adding the adsorbents such as metals oxide or metal hydroxide in water containing fluoride, it will happen the formation of a functional group (hydroxyl compounds) on the surface of adsorbents due to the chemical reaction of hydration, the (− OH) formed will be exchanged with fluoride ions and finally released into the water [22]. This mechanism commonly known as ligand exchange is very well explained by Valdivieso et al. [243] in their study of removing fluoride using α-Al2O3 as an adsorbent in the Eq. (16). Han et al. [244] revealed that fluoride can be removed by the influence mechanism of the Lewis acid–base which is the same as ligand exchange, the only difference is that there is a weak interaction betwixt fluoride ion (Lewis bases) and metal (Lewis acids). The surface of hydroxyl compounds shows that it is an effective agent for removing fluoride, however, if this surface is increased also the adsorption performance is increased.
Many studies showed that fluoride ions can be swapped with different anions like sulfate, carbonate, and nitrate on the surface of adsorbent in the mechanism known as ion exchange. For instance, He et al. [242] confirm the ion exchange mechanism between fluoride ions and sulfate ions in Eq. (17).
The study done by Kumari et al. [245], show that the electrostatic attraction force produces an active region to adsorb fluoride ion by protonating the surface of the adsorbent at low pH in the mechanism named electrostatic attraction as presented in Eq. (18). Additionally, fluoride can be adsorbed on the adsorbent surface by exchanging fluoride ions with hydroxyl and sulfate function compound as presented in Eqs. (19) and (20), where ≡ S symbolize the surface of the adsorbent [246, 247].
Electrostatic attraction
Ion exchange
4.6.2 Factors Affecting the Process of Fluoride Removal
The performance of the adsorption method on water defluoridation is determined by various elements such as pH, fluoride concentration, adsorbent dose, contact time, mixing speed, and temperature. All these aspects are depicted in (Fig. 14) and thoroughly addressed.
4.6.2.1 Effect of pH
The pH is a principal parameter to consider while removing fluoride from water using the adsorption process, because it affects the adhesion of fluoride to the surface of adsorbents. The performance of adsorbents to attract fluoride ions in different pH of water such as acidic, neutral, or basic depends on the surface charge of adsorbents. In acidic water, the electrostatic attraction force produce an active region to adsorb fluoride ion by protonating the surface of the adsorbent which enhance the de-fluoridation [245], whereas in basic water, the surface of the adsorbent have a tendency to be negatively charged which create a repulsive force between fluoride ions hydroxide ions, so the fluoride ions are captured by ion exchange mechanism [248] as shown in Eq. (16). Therefore, by increasing the pH the adsorption performance decreases. The influence of water pH should be evaluated in conjunction with the adsorbent's pHpzc. The adsorbent surface less than pHpzc is always positively charged and negatively charged more than it. This is supported by Issabayeva et al. [249] in their study, the high de-fluoridation efficiency was 46% at a pH of 2 whereas at a pH of 3 the de-fluoridation efficiency decreased to 10%. This indicates the shift in adsorbent surface charge over the pH of the point of zero charges from positive to negative.
4.6.2.2 Effect of Adsorbent Dose
Adsorbent dose plays a significant role in removing fluoride, because increasing the adsorbents doses, it increase also the surface of adsorbents and that create the more available active site on the surface of the adsorbent resulting in high de-fluoridation [248]. In the study done by Jeyaseelan et al. [250] noticed that fluoride removal gradually increased with increasing adsorbent dose from 0.05 to 0.1 g. After 0.1 g there is no increase observed beyond this optimum dose because there is no active site on the surface of the adsorbent to attract fluoride, which means the surface of the adsorbent is saturated by fluoride ions. Another study showed that increasing the dose of adsorbent from 5 to 10 g, resulted in fluoride removal efficiency being increased by 24%, which means from 46 to 70% [249]. Additionally, the increase of the adsorbent dose from 1.67 to 5 g/L resulted in the increase of de-fluoridation efficiency from 72.4% to 99.5% and remain constant at 99.6% for adsorbent dose greater than 5 g/L [251].
4.6.2.3 Effect of Concentration
In enhancing fluoride removal using adsorption, the concentration of fluoride is one of the factors to consider. By increasing the concentration of fluoride, the efficiency removal decrease. At low level of the fluoride, the de-fluoridation efficiency is high because the more functional groups are available on the surface of the adsorbent to attract fluoride which is reduced by increasing the fluoride concentration [248]. This is noticed by Araga et al. [252] in their experimental work, which observed a decrease of adsorption capacity from 0.1 mg/g to 1.25 mg/g while increasing the concentration of F from 2.7 mg/L to 20.04 mg/L.
4.6.2.4 Effect of Contact Time
The contact time is another factor to consider while enhancing the performance of the adsorption process, it was observed that while increasing the contact time also the de-fluoridation efficiency increase until reaching the equilibrium [248]. There is no significant fluoride removal once the equilibrium is reached due to there are no more active sites of adsorbent to capture fluoride. Adsorption of fluoride was investigated by Issabayeva et al. [249], and they realized that fluoride removal efficiency was 43% after a contact time of 60 min. Remarkably, after the contact time of 120 min, the efficiency removal increased up to 55%. However, there is no other significant increase observed after this time (120 min). However, no further major rise has been reported since this time. (120 min). Indeed, a drop in adsorption effectiveness can be detected after the equilibrium due to fluoride release from the surface, which is then stabilized after some time.
4.6.2.5 Effect of Temperature
The temperature significantly affects the fluoride removal efficiency in the adsorption process by influencing the physical binding mechanism of fluoride ion to the surface of the adsorbent. Furthermore, temperature can influence the physical characteristic of the adsorbent, varying the temperature of water considerably alters the adsorption capacity. Most of the adsorption research is carried out in laboratories at room temperature. By increasing the temperature, the efficiency of adsorption is reduced because it enhances the deprotonation and the hydroxylation of the adsorbent surface which result in more negative charge on it [248]. That is contradicted in a study carried out by Gao et al. [253], who observed that the fluoride removal efficiency increased from 89.46% to 94.83% by rising temperature from 5 to 25 °C. Therefore, increasing the temperature can create new active sites on the surface of the adsorbent.
4.6.2.6 Effect of Mixing
Mixing speed is an important factor to consider while enhancing the adsorption efficiency, it was observed that the kinetics and adsorption equilibrium depend on agitation speed [254]. While increasing the mixing speed up to optimum, it increases also the efficiency of adsorption. A study carried out by Papari et al. [255] to remove fluoride from water using an adsorption process prove the influence of agitation speed (0–150 rpm), the efficiency of removal without mixing was 53.25%, by increasing the mixing speed to 150 rpm, the efficiency fluoride removal increased to 99.7%. This could be the result of enhanced fluoride ion distribution toward the surface of the absorbent and improved interaction between fluoride and adsorbent.
4.6.3 Adsorbents
Adsorbents are porous materials that absorb contaminants to their surface. There are two types of adsorbents viz. natural absorbent and synthetic absorbent. Natural adsorbents include clay, zeolite, siliceous materials, calcite, soil, sediment, biomaterials, etc. [240]. Whereas synthetic adsorbents are synthesized by researchers such as activated carbon solid waste, by product, and others modified naturals and biological materials [256]. The classification of various types of adsorbents according to there is compiled in (Fig. 15).
4.6.4 Natural Adsorbent
Natural adsorbents are defined as a single natural material or compound that was created through a natural process and has largely constant chemical components [240]. Natural adsorbents have distinct benefits in scale, affordability, operation, environmentally friendly, and abundantly available [22]. Generally, these type of naturally occurring adsorbents is exciting in the adsorption process for water treatment because they contain metal atoms that are electrostatically charged or in different stages of oxidation like aluminum, iron, silicon, etc.[240]. These properties enhance the adhesion of the contaminants to the surface of the adsorbent, which increases the efficiency removal [22]. There are much research on the application of natural adsorbents in de-fluoridation from water. Some natural adsorbents resulted in high efficiency removal up to the advised level of fluoride for drinking water. Nabbou et al. [39] investigated the capacity of Algerian natural clay (kaolinite) to remove fluoride with the initial concentration of 5 mg/L from groundwater, they found that the adsorption capacity of kaolinite was 0.48 mg/g at a pH range from 4.5 to 6 and at the temperature of 55 °C. Furthermore, clay has tiny particle sizes, often less than 2 µm, and highly active site due to its complex porosity architectures, which allow physical and chemical interactions with contaminants like fluoride. Zeolite showed high performance in adsorption, generally, the surface of zeolite has a negative charge in acidic and basic condition (all pH), which make it to be more attractive on cations and less attractive on anions due to electrostatic repulsions [240]. Numerous studies have removed fluoride modified zeolite using metallic cations such as Al3+ to create the positive charge on the zeolite surface, however, there are limited studies using natural zeolite as adsorbents. Gómez et al. [257] examined the adsorption of fluoride using Ethiopian natural zeolite (analcime and mordenite), they observed that natural zeolite was able to remove fluoride up to the permissible limit for drinking water, and the maximum fluoride removal capacity was 0.47 mg (F−)/g which is equivalent to 87%. Similarly, another study done by Cai et al. [258] showed the efficiency of natural zeolite in de-fluoridation of 95% at 20 °C and pH of 9 in 84 min, with the initial concentration of 200 mg/L. Iriel et al. [259] studied the adsorption efficiency of lateritic soil from Argentina to remove fluoride from groundwater (2.5 mg F−/l), they observed that lateritic soils are likely adsorbents on a domestic scale for drinking water with the efficient removal of 30% at pH of 8 in 30 min. Additionally, Chandraker et al. [260] showed the performance removal of fly ash as an adsorbent in their research of 62.2% with an initial fluoride concentration of 50 mg/L, while the optimum fluoride removal was pH:5 and time: 4 h. However, fly ash is an abundantly available adsorbent, like other natural adsorbents that can be utilized after being washed and dried, or activated to enhance its removal capacity.
Moreover, bioadsorbents have the high performance to adsorb fluoride from water and wastewater, generally, bioadsorbents also called biomass based adsorbents are biological materials that are able to accumulate different contaminants on their surface [240]. Those materials include living materials such as algae, bacteria, fungal, yeast and non-living materials such as animal materials, agriculture waste, as well as household waste (sawdust, seaweed, bones, vegetables, coconut shell, corncob waste, tea waste, rice hulls, wool, peat, and chitosan) [256]. Researchers focused on the bioadsorbents because they are abundantly available and eco-friendly. Applications of bioadsorbents for de-fluoridation from water have been done by numerous researchers. In recent years, Shanker et al. [261] employed a study focusing on the use of microorganisms (bacterial) as adsorbents to remove fluoride from the water of Mahbubnagar, they observed that acinetobacter sp (GU566361) removed fluoride up to 57.3% at 35 °C and pH of 7.5 after 10 h of incubation, while the fluoride concentration was 20 mg/L. Mondal [262] studied the potential of natural banana peel as bioadsorbent for de-fluoridation from water collected in West Bengal State with 30.08 mg/L, it was observed that at a temperature of 28.85 °C and pH of 4, the removal efficiency of fluoride by natural banana peel was 98.8%. The natural and synthetic adsorbent’s efficient removal of fluoride with optimum factors affecting adsorption are presented in (Table 10).
4.6.4.1 Synthetic Adsorbents
Synthetic adsorbents are synthesized materials to enhance their adsorption capacity compared to their natural raw materials, many researchers show that modification of natural materials like metal impregnation to create a positive ion, as well as treatment using acid–base activation or thermal, can increase the specific site for contaminant removal on the surface of adsorbents [239]. Generally, the synthetic adsorbent can be synthesized via inorganic materials such as clay, calcareous soils, slags, fly ash, and zeolites or organic materials such as agricultural waste, industrial waste, and household waste [256]. Several researchers revealed that synthetic adsorbents can be used in water treatment especially in removing fluoride from groundwater due to its high removal capacity up to the advised concentration for drinking water [269]. Gao et al. [253] synthesize micron zirconia modified zeolite molecular sieve to remove fluoride from groundwater in the adsorption batch experiment, they observed that the efficiency of fluoride removal of ZrO2-Ze adsorbents is 94.89% at pH of 6, the temperature of 25 °C while fluoride concentration was 5 mg/L and this process reaches equilibrium in 8 h. Combining modified graphene oxide (GO) and chitosan (CS) gives a hybrid composite bead (GO/CS) which are used as an adsorbent in de-fluoridation [250]. Employing (GO/CS) adsorbent on water containing 10 mg/L, the efficient fluoride removal observed was greater than 80% in 30 min at a pH of 6. The result shows that this adsorbent can be used for up to six rounds without losing its adsorption efficiency. Additionally, Dhanasekaran and Sahu [265] synthesize an adsorbent from ‘Anjili’ tree sawdust impregnated by ferric hydroxide and activated alumina (SFAA), and they employ it in de-fluoridation from water containing 20 mg/L of fluoride, they found the adsorption capacity of 2.42 mg/g at pH between 6–7 and at the temperature of 29.85 °C. It was observed that SFAA also can remove other contaminants in water such as arsenic to the standard of drinking water. Some synthesized adsorbents can result in high adsorption capacity on the high concentrations of fluoride in water, but don’t remove it to the level of drinking water standard. Shao et al. [266] showed that synthesized CaSO4⋅2H2O nanorods can remove fluoride from 200 mg/L to 7.88 mg/L with is beyond the permissible limit for drinking water.
5 Cost of Defluoridation Technologies
The cost of the water treatment technology may be affected by a number of variables that require specialized knowledge in order to provide an accurate and complete estimate when comparing alternative treatments. Material considerations, chemical use, energy usage, and life cycles are some of the aspects that must be taken into account, along with their accompanying costs [208]. The result of the techno-economic analysis conducted by Bhaskar et al. [270] showed that for the adsorption of fluoride using activated soil-clay mixture, the cost involved was 0.17 $/m3. The basic economic evaluation carried out by Mena et al. [271] resulted that the associated price of fluoride removal using EC is 0.27 $/m3. Additionally, Bhagawan et al. [272] reported that the operation cost of EC in fluoride removal range between 0.28 and 0.98 $/m3. The cost of EC process mostly depends on electrode materials, electricity consumed and fluoride initial concentration. The relevant studies, which offer a detailed estimate of operational treatment costs of fluoride removal, have been summarized in (Table 11).
Among the developed fluoride removal techniques, the estimated cost of the adsorption process is low compared to other technologies; this is due to the low cost of the materials used in the adsorption process. It was observed that the use of natural adsorbents reduces the cost of the adsorption process because they are abundantly available and can be regenerated [22]. On the other hand, IE has a high-cost estimation compared to others; this may be linked to the high cost of IER.
6 Summary
Contaminant water is a serious threat to human health, necessitating extremely effective protection of the environment and water management. Some investigators can strive to create specific approaches since they mix several strategies to generate collaborative fluoride removal solutions. This unit is the role of contributing to development and advancement in engineering technology by applying current and appropriate approaches, outperforming smaller attempts for practical usage. The fluoride separation technology from water system is the sole subject of this study's narrative and investigation. Due to location-specific physicochemical, geological, and socioeconomic factors that can affect fluoride removal effectiveness, actual implementations may differ from the estimates given. To remove fluoride from drinkable water and industrial effluent, a variety of techniques have been utilized in the past, and this analysis has tried to take them all into account. Both fluoride precipitation and the traditional method of removing fluoride from water supplies have limitations. The shortcomings of the great majority of these solutions include expensive operating and maintenance expenses, supplementary pollution, such as the formation of hazardous sludge, and so on, as well as the treatment's sophisticated procedure. Ultimately, by adopting a comprehensive strategy to investigating the complex difficulties of fluoride pollution, this work unequivocally demonstrates the significance of various exposure routes and dangers. As a result, it is urged to aspiring researchers that they use a more diligent and comprehensive strategy to evaluate fluoride concentrations.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
References
Bin HS, Hossain MB, Hossain MS (2021) Ecological risk evaluation in bottom-surface sediments and sub-surface water in the subtropical Meghna estuarine system. Heliyon 7:e08324. https://doi.org/10.1016/j.heliyon.2021.e08324
Vural A, Gündoğdu A, Saka F (2022) The heavy metals and minor elements effects of mineralization and alteration areas with buried ore deposits potential on the surface waters. J Investig Eng Technol 5:21–33
Luo X, Tong X, Hu Z (2021) An applicable and automatic method for earth surface water mapping based on multispectral images. Int J Appl Earth Obs Geoinf 103:102472. https://doi.org/10.1016/j.jag.2021.102472
Vural A, Gündoğdu A, Saka F (2022) Geochemical investigation of the potability of surface water in Çit River and related creeks in Avliyana Basin (Gümüşhane, NE Turkey). Turkish J Anal Chem. https://doi.org/10.51435/turkjac.1102045
Drouiche N, Ghaffour N, Naceur MW (2012) Towards sustainable water management in Algeria. Desalin Water Treat 50:272–284. https://doi.org/10.1080/19443994.2012.719477
Eunice Jayashree D, Pooja G, Senthil Kumar P, Prasannamedha G (2020) A review on fluoride: Treatment strategies and scope for further research. Desalin Water Treat 200:167–186. https://doi.org/10.5004/dwt.2020.26010
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manage 92:2355–2388. https://doi.org/10.1016/j.jenvman.2011.06.009
Mitra S, Corsolini S, Pozo K (2019) Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere 221:154–165. https://doi.org/10.1016/j.chemosphere.2018.12.173
Wilson JM, Wang Y, VanBriesen JM (2014) Sources of high total dissolved solids to drinking water supply in Southwestern Pennsylvania. J Environ Eng. https://doi.org/10.1061/(asce)ee.1943-7870.0000733
Broséus R, Cigana J, Barbeau B (2009) Removal of total dissolved solids, nitrates and ammonium ions from drinking water using charge-barrier capacitive deionisation. Desalination 249:217–223. https://doi.org/10.1016/j.desal.2008.12.048
Rafiqul Islam M (2016) A study on the TDS level of drinking mineral water in Bangladesh. Am J Appl Chem 4:164. https://doi.org/10.1164/j.ajac.20160405.11
Hussain I, Shakeel M, Faisal M (2014) Distribution of total dissolved solids in drinking water by means of Bayesian Kriging and Gaussian spatial predictive process. Water Qual Expo Heal 6:177–185. https://doi.org/10.1007/s12403-014-0123-9
Karunanithi M, Agarwal R, Qanungo K (2019) A review of fluoride removal from groundwater. Period Polytech Chem Eng 63:425–437. https://doi.org/10.3311/PPch.12076
Vural A, Gündoğdu A (2020) High-fluoride risk and toxicity in surface waters in Gümüşhane-Gökdere valley drainage network (NE Turkey). J Eng Res Appl Sci 9:1323–1334
Vinati A, Mahanty B, Behera SK (2015) Clay and clay minerals for fluoride removal from water: a state-of-the-art review. Appl Clay Sci 114:340–348. https://doi.org/10.1016/j.clay.2015.06.013
Abiye T, Bybee G, Leshomo J (2018) Fluoride concentrations in the arid Namaqualand and the Waterberg groundwater, South Africa: Understanding the controls of mobilization through hydrogeochemical and environmental isotopic approaches. Groundw Sustain Dev 6:112–120. https://doi.org/10.1016/j.gsd.2017.12.004
Meenakshi MRC (2006) Fluoride in drinking water and its removal. J Hazard Mater 137:456–463. https://doi.org/10.1016/j.jhazmat.2006.02.024
Duggal V, Sharma S (2022) Fluoride contamination in drinking water and associated health risk assessment in the Malwa Belt of Punjab, India. Environ Adv 8:100–242. https://doi.org/10.1016/j.envadv.2022.100242
Shivaraju HP, Egumbo H, Madhusudan P (2019) Preparation of affordable and multifunctional clay-based ceramic filter matrix for treatment of drinking water. Environ Technol (United Kingdom) 40:1633–1643. https://doi.org/10.1080/09593330.2018.1430853
Solanki YS, Agarwal M, Gupta AB (2022) Fluoride occurrences, health problems, detection, and remediation methods for drinking water: a comprehensive review. Sci Total Environ 807:150601. https://doi.org/10.1016/J.SCITOTENV.2021.150601
Kimambo V, Bhattacharya P, Mtalo F (2019) Fluoride occurrence in groundwater systems at global scale and status of defluoridation – State of the art. Groundw Sustain Dev 9:100223. https://doi.org/10.1016/j.gsd.2019.100223
Gai WZ, Deng ZY (2021) A comprehensive review of adsorbents for fluoride removal from water: performance, water quality assessment and mechanism. Environ Sci Water Res Technol 7:1362–1386. https://doi.org/10.1039/d1ew00232e
Singh G, Kumari B, Sinam G (2018) Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective– a review. Environ Pollut 239:95–108. https://doi.org/10.1016/j.envpol.2018.04.002
Aoun A, Darwiche F, Al Hayek S, Doumit J (2018) The fluoride debate: the pros and cons of fluoridation. Prev Nutr Food Sci 23:171–180. https://doi.org/10.3746/pnf.2018.23.3.171
Sivasankar V, Murugesh S, Rajkumar S, Darchen A (2013) Cerium dispersed in carbon (CeDC) and its adsorption behavior: a first example of tailored adsorbent for fluoride removal from drinking water. Chem Eng J 214:45–54. https://doi.org/10.1016/j.cej.2012.10.023
Stephen P, Niyi A (2014) 水的氟化:对作为公共卫生干预的摄入氟化物的生理影响的批判性评论Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention. Sci World J 2014:1–10
Ullah R, Zafar MS, Shahani N (2017) Potential fluoride toxicity from oral medicaments: a review. Iran J Basic Med Sci 20:841–848. https://doi.org/10.2203/ijbms.2017.9104
Harter T (2003) Basic concepts of groundwater hydrology. Basic Concepts Groundw Hydrol. https://doi.org/10.3733/ucanr.8083
Bierkens MFP, Wada Y (2019) Non-renewable groundwater use and groundwater depletion: a review. Environ Res Lett. https://doi.org/10.1088/1748-9326/ab1a5f
Bennett G, Van Camp M, Shemsanga C (2022) Delineation of the aquifer structure and estimation of hydraulic properties on the flanks of Mount Meru Northern Tanzania. J African Earth Sci 196:104673. https://doi.org/10.1016/J.JAFREARSCI.2022.104673
Rashid A, Guan DX, Farooqi A (2018) Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat, Pakistan. Sci Total Environ 635:203–215. https://doi.org/10.1016/j.scitotenv.2018.04.064
Yadav M, Singh G, Jadeja RN (2021) Fluoride contamination in groundwater, impacts, and their potential remediation techniques. Groundw Geochem. https://doi.org/10.1002/9781119709732.ch2
Vithanage M, Bhattacharya P (2015) Fluoride in the environment: sources, distribution and defluoridation. Environ Chem Lett 13:131–147. https://doi.org/10.1007/s10311-015-0496-4
Nizam S, Virk HS, Sen IS (2022) High levels of fluoride in groundwater from Northern parts of Indo-Gangetic plains reveals detrimental fluorosis health risks. Environ Adv 8:100200. https://doi.org/10.1016/j.envadv.2022.100200
Walker DB, Baumgartner DJ, Gerba CP, Fitzsimmons K (2019) Surface Water Pollution, 3 本. Elsevier Inc.
Brunner P, Simmons CT, Cook PG (2009) Spatial and temporal aspects of the transition from connection to disconnection between rivers, lakes and groundwater. J Hydrol 376:159–169. https://doi.org/10.1016/j.jhydrol.2009.07.023
Islam MS, Idris AM, Islam ARMT (2021) Hydrological distribution of physicochemical parameters and heavy metals in surface water and their ecotoxicological implications in the Bay of Bengal coast of Bangladesh. Environ Sci Pollut Res 28:68585–68599. https://doi.org/10.1007/s11356-021-15353-9
Dartan G, Taspinar F, İs T (2017) Analysis of fluoride pollution from fertilizer industry and phosphogypsum piles in agricultural area. J Ind Pollut Control 33:662–669
Nabbou N, Belhachemi M, Boumelik M (2019) Removal of fluoride from groundwater using natural clay (kaolinite): Optimization of adsorption conditions. Comptes Rendus Chim 22:105–112. https://doi.org/10.1016/j.crci.2018.09.010
Ali S, Thakur SK, Sarkar A, Shekhar S (2016) Worldwide contamination of water by fluoride. Environ Chem Lett 14:291–315. https://doi.org/10.1007/s10311-016-0563-5
Shah MT, Danishwar S (2003) Potential fluoride contamination in the drinking water of Naranji area, NorthWest Frontier Province, Pakistan. Environ Geochem Health 25:475–481. https://doi.org/10.1023/B:EGAH.0000004579.54947.e7
Rango T, Bianchini G, Beccaluva L, Tassinari R (2010) Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J African Earth Sci 57:479–491. https://doi.org/10.1016/j.jafrearsci.2009.12.005
Ram S, Thakkar V, Machale P (2017) Determination of fluoride level in drinking water from water samples in Navi Mumbai, Maharashtra. J Indian Assoc Public Heal Dent 15:395. https://doi.org/10.4103/jiaphd.jiaphd_95_17
Sahu P (2020) Environmental concerns and sustainable development. Environ Concerns Sustain Dev. https://doi.org/10.1007/978-981-13-5889-0
Datta AS, Chakrabortty A, De Dalal SS, Lahiri SC (2014) Fluoride contamination of underground water in West Bengal, India. Fluoride 47:241–248
Maheswari E, Meignana Arumugham I, Pradeep Kumar R, Sri Sakthi D (2017) Fluoride content in various sources of drinking water in Chennai. J Adv Pharm Educ Res 7:142–145
Dasaiah S, Kurakalva RM, Pindi PK (2020) Data on fluoride concentration profile in groundwater of rural habitats in Mahabubnagar district, Telangana India. Data Br 32:106165. https://doi.org/10.1016/j.dib.2020.106165
Samal AK, Mishra PK, Biswas A (2020) Assessment of origin and distribution of fluoride contamination in groundwater using an isotopic signature from a part of the Indo-Gangetic Plain (IGP), India. HydroResearch 3:75–84. https://doi.org/10.1016/j.hydres.2020.05.001
Waziri M, Musa U, S. Hati S, (2012) Assessment of fluoride concentrations in surface waters and groundwater sources in Northeastern Nigeria. Resour Environ 2:67–72. https://doi.org/10.5923/j.re.20120202.10
Hayat E, Baba A (2017) Quality of groundwater resources in Afghanistan. Environ Monit Assess. https://doi.org/10.1007/s10661-017-6032-1
Berger T, Mathurin FA, Drake H, Åström ME (2016) Fluoride abundance and controls in fresh groundwater in Quaternary deposits and bedrock fractures in an area with fluorine-rich granitoid rocks. Sci Total Environ 569–570:948–960. https://doi.org/10.1016/j.scitotenv.2016.06.002
D’Alessandro W, Bellomo S, Parello F (2008) Survey on fluoride, bromide and chloride contents in public drinking water supplies in Sicily (Italy). Environ Monit Assess 145:303–313. https://doi.org/10.1007/s10661-007-0039-y
Vitor Portugal Gonçalves M, Alves Santos R, Alberto Machado Coutinho C, Jerônimo Moreira Cruz M (2020) Fluoride levels in the groundwater and prevalence of dental fluorosis in the Municipality of Santana , in Region Karstic of West Bahia, Brazil. Groundw Hydrol. https://doi.org/10.5772/intechopen.85007
Desbarats AJ (2009) On elevated fluoride and boron concentrations in groundwaters associated with the Lake Saint-Martin impact structure, Manitoba. Appl Geochem 24:915–927. https://doi.org/10.1016/j.apgeochem.2009.02.016
Hu Y, You M, Liu G, Dong Z (2021) Spatial distribution and potential health risk of fluoride in drinking groundwater sources of Huaibei, Anhui Province. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-87699-6
Liu J, Peng Y, Li C (2021) A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province North China. Ecotoxicol Environ Saf 207:111512. https://doi.org/10.1016/j.ecoenv.2020.111512
Haji M, Wang D, Li L (2018) Geochemical evolution of Fluoride and implication for F- enrichment in groundwater: Example from the Bilate River Basin of Southern Main Ethiopian Rift. Water (Switzerland) 10:1–20. https://doi.org/10.3390/w10121799
Habiyakare T, Schurer JM, Poole B (2021) Dental fluorosis among people and livestock living on Gihaya Island in Lake Kivu, Rwanda. One Heal Outlook. https://doi.org/10.1186/s42522-021-00054-7
Rusiniak P, Sekuła K, Sracek O, Stopa P (2021) Fluoride ions in groundwater of the Turkana County, Kenya, East Africa. Acta Geochim 40:945–960. https://doi.org/10.1007/s11631-021-00481-3
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County, Wisconsin. Environ Geol 50:132–138. https://doi.org/10.1007/s00254-006-0192-6
Hossain S, Hosono T, Yang H, Shimada J (2016) Geochemical processes controlling fluoride enrichment in groundwater at the Western Part of Kumamoto Area, Japan. Water Air Soil Pollut. https://doi.org/10.1007/s11270-016-3089-3
Ahmed AA (2014) Fluoride in quaternary groundwater aquifer, Nile Valley, Luxor. Egypt Arab J Geosci 7:3069–3083. https://doi.org/10.1007/s12517-013-0962-x
Kahsai F, Fisahatsion A, Asmellash M (2001) Fluoride in groundwater in selected villages in Eritrea (North East Africa). Environ Monitor Assess 75:169–177
Davraz A, Sener E, Sener S (2008) Temporal variations of fluoride concentration in Isparta public water system and health impact assessment (SW-Turkey). Environ Geol 56:159–170. https://doi.org/10.1007/s00254-007-1148-1
Essebbahi I, Ouazzani C, Moustaghfir A (2022) Analysis of the fluoride levels of well water and tea consumed by the Moroccan population in different rural areas. Mater Today Proc. https://doi.org/10.1016/j.matpr.2022.07.360
Msonda KWM, Masamba WRL, Fabiano E (2007) A study of fluoride groundwater occurrence in Nathenje, Lilongwe, Malawi. Phys Chem Earth 32:1178–1184. https://doi.org/10.1016/j.pce.2007.07.050
Rondano Gómez K, López Pasquali CE, Paniagua González G (2020) Statistical evaluation of fluoride contamination in groundwater resources of Santiago del Estero Province, Argentina. Geosci Front 11:2197–2205. https://doi.org/10.1016/j.gsf.2020.02.018
Jha SK, Singh RK, Damodaran T (2013) Fluoride in groundwater: toxicological exposure and remedies. J Toxicol Environ Heal Part B Crit Rev 16:52–66. https://doi.org/10.1080/10937404.2013.769420
Wang F, Li Y, Tang D (2021) Effects of water improvement and defluoridation on fluorosis-endemic areas in China: a meta-analysis. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.116227
Bretzler A, Johnson CA (2015) The geogenic contamination handbook: addressing arsenic and fluoride in drinking water. Appl Geochem 63:642–646. https://doi.org/10.1016/j.apgeochem.2015.08.016
Shen J, Mkongo G, Abbt-Braun G (2015) Renewable energy powered membrane technology: fluoride removal in a rural community in northern Tanzania. Sep Purif Technol 149:349–361. https://doi.org/10.1016/j.seppur.2015.05.027
Joseph A, Rajan R, Paul J (2022) The continuing crippling challenge of skeletal fluorosis – Case series and review of literature. J Clin Transl Endocrinol Case Rep 24:100114. https://doi.org/10.1016/j.jecr.2022.100114
Mousazadeh M, Alizadeh SM, Frontistis Z (2021) La electrocoagulación como tecnología prometedora de desfluoración del agua: una revisión del estado del arte de los mecanismos de eliminación y las tendencias de rendimiento. Water (Switzerland). https://doi.org/10.3390/w13050656
Adlard ER, Gupta AK, Ayoob S (2016) Fluoride in drinking water. Status Issues Solut 79:1395–1396. https://doi.org/10.1007/s10337-016-3128-7
Trikha R, Sharma BK (2014) Studies on factors affecting fluoride removal from water using passive system. J Environ Chem Eng 2:172–176. https://doi.org/10.1016/j.jece.2013.12.006
Aggarwal A, Mehta S, Gupta D (2012) Clinical & immunological erythematosus patients characteristics in systemic lupus Maryam. J Dent Educ 76:1532–1539. https://doi.org/10.4103/ijmr.IJMR
Goodman C, Hall M, Green R (2022) Maternal fluoride exposure, fertility and birth outcomes: the MIREC cohort. Environ Adv 7:100135. https://doi.org/10.1016/j.envadv.2021.100135
Xu K, An N, Huang H (2020) Fluoride exposure and intelligence in school-age children: evidence from different windows of exposure susceptibility. BMC Public Health 20:1–8. https://doi.org/10.1186/s12889-020-09765-4
Martínez-Mier EA (2012) Fluoride: its metabolism, toxicity, and role in dental health. J Evid Based Complement Altern Med 17:28–32. https://doi.org/10.1177/2156587211428076
Choubisa SL (2015) Industrial fluorosis in domestic goats. Fluoride 48:105–112
Gupta R, Khan T, Agrawal D, Kachhawa J (2007) The toxic effects of sodium fluoride on the reproductive system of male rats. Toxicol Ind Health 23:507–513. https://doi.org/10.1177/0748233708089041
Patra RC, Dwivedi SK, Bhardwaj B, Swarup D (2000) Industrial fluorosis in cattle and buffalo around Udaipur, India. Sci Total Environ 253:145–150. https://doi.org/10.1016/S0048-9697(00)00426-5
Tao X, Xu ZR, Wang YZ (2006) Effects of dietary fluoride levels on growth, serum indexes and antioxidant systems in growing pigs. Turkish J Vet Anim Sci 30:65–70
Miao LP, Li LL, Zhu MK (2019) Excess dietary fluoride affects laying performance, egg quality, tissue retention, serum biochemical indices, and reproductive hormones of laying hens. Poult Sci 98:6873–6879. https://doi.org/10.3382/ps/pez443
Choubisa SL (2013) Fluorotoxicosis in diverse species of domestic animals inhabiting areas with high fluoride in drinking water of Rajasthan, India. Proc Natl Acad Sci India Sect B Biol Sci 83:317–321. https://doi.org/10.1007/s40011-012-0138-6
Alonso Á, Camargo JA (2011) Subchronic toxic effects of fluoride ion on the survival and behaviour of the aquatic snail Potamopyrgus antipodarum (hydrobiidae, mollusca). Arch Environ Contam Toxicol 60:511–517. https://doi.org/10.1007/s00244-010-9562-x
Choudhary S, Rani M, Devika S (2019) Impact of fluoride on agriculture: a review on it’s sources, toxicity in plants and mitigation strategies. Int J Chem Stud 7:1675–1680
Chatterjee N, Sahu G, Bag AG (2020) Role of fluoride on soil, plant and human health: a review on its sources, toxicity and mitigation strategies. Int J Environ Clim Chang. https://doi.org/10.9734/ijecc/2020/v10i830220
Domingos M, Klumpp A, Rinaldi MCS (2003) Combined effects of air and soil pollution by fluoride emissions on Tibouchina pulchra Cogn., at Cubatão, SE Brazil, and their relations with aluminium. Plant Soil 249:297–308. https://doi.org/10.1023/A:1022800225753
Jarosz Z, Pitura K (2021) Fluoride toxicity limit—can the element exert a positive effect on plants? Sustain. https://doi.org/10.3390/su132112065
Elloumi N, Ben Abdallah F, Mezghani I (2005) Effect of flouride on almond seedlings in culture solution. Fluoride 38:193–198
de Lima e Silva IMH, Rodrigues AA, de Fátima Sales J, (2022) Fluoride effect indicators in Phaseolus vulgaris seeds and seedlings. PeerJ 10:1–19. https://doi.org/10.7717/peerj.13434
Mondal NK (2017) Effect of fluoride on photosynthesis, growth and accumulation of four widely cultivated rice (Oryza sativa L.) varieties in India. Ecotoxicol Environ Saf 144:36–44. https://doi.org/10.1016/j.ecoenv.2017.06.009
Fina BL, Lupo M, Dri N (2016) Comparison of fluoride effects on germination and growth of Zea mays, Glycine max and Sorghum vulgare. J Sci Food Agric 96:3679–3687. https://doi.org/10.1002/jsfa.7551
Bustingorri C, Balestrasse K, Lavado RS (2015) Effects of high arsenic and fluoride soil concentrations on soybean plants. Phyton-Int J Exp Bot 84:407–416. https://doi.org/10.32604/phyton.2015.84.407
Jha SK, Nayak AK, Sharma YK (2009) Fluoride toxicity effects in onion (Allium cepa L.) grown in contaminated soils. Chemosphere 76:353–356. https://doi.org/10.1016/j.chemosphere.2009.03.044
Riley HCF, Bleken MA, Abrahamsen S (2005) Effects of alternative tillage systems on soil quality and yield of spring cereals on silty clay loam and sandy loam soils in the cool, wet climate of central Norway. Soil Tillage Res 80:79–93. https://doi.org/10.1016/j.still.2004.03.005
Hong B, Joo R, Lee K (2016) Fluoride in soil and plant. Korean J Agric Sci 43:522–536. https://doi.org/10.7744/kjoas.20160054
Bhat N, Jain S, Asawa K (2015) Assessment of fluoride concentration of soil and vegetables in vicinity of zinc smelter, Debari. J Clin Diagn Res 9:ZC63–ZC66. https://doi.org/10.7860/JCDR/2015/13902.6667
Loganathan P, Gray CW, Hedley MJ, Roberts AHC (2006) Total and soluble fluorine concentrations in relation to properties of soils in New Zealand. Eur J Soil Sci 57:411–421. https://doi.org/10.1111/j.1365-2389.2005.00751.x
Wang W, Li R, Tan J (2002) Adsorption and leaching of fluoride in soils of China. Fluoride 35:122–129
Loganathan P, Hedley MJ, Grace ND (2008) Pasture soils contaminated with fertilizer-derived cadmium and fluorine: livestock effects. Rev Environ Contam Toxicol 192:29–66. https://doi.org/10.1007/978-0-387-71724-1_2
Moirana RL, Mkunda J, Perez MP (2021) The influence of fertilizers on the behavior of fluoride fractions in the alkaline soil. J Fluor Chem 250:109883. https://doi.org/10.1016/j.jfluchem.2021.109883
Yang J, yan, Wang M, Lu J, (2020) Fluorine in the environment in an endemic fluorosis area in Southwest China. Environ Res 184:109300. https://doi.org/10.1016/j.envres.2020.109300
Yin N, Li Y, Yang Y (2021) Human health risk assessment in aluminium smelting site: Soil fluoride bioaccessibility and relevant mechanism in simulated gastrointestinal tract. J Hazard Mater 416:125899. https://doi.org/10.1016/j.jhazmat.2021.125899
Mahmoudabadi ZS, Rashidi A, Maklavany DM (2022) Optimizing treatment of alcohol vinasse using a combination of advanced oxidation with porous α-Fe2O3 nanoparticles and coagulation-flocculation. Ecotoxicol Environ Saf 234:113354. https://doi.org/10.1016/j.ecoenv.2022.113354
Garg A, Mishra IM, Chand S (2010) Effectiveness of coagulation and acid precipitation processes for the pre-treatment of diluted black liquor. J Hazard Mater 180:158–164. https://doi.org/10.1016/j.jhazmat.2010.04.008
Ozairi N, Mousavi SA, Samadi MT (2020) Removal of fluoride from water using coagulation–flocculation procea comparative study. Desalin Water Treat 180:265–270. https://doi.org/10.5004/dwt.2020.25064
Wamalwa Wambu E, Frau F, Machunda R (2022) Water defluoridation methods applied in rural areas over the world. Fluoride. https://doi.org/10.5772/intechopen.105102
Teshome LY, Jim FC, Feleke ZB, David AS (2018) Performance enhancement of Nalgonda technique and pilot testing electrolytic defluoridation system for removing fluoride from drinking water in East Africa. African J Environ Sci Technol 12:357–369. https://doi.org/10.5897/ajest2018.2545
Pillai P, Dharaskar S, Pandian S, Panchal H (2021) Overview of fluoride removal from water using separation techniques. Environ Technol Innov 21:101246. https://doi.org/10.1016/j.eti.2020.101246
Kashyap SJ, Sankannavar R, Madhu GM (2021) Fluoride sources, toxicity and fluorosis management techniques—A brief review. J Hazard Mater Lett 2:100033. https://doi.org/10.1016/j.hazl.2021.100033
Saritha V, Srinivas N, Srikanth Vuppala NV (2017) Analysis and optimization of coagulation and flocculation process. Appl Water Sci 7:451–460. https://doi.org/10.1007/s13201-014-0262-y
Saxena K, Brighu U, Choudhary A (2018) Parameters affecting enhanced coagulation: a review. Environ Technol Rev 7:156–176. https://doi.org/10.1080/21622515.2018.1478456
Liu R, Zhu L, Gong W (2013) Effects of fluoride on coagulation performance of aluminum chloride towards Kaolin suspension. Colloids Surf A Physicochem Eng Asp 421:84–90. https://doi.org/10.1016/j.colsurfa.2012.12.047
Sun Y, Ren M, Zhu C (2016) UV-Initiated graft copolymerization of cationic chitosan-based flocculants for treatment of zinc phosphate-contaminated wastewater. Ind Eng Chem Res 55:10025–10035. https://doi.org/10.1021/acs.iecr.6b02855
Aoudj S, Drouiche N, Hecini M (2012) Coagulation as a post-treatment method for the defluoridation of photovoltaic cell manufacturing wastewater. Proced Eng 33:111–120. https://doi.org/10.1016/j.proeng.2012.01.1183
Zhang J, Brutus TE, Cheng J, Meng X (2017) Fluoride removal by Al, Ti, and Fe hydroxides and coexisting ion effect. J Environ Sci (China) 57:190–195. https://doi.org/10.1016/j.jes.2017.03.015
Dubey S, Agarwal M, Gupta AB (2018) Experimental investigation of Al-F species formation and transformation during coagulation for fluoride removal using alum and PACl. J Mol Liq 266:349–360. https://doi.org/10.1016/j.molliq.2018.06.080
Xiang Y, Xu H, Li C (2022) Impact of M. aeruginosa on fluoride removal efficiency of AlCl3and FeCl3coagulants and the mechanism. J Environ Chem Eng 10:107691. https://doi.org/10.1016/j.jece.2022.107691
Lim HR, Choo CM, Chong CH, Wong VL (2021) Optimization studies for water defluoridation with two-stage coagulation processes using new industrial-based chemical coagulants. J Water Process Eng 42:102179. https://doi.org/10.1016/j.jwpe.2021.102179
Li S, Liu M, Meng F (2021) Removal of F− and organic matter from coking wastewater by coupling dosing FeCl3 and AlCl3. J Environ Sci (China) 110:2–11. https://doi.org/10.1016/j.jes.2021.03.009
da Conceição VM, Yamaguchi NU, de Jesus BF, Bergamasco R (2021) Process performance combining natural coagulant Moringa oleifera Lam and ultrafiltration for groundwater defluoridation. Water Air Soil Pollut 232:322. https://doi.org/10.1007/s11270-021-05120-4
Wang X, Xu H, Wang D (2020) Mechanism of fluoride removal by AlCl3 and Al13: The role of aluminum speciation. J Hazard Mater 398:122987. https://doi.org/10.1016/j.jhazmat.2020.122987
Solanki YS, Agarwal M, Maheshwari K (2020) Investigation of plausible mechanism of the synthesized inorganic polymeric coagulant and its application toward fluoride removal from drinking water. Ind Eng Chem Res 59:9679–9687. https://doi.org/10.1021/acs.iecr.0c00760
Gan Y, Wang X, Zhang L (2019) Coagulation removal of fluoride by zirconium tetrachloride: Performance evaluation and mechanism analysis. Chemosphere 218:860–868. https://doi.org/10.1016/j.chemosphere.2018.11.192
Venditti F, Cuomo F, Giansalvo G (2018) Fluorides decontamination by means of aluminum polychloride based commercial coagulant. J Water Process Eng 26:182–186. https://doi.org/10.1016/j.jwpe.2018.10.012
Dahman Y, Yaser D, Kevin D, Timothy D, Andrew I (2017) Nanopolymers. Nanotechnol Funct Mater Eng. https://doi.org/10.1016/B978-0-323-51256-5.00006-X
Ali AF, Atwa SM, El-Giar EM (2017) Development of magnetic nanoparticles for fluoride and organic matter removal from drinking water. Elsevier Inc. https://doi.org/10.1016/B978-0-12-804300-4/00006-X
Matthews JA (2014) Chemical precipitation. Encycl Environ Chang 3:141–142. https://doi.org/10.4135/9781446247501.n624
Renuka P, Pushpanji K (2013) Review on defluoridation techniques of water. Int J Eng Sci 2:86–94
Wang L, Zhang Y, Sun N (2019) Precipitation methods using calcium-containing ores for fluoride removal in wastewater. Minerals 9:30–33. https://doi.org/10.3390/min9090511
Huang H, Liu J, Zhang P (2017) Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem Eng J 307:696–706. https://doi.org/10.1016/j.cej.2016.08.134
Lacson CFZ, Lu MC, Huang YH (2022) Calcium-based seeded precipitation for simultaneous removal of fluoride and phosphate: Its optimization using BBD-RSM and defluoridation mechanism. J Water Process Eng 47:102658. https://doi.org/10.1016/J.JWPE.2022.102658
Zhu J, Wang Y, Zhang Y, Huang K (2021) Defluoridation efficiency assessment of spiny hierarchical-structured calcium hydroxyphosphate particles. Colloids Surf Physicochem Eng Asp 627:127219. https://doi.org/10.1016/j.colsurfa.2021.127219
Xia L, Zhang W, Che J (2021) Stepwise removal and recovery of phosphate and fluoride from wastewater via pH-dependent precipitation: Thermodynamics, experiment and mechanism investigation. J Clean Prod 320:128872. https://doi.org/10.1016/j.jclepro.2021.128872
Hu X, Zhu F, Kong L, Peng X (2021) A novel precipitant for the selective removal of fluoride ion from strongly acidic wastewater: Synthesis, efficiency, and mechanism. J Hazard Mater 403:124039. https://doi.org/10.1016/j.jhazmat.2020.124039
Mohan R, Bora AJ, Dutta RK (2018) Fluoride removal from water by lime-sludge waste. Desalin Water Treat 112:19–33. https://doi.org/10.5004/dwt.2018.21918
Ezzeddine A, Bedoui A, Hannachi A, Bensalah N (2015) Removal of fluoride from aluminum fluoride manufacturing wastewater by precipitation and adsorption processes. Desalin Water Treat 54:2280–2292. https://doi.org/10.1080/19443994.2014.899515
Ben NA, Walha K, Puel F (2014) Precipitation and adsorption during fluoride removal from water by calcite in the presence of acetic acid. Desalin Water Treat 52:2231–2240. https://doi.org/10.1080/19443994.2013.799441
Rezai B, Allahkarami E (2021) Wastewater treatment processes—techniques, technologies, challenges faced, and alternative solutions. Soft Comput Tech Solid Waste Wastewater Manag. https://doi.org/10.1016/B978-0-12-824463-0.00004-5
Ilmasari D, Sahabudin E, Riyadi FA (2022) Future trends and patterns in leachate biological treatment research from a bibliometric perspective. J Environ Manage 318:115594. https://doi.org/10.1016/j.jenvman.2022.115594
Abujayyab MA, Hamouda M, Aly Hassan A (2022) Biological treatment of produced water: a comprehensive review and metadata analysis. J Pet Sci Eng 209:109914. https://doi.org/10.1016/J.PETROL.2021.109914
Roy M, Saha R (2020) Dyes and their removal technologies from wastewater: A critical review. Elsevier Inc 127–160. https://doi.org/10.1016/B978-0-12-819671-7.00006-3
Ge H, Batstone DJ, Mouiche M (2017) Nutrient removal and energy recovery from high-rate activated sludge processes – Impact of sludge age. Bioresour Technol 245:1155–1161. https://doi.org/10.1016/j.biortech.2017.08.115
Kumar R, Qureshi M, Vishwakarma DK (2022) A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud Chem Environ Eng 6:100219. https://doi.org/10.1016/j.cscee.2022.100219
Davidson J, Summerfelt S, Schrader KK, Good C (2019) Integrating activated sludge membrane biological reactors with freshwater RAS: Preliminary evaluation of water use, water quality, and rainbow trout Oncorhynchus mykiss performance. Aquac Eng 87:102022. https://doi.org/10.1016/j.aquaeng.2019.102022
Iorhemen OT, Hamza RA, Tay JH (2016) Membrane bioreactor ( MBR ) technology for wastewater treatment and reclamation : membrane fouling. Membranes. https://doi.org/10.3390/membranes6020033
Hoinkis J, Deowan SA, Panten V (2012) Membrane bioreactor (MBR) technology - a promising approach for industrial water reuse. Proced Eng 33:234–241. https://doi.org/10.1016/j.proeng.2012.01.1199
Mengesha A, Sahu O (2021) Relevance of membrane biological reactor in heavy metals recovery: diminutive review. Environ Qual Manag 31:441–452. https://doi.org/10.1002/tqem.21805
Xiao K, Liang S, Wang X (2019) Current state and challenges of full-scale membrane bioreactor applications: a critical review. Bioresour Technol 271:473–481. https://doi.org/10.1016/j.biortech.2018.09.061
Gao T, Xiao K, Zhang J (2022) Techno-economic characteristics of wastewater treatment plants retrofitted from the conventional activated sludge process to the membrane bioreactor process. Front Environ Sci Eng. https://doi.org/10.1007/s11783-021-1483-6
Konbr U, Bayoumi W, Ali MN, Shiba ASE (2022) Sustainability of Egyptian cities through utilizing sewage and sludge in softscaping and biogas production. Sustain 14:1–14. https://doi.org/10.3390/su14116675
Judd SJ (2016) The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem Eng J 305:37–45. https://doi.org/10.1016/j.cej.2015.08.141
Hao XD, Li J, Van Loosdrecht MCM, Li TY (2018) A sustainability-based evaluation of membrane bioreactors over conventional activated sludge processes. J Environ Chem Eng 6:2597–2605. https://doi.org/10.1016/j.jece.2018.03.050
Rajaei M, Nazif S (2022) Improving wastewater treatment plant performance based on effluent quality, operational costs, and reliability using control strategies for water and sludge lines. Process Saf Environ Prot 167:398–411. https://doi.org/10.1016/J.PSEP.2022.09.012
Sam T, Le Roes-Hill M, Hoosain N, Welz PJ (2022) Strategies for controlling filamentous bulking in activated sludge wastewater treatment plants: the old and the new. Water (Switzerland) 14:1–21. https://doi.org/10.3390/w14203223
Foladori P, Andreottola G, Ziglio G (2010) Sludge reduction technologies in wastewater treatment plants. IWA publishing. https://library.oapen.org/handle/20.500.12657/24365
Koul B, Yadav D, Singh S (2022) Insights into the domestic wastewater treatment (DWWT) Regimes: a review. Water (Switzerland) 14:21. https://doi.org/10.3390/w14213542
Kabir M, Suzuki M, Yoshimur N (2011) Excess sludge reduction in waste water treatment plants. Waste Water Treat Reutil. https://doi.org/10.5772/16082
Deowan SA, Bouhadjar SI, Hoinkis J (2015) Membrane bioreactors for water treatment. Adv Membr Technol Water Treat Mater Process Appl. https://doi.org/10.1016/B978-1-78242-121-4.00005-8
Azanaw A, Birlie B, Teshome B, Jemberie M (2022) Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud Chem Environ Eng 6:100230. https://doi.org/10.1016/j.cscee.2022.100230
Farmer JK (2017) Wastewater treatment technologies. Pollut Eng 23:249–293. https://doi.org/10.1016/B978-0-12-811989-1.00012-9
Lian Y, Coggins LX, Hay J (2022) Effect of attached growth on treatment performance in waste stabilization ponds. Water (Switzerland) 14:20. https://doi.org/10.3390/w14203245
Gupta S, Mittal Y, Panja R (2021) Conventional wastewater treatment technologies. Curr Dev Biotechnol Bioeng Strateg Perspect Solid Waste Wastewater Manag. https://doi.org/10.1016/B978-0-12-821009-3.00012-9
Mahapatra S, Samal K, Dash RR (2022) Waste Stabilization Pond (WSP) for wastewater treatment: a review on factors, modelling and cost analysis. J Environ Manage 308:114668. https://doi.org/10.1016/J.JENVMAN.2022.114668
Kadri SUT et al (2021) Effect of attached growth on treatment performance in waste stabilization ponds. Water. https://doi.org/10.3390/w14203245
Khatri N, Vyas AK, Abdul-Qawy ASH, Rene ER (2022) Artificial neural network based models for predicting the effluent quality of a combined upflow anaerobic sludge blanket and facultative pond: Performance evaluation and comparison of different algorithms. Environ Res. https://doi.org/10.1016/J.ENVRES.2022.114843
Alexiou GE, Mara DD (2003) Anaerobic waste stabilization ponds. Appl Biochem Biotechnol 109:241–252
Butler E, Hung Y-T, Suleiman Al Ahmad M (2017) Oxidation pond for municipal wastewater treatment. Appl Water Sci 7:31–51. https://doi.org/10.1007/s13201-015-0285-z
Alavi J, Ansari S (2022) Kinetic models evaluation for chemical organic matter removal prediction in a full-scale primary facultative pond treating municipal wastewater. Water Sci Technol 85:1720–1735. https://doi.org/10.2166/wst.2022.074
Xu J, Huang Y, Li Z (2022) Demonstration study of bypass stabilization pond system in the treatment of eutrophic water body. Water Sci Technol 85:2601–2612. https://doi.org/10.2166/wst.2022.130
de Mendonça HV, Otenio MH, Lomeu AA, Rita AVS (2022) Post-treatment of an aerated facultative pond with constructed wetland: First two years of operation in a dairy industry. Ecol Eng 179:106623. https://doi.org/10.1016/J.ECOLENG.2022.106623
Nam TS, Van Thao H, Luan NT (2022) Optimizing hydraulic retention time and area of biological settling ponds for super-intensive shrimp wastewater treatment systems. Water (Switzerland) 14:6. https://doi.org/10.3390/w14060932
Kaid M, Ali AE, Shamsan AQS (2022) Efficiency of maturation oxidation ponds as a post-treatment technique of wastewater. Curr Chem Lett 11:415–422. https://doi.org/10.5267/j.ccl.2022.4.005
Passos RG, Dias DFC, von Sperling M (2022) Simple mid-depth transverse baffles to improve bacterial disinfection in a shallow maturation pond–performance evaluation and CFD simulation. Environ Technol (United Kingdom) 43:1437–1445. https://doi.org/10.1080/09593330.2020.1832584
Dias DFC, Von Sperling M (2017) Solar radiation (PAR, UV-A, UV-B) penetration in a shallow maturation pond operating in a tropical climate. Water Sci Technol 76:182–191. https://doi.org/10.2166/wst.2017.203
Liu J, Su J, Ali A (2022) Potential of a novel facultative anaerobic denitrifying Cupriavidus sp. W12 to remove fluoride and calcium through calcium bioprecipitation. J Hazard Mater 423:126976. https://doi.org/10.1016/J.JHAZMAT.2021.126976
Ali A, Wu Z, Li M, Su J (2021) Carbon to nitrogen ratios influence the removal performance of calcium, fluoride, and nitrate by Acinetobacter H12 in a quartz sand-filled biofilm reactor. Bioresour Technol 333:125154. https://doi.org/10.1016/j.biortech.2021.125154
Wang Z, Su J, Ali A (2021) Microbially induced calcium precipitation based simultaneous removal of fluoride, nitrate, and calcium by Pseudomonas sp. WZ39: mechanisms and nucleation pathways. J Hazard Mater 416:125914. https://doi.org/10.1016/j.jhazmat.2021.125914
Feng SJ, Zhen WZ, Lin HT (2020) A new technology for simultaneous calcium–nitrate and fluoride removal in the biofilm reactor. J Hazard Mater 399:122846. https://doi.org/10.1016/j.jhazmat.2020.122846
Demirel S, Uyanık İ (2019) Simultaneous fluoride and nitrate removal from drinking water using mixotrophic denitrification processes in a fixed bed column reactor. Desalin Water Treat 164:56–61. https://doi.org/10.5004/dwt.2019.24474
Biswas G, Thakurta SG, Chakrabarty J (2018) Evaluation of fluoride bioremediation and production of biomolecules by living cyanobacteria under fluoride stress condition. Ecotoxicol Environ Saf 148:26–36. https://doi.org/10.1016/j.ecoenv.2017.10.019
Mukherjee S, Sahu P, Halder G (2017) Microbial remediation of fluoride-contaminated water via a novel bacterium Providencia vermicola (KX926492). J Environ Manage 204:413–423. https://doi.org/10.1016/J.JENVMAN.2017.08.051
Gouider M, Mlaik N, Feki M, Sayadi S (2011) Integrated physicochemical and biological treatment process for fluoride and phosphorus removal from fertilizer plant wastewater. Water Environ Res 83:731–738. https://doi.org/10.2175/106143011x12928814444772
Mekonen A, Kumar P, Kumar A (2001) Integrated biological and physiochemical treatment process for nitrate and fluoride removal. Water Res 35:3127–3136. https://doi.org/10.1016/S0043-1354(01)00019-7
Ezugbe EO, Rathilal S (2020) Membrane technologies in wastewater treatment: a review. Membranes (Basel) 10:5. https://doi.org/10.3390/membranes10050089
Damtie MM, Woo YC, Kim B (2019) Removal of fluoride in membrane-based water and wastewater treatment technologies: performance review. J Environ Manage 251:109524. https://doi.org/10.1016/j.jenvman.2019.109524
Koriem OA, Showman MS, El-Shazly AH, Elkady MF (2022) Cellulose acetate/polyvinylidene fluoride based mixed matrix membranes impregnated with UiO-66 nano-MOF for reverse osmosis desalination. Cellulose. https://doi.org/10.1007/s10570-022-04889-9
Odabaşı Ç, Dologlu P, Gülmez F (2022) Investigation of the factors affecting reverse osmosis membrane performance using machine-learning techniques. Comput Chem Eng 159:107669. https://doi.org/10.1016/J.COMPCHEMENG.2022.107669
Ketharani J, Hansima MACK, Indika S (2022) A comparative study of community reverse osmosis and nanofiltration systems for total hardness removal in groundwater. Groundw Sustain Dev 18:100800. https://doi.org/10.1016/J.GSD.2022.100800
Bejaoui I, Mnif A, Hamrouni B (2014) Performance of reverse osmosis and nanofiltration in the removal of fluoride from model water and metal packaging industrial effluent. Sep Sci Technol 49:1135–1145. https://doi.org/10.1080/01496395.2013.878956
Shen J, Schäfer A (2014) Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere 117:679–691. https://doi.org/10.1016/j.chemosphere.2014.09.090
Qiu Y, Ren LF, Shao J (2022) An integrated separation technology for high fluoride-containing wastewater treatment: Fluoride removal, membrane fouling behavior and control. J Clean Prod 349:131225. https://doi.org/10.1016/J.JCLEPRO.2022.131225
Ayala LIM, Paquet M, Janowska K (2018) Water defluoridation: nanofiltration vs membrane distillation. Ind Eng Chem Res 57:14740–14748. https://doi.org/10.1021/acs.iecr.8b03620
Lu NC, Liu JC (2010) Removal of phosphate and fluoride from wastewater by a hybrid precipitation-microfiltration process. Sep Purif Technol 74:329–335. https://doi.org/10.1016/j.seppur.2010.06.023
Guigui C, Roucha JC, Durand-Bourlierb L (2002) Impact of coagulation conditions on the in-line coagulationKJF process for drinking water production. Desalination 147:95–100
Zevenhuizen E, Reed VA, Rahman MS, Gagnon GA (2015) In-line coagulation to reduce high-pressure membrane fouling in an integrated membrane system: a case study. Desalin Water Treat 56:1987–1998. https://doi.org/10.1080/19443994.2014.958106
da Conceição VM, Ambrosio Ugri MCB, Silveira C (2015) Removal of excess fluoride from groundwater using natural coagulant Moringa oleifera Lam and microfiltration. Can J Chem Eng 93:37–45. https://doi.org/10.1002/cjce.22101
Chen Y, Kong H, Guo L, Wei G (2022) Biomimetic organic-inorganic hybrid membranes for removal of fluoride ions. Materials (Basel) 15:1–13. https://doi.org/10.3390/ma15103457
Owusu-Agyeman I, Reinwald M, Jeihanipour A, Schäfer AI (2019) Removal of fluoride and natural organic matter from natural tropical brackish waters by nanofiltration/reverse osmosis with varying water chemistry. Chemosphere 217:47–58. https://doi.org/10.1016/J.CHEMOSPHERE.2018.10.135
Grzegorzek M, Majewska-Nowak K (2018) The use of micellar-enhanced ultrafiltration (MEUF) for fluoride removal from aqueous solutions. Sep Purif Technol 195:1–11. https://doi.org/10.1016/J.SEPPUR.2017.11.022
Gaikwad MS, Balomajumder C (2017) Simultaneous rejection of fluoride and Cr(VI) from synthetic fluoride-Cr(VI) binary water system by polyamide flat sheet reverse osmosis membrane and prediction of membrane performance by CFSK and CFSD models. J Mol Liq 234:194–200. https://doi.org/10.1016/j.molliq.2017.03.073
Shen J, Richards BS, Schäfer AI (2016) Renewable energy powered membrane technology: Case study of St. Dorcas borehole in Tanzania demonstrating fluoride removal via nanofiltration/reverse osmosis. Sep Purif Technol 170:445–452. https://doi.org/10.1016/j.seppur.2016.06.042
Ezzeddine A, Meftah N, Hannachi A (2015) Removal of fluoride from an industrial wastewater by a hybrid process combining precipitation and reverse osmosis. Desalin Water Treat 55:2618–2625. https://doi.org/10.1080/19443994.2014.959737
Shen J, Schäfer AI (2015) Factors affecting fluoride and natural organic matter (NOM) removal from natural waters in Tanzania by nanofiltration/reverse osmosis. Sci Total Environ 527–528:520–529. https://doi.org/10.1016/J.SCITOTENV.2015.04.037
Campione A, Gurreri L, Ciofalo M (2018) Electrodialysis for water desalination: a critical assessment of recent developments on process fundamentals, models and applications. Desalination 434:121–160. https://doi.org/10.1016/j.desal.2017.12.044
Lacson CFZ, Lu MC, Huang YH (2021) Fluoride-containing water: A global perspective and a pursuit to sustainable water defluoridation management -An overview. J Clean Prod 280:124236. https://doi.org/10.1016/j.jclepro.2020.124236
Galama AH, Saakes M, Bruning H (2014) Seawater predesalination with electrodialysis. Desalination 342:61–69. https://doi.org/10.1016/J.DESAL.2013.07.012
Gangani N, Joshi VC, Sharma S, Bhattacharya A (2022) Fluoride contamination in water: remediation strategies through membranes. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2022.100751
Bhadja V, Trivedi JS, Chatterjee U (2016) Efficacy of polyethylene Interpolymer membranes for fluoride and arsenic ion removal during desalination of water: Via electrodialysis. RSC Adv 6:67118–67126. https://doi.org/10.1039/c6ra11450d
Gmar S, Ben Salah Sayadi I, Helali N (2015) Desalination and defluoridation of tap water by electrodialysis. Environ Process 2:S209–S222. https://doi.org/10.1007/s40710-015-0112-4
Bagastyo AY, Anggrainy AD, Nindita CS (2017) Electrodialytic removal of fluoride and calcium ions to recover phosphate from fertilizer industry wastewater. Sustain Environ Res 27:230–237. https://doi.org/10.1016/j.serj.2017.06.002
Tekinalp Ö, Zimmermann P, Burheim OS, Deng L (2022) Designing monovalent selective anion exchange membranes for the simultaneous separation of chloride and fluoride from sulfate in an equimolar ternary mixture. J Memb Sci 666:121148. https://doi.org/10.1016/j.memsci.2022.121148
Yi PC, Feng LH, Huan QH (2020) Evaluation of the feasibility of short-term electrodialysis for separating naturally occurring fluoride from instant brick tea infusion. J Sci Food Agric 100:168–176. https://doi.org/10.1002/jsfa.10011
Yangbo Q, Long-F R, Lei X, Changmei Z, Jiahui S, Yan Z (2022) Recovery of fluoride-rich and silica-rich wastewaters as valuable resources: a resource capture ultrafiltration-bipolar membrane electrodialysis-based closed-loop process. Environ Sci Technol. https://doi.org/10.1021/acs.est.2c04704
Grzegorzek M, Majewska-Nowak K, Ahmed AE (2020) Removal of fluoride from multicomponent water solutions with the use of monovalent selective ion-exchange membranes. Sci Total Environ 722:137681. https://doi.org/10.1016/j.scitotenv.2020.137681
Djouadi Belkada F, Kitous O, Drouiche N (2018) Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Sep Purif Technol 204:108–115. https://doi.org/10.1016/j.seppur.2018.04.068
Sharma PP, Yadav V, Maru PD (2018) Mitigation of fluoride from brackish water via electrodialysis: an environmentally friendly process. ChemistrySelect 3:779–784. https://doi.org/10.1002/slct.201701170
Grzegorzek M, Majewska-Nowak K (2017) The influence of organic matter on fluoride removal efficiency during the electrodialysis process. Desalin Water Treat 69:153–162. https://doi.org/10.5004/dwt.2017.20327
Magnisali E, Yan Q, Vayenas DV (2022) Electrocoagulation as a revived wastewater treatment method-practical approaches: a review. J Chem Technol Biotechnol 97:9–25. https://doi.org/10.1002/jctb.6880
Chandraker N, Chaudhari PK, Jyoti G (2021) Removal of fluoride from water by electrocoagulation using Mild Steel electrode. J Indian Chem Soc 98:100026. https://doi.org/10.1016/j.jics.2021.100026
Das PP, Sharma M, Purkait MK (2022) Recent progress on electrocoagulation process for wastewater treatment: a review. Sep Purif Technol 292:121058. https://doi.org/10.1016/j.seppur.2022.121058
Govindan K, Raja M, Uma Maheshwari S (2015) Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+ and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes. J Environ Chem Eng 3:1784–1793. https://doi.org/10.1016/j.jece.2015.06.014
Goren AY, Recepoğlu YK, Khataee A (2022) Language of response surface methodology as an experimental strategy for electrochemical wastewater treatment process optimization. Artif Intell Data Sci Environ Sens. https://doi.org/10.1016/B978-0-323-90508-4.00009-5
Priya T, Mishra BK, Prasad MNV (2020) Physico-chemical techniques for the removal of disinfection by-products precursors from water. Disinfect By-products Drink Water. https://doi.org/10.1016/B978-0-08-102977-0.00002-0
Nyangi MJ, Chebude Y, Kilulya KF, Salim CJ (2021) Comparative study on adsorption isotherm and kinetics of defluoridation using aluminum and iron electrodes in electrocoagulation. Chem Africa 4:391–398. https://doi.org/10.1007/s42250-021-00228-w
Sandoval MA, Fuentes R, Thiam A, Salazar R (2021) Arsenic and fluoride removal by electrocoagulation process: a general review. Sci Total Environ 753:142108. https://doi.org/10.1016/j.scitotenv.2020.142108
López-Guzmán M, Alarcón-Herrera MT, Irigoyen-Campuzano JR (2019) Simultaneous removal of fluoride and arsenic from well water by electrocoagulation. Sci Total Environ 678:181–187. https://doi.org/10.1016/j.scitotenv.2019.04.400
Kang J, Li J, Ma C (2022) Goethite/montmorillonite adsorption coupled with electrocoagulation for improving fluoride removal from aqueous solutions. RSC Adv 12:7475–7484. https://doi.org/10.1039/d1ra08503d
Levchuk I, Rueda Márquez JJ, Sillanpää M (2018) Removal of natural organic matter (NOM) from water by ion exchange—a review. Chemosphere 192:90–104. https://doi.org/10.1016/J.CHEMOSPHERE.2017.10.101
Hailu Y, Tilahun E, Brhane A (2019) Ion exchanges process for calcium, magnesium and total hardness from ground water with natural zeolite. Groundw Sustain Dev 8:457–467. https://doi.org/10.1016/j.gsd.2019.01.009
Sairam Sundaram C, Meenakshi S (2009) Fluoride sorption using organic-inorganic hybrid type ion exchangers. J Colloid Interface Sci 333:58–62. https://doi.org/10.1016/j.jcis.2009.01.022
Singh S, German M, Chaudhari S, Sengupta AK (2020) Fluoride removal from groundwater using Zirconium Impregnated Anion Exchange Resin. J Environ Manage 263:110415. https://doi.org/10.1016/j.jenvman.2020.110415
Rodríguez-Iglesias J, Alcalá L, Megido L, Castrillón L (2022) Removal of fluoride from coke wastewater by aluminum doped chelating ion-exchange resins: a tertiary treatment. Environ Sci Pollut Res 29:8705–8715. https://doi.org/10.1007/s11356-021-16299-8
Wan K, Huang L, Yan J (2021) Removal of fluoride from industrial wastewater by using different adsorbents: a review. Sci Total Environ 773:145535. https://doi.org/10.1016/j.scitotenv.2021.145535
Ibrahim Q, Creedon L, Gharbia S (2022) A literature review of modelling and experimental studies of water treatment by adsorption processes on nanomaterials. Membranes (Basel) 12:4. https://doi.org/10.3390/membranes12040360
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988. https://doi.org/10.1016/j.jece.2020.103988
Vieira Y, Netto MS, Lima ÉC (2022) An overview of geological originated materials as a trend for adsorption in wastewater treatment. Geosci Front 13:101150. https://doi.org/10.1016/j.gsf.2021.101150
He J, Yang Y, Wu Z (2020) Review of fluoride removal from water environment by adsorption. Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104516
Zhu F, Guo Z, Hu X (2020) Fluoride removal efficiencies and mechanism of schwertmannite from KMnO4/MnO2–Fe(II) processes. J Hazard Mater 397:122789. https://doi.org/10.1016/J.JHAZMAT.2020.122789
Cai H, Chen G, Peng C (2015) Enhanced removal of fluoride by tea waste supported hydrous aluminium oxide nanoparticles: Anionic polyacrylamide mediated aluminium assembly and adsorption mechanism. RSC Adv 5:29266–29275. https://doi.org/10.1039/c5ra01560j
López Valdivieso A, Reyes Bahena JL, Song S, Herrera Urbina R (2006) Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. J Colloid Interface Sci 298:1–5. https://doi.org/10.1016/j.jcis.2005.11.060
Han M, Zhang J, Hu Y, Han R (2019) Preparation of novel magnetic microspheres with the la and Ce-bimetal oxide shell for excellent adsorption of fluoride and phosphate from solution. J Chem Eng Data 64:3641–3651. https://doi.org/10.1021/acs.jced.9b00434
Kumari U, Siddiqi H, Bal M, Meikap BC (2020) Calcium and zirconium modified acid activated alumina for adsorptive removal of fluoride: Performance evaluation, kinetics, isotherm, characterization and industrial wastewater treatment. Adv Powder Technol 31:2045–2060. https://doi.org/10.1016/j.apt.2020.02.035
Gasparotto JM, Roth D, de Perilli AL (2021) A novel Fe-Al-La trioxide composite: Synthesis, characterization, and application for fluoride ions removal from the water supply. J Environ Chem Eng 9:106350. https://doi.org/10.1016/j.jece.2021.106350
Wang M, Yu X, Yang C (2017) Removal of fluoride from aqueous solution by Mg-Al-Zr triple-metal composite. Chem Eng J 322:246–253. https://doi.org/10.1016/J.CEJ.2017.03.155
Yadav KK, Gupta N, Kumar V (2018) A review of emerging adsorbents and current demand for defluoridation of water: bright future in water sustainability. Environ Int 111:80–108. https://doi.org/10.1016/j.envint.2017.11.014
Issabayeva G, Wong SH, Pang CY (2022) Fluoride removal by low-cost palm shell activated carbon modified with prawn shell chitosan adsorbents. Int J Environ Sci Technol 19:3731–3740. https://doi.org/10.1007/s13762-021-03448-2
Jeyaseelan A, Mezni A, Viswanathan N (2022) Facile hydrothermal fabrication of functionalized multi-layer graphene oxide encapsulated chitosan beads for enriched fluoride adsorption. J Appl Polym Sci 139:9. https://doi.org/10.1002/app.51703
Choi MY, Lee CG, Park SJ (2022) Conversion of organic waste to novel adsorbent for fluoride removal: efficacy and mechanism of fluoride adsorption by calcined venerupis philippinarum shells. Water Air Soil Pollut. https://doi.org/10.1007/s11270-022-05757-9
Araga R, Soni S, Sharma CS (2017) Fluoride adsorption from aqueous solution using activated carbon obtained from KOH-treated jamun (Syzygium cumini) seed. J Environ Chem Eng 5:5608–5616. https://doi.org/10.1016/j.jece.2017.10.023
Gao Y, Li M, Ru Y, Fu J (2021) Fluoride removal from water by using micron zirconia/zeolite molecular sieve: characterization and mechanism. Groundw Sustain Dev 13:100567. https://doi.org/10.1016/j.gsd.2021.100567
Kuśmierek K, ͆wia¸tkowski A, (2015) The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React Kinet Mech Catal 116:261–271. https://doi.org/10.1007/s11144-015-0889-1
Papari F, Najafabadi PR, Ramavandi B (2017) Fluoride ion removal from aqueous solution, groundwater, and seawater by granular and powdered Conocarpus erectus biochar. Desalin Water Treat 65:375–386. https://doi.org/10.5004/dwt.2017.20271
Pfeifer A, Škerget M (2020) A review: a comparison of different adsorbents for removal of Cr (VI), Cd (II) and Ni (II). Turkish J Chem 44:859–883. https://doi.org/10.3906/KIM-2002-21
Gómez-Hortigüela L, Pérez-Pariente J, García R (2013) Natural zeolites from Ethiopia for elimination of fluoride from drinking water. Sep Purif Technol 120:224–229. https://doi.org/10.1016/j.seppur.2013.10.006
Cai Q, Turner BD, Sheng D, Sloan S (2015) The kinetics of fluoride sorption by zeolite: effects of cadmium, barium and manganese. J Contam Hydrol 177–178:136–147. https://doi.org/10.1016/j.jconhyd.2015.03.006
Iriel A, Bruneel SP, Schenone N, Cirelli AF (2018) The removal of fluoride from aqueous solution by a lateritic soil adsorption: kinetic and equilibrium studies. Ecotoxicol Environ Saf 149:166–172. https://doi.org/10.1016/j.ecoenv.2017.11.016
Chandraker N, Jyoti G, Thakur RS, Chaudhari PK (2020) Removal of fluoride using fly ash adsorbent. IOP Conf Ser Earth Environ Sci 597:1. https://doi.org/10.1088/1755-1315/597/1/012009
Shanker AS, Srinivasulu D, Pindi PK (2020) A study on bioremediation of fluoride-contaminated water via a novel bacterium Acinetobacter sp. (GU566361) isolated from potable water. Results Chem 2:100070. https://doi.org/10.1016/j.rechem.2020.100070
Mondal NK (2017) Natural banana (Musa acuminate) peel: an unconventional adsorbent for removal of fluoride from aqueous solution through batch study. Water Conserv Sci Eng 1:223–232. https://doi.org/10.1007/s41101-016-0015-x
Diop SN, Dieme MM, Diallo MA, Diawara CK (2022) Fluoride excess removal from brackish drinking water in senegal by using KSF and K10 montmorillonite clays. J Water Resour Prot 14:21–34. https://doi.org/10.4236/jwarp.2022.141002
Yapo NS, Aw S, Briton BGH (2022) Removal of fluoride in groundwater by adsorption using hydroxyapatite modified Corbula trigona shell powder. Chem Eng J Adv. https://doi.org/10.1016/j.ceja.2022.100386
Dhanasekaran P, Sahu O (2021) Arsenate and fluoride removal from groundwater by sawdust impregnated ferric hydroxide and activated alumina (SFAA). Groundw Sustain Dev 12:100490. https://doi.org/10.1016/j.gsd.2020.100490
Shao S, Ma B, Chen Y (2021) Behavior and mechanism of fluoride removal from aqueous solutions by using synthesized CaSO4·2H2O nanorods. Chem Eng J 426:131364. https://doi.org/10.1016/j.cej.2021.131364
Hashemkhani M, Rezvani Ghalhari M, Bashardoust P (2022) Fluoride removal from aqueous solution via environmentally friendly adsorbent derived from seashell. Sci Rep 12:1–13. https://doi.org/10.1038/s41598-022-13756-3
Iwar RT, Ogedengbe K, Ugwudike BO (2022) Groundwater fluoride removal by novel activated carbon/aluminium oxide composite derived from raffia palm shells: Optimization of batch operations and field-scale point of use system evaluation. Results Eng 14:100407. https://doi.org/10.1016/j.rineng.2022.100407
Akhbari-Shad MH, Amini-Fazl A, Amini-Fazl MS et al (2023) Polymeric hydrogel for removing water pollutants: optimizing synthesis conditions and investigating antibacterial activity. Chem Africa 6:153–161. https://doi.org/10.1007/s42250-022-00464-8
Bishayee B, Ruj B, Chakrabortty S, Nayak J (2021) Facile synthesis, characterization and application of heterogeneous Al@Si materials for adsorptive mitigation of fluoride: optimization and cost analysis. Environ Nanotechnol Monit Manag 16:100490. https://doi.org/10.1016/j.enmm.2021.100490
Mena VF, Betancor-Abreu A, González S (2019) Fluoride removal from natural volcanic underground water by an electrocoagulation process: Parametric and cost evaluations. J Environ Manage 246:472–483. https://doi.org/10.1016/j.jenvman.2019.05.147
Bhagawan D, Saritha P, Shankaraiah G, Himabindu V (2019) Fluoride removal from groundwater using hybrid cylindrical electrocoagulation reactor. J Water Chem Technol 41:164–169. https://doi.org/10.3103/s1063455x19030056
El Diwani G, Amin SK, Attia NK, Hawash SI (2022) Fluoride pollutants removal from industrial wastewater. Bull Natl Res Cent 46:1. https://doi.org/10.1186/s42269-022-00833-w
Elazhar F, Tahaikt M, Achatei A (2009) Economical evaluation of the fluoride removal by nanofiltration. Desalination 249:154–157. https://doi.org/10.1016/j.desal.2009.06.017
Acknowledgements
The author is grateful to the Department of Civil Engineering and Chemical Engineering, Chandigarh University for their assistance with the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patrick, M., Sahu, O. Origins, Mechanisms, and Remedies of Fluoride Ions from Ground and Surface Water: A Review. Chemistry Africa 6, 2737–2768 (2023). https://doi.org/10.1007/s42250-023-00694-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00694-4