Abstract
Heavy metal contamination emanating from increased industrial and anthropogenic activities poses considerable environmental risks to water resources, aquatic biodiversity, and human health. These metals are major sources of various unfavorable environmental changes and human health hazards. This research investigated the potential contamination level and health risk assessment of heavy metals (Fe, Zn, Ni, Cu and Cr) in five highly edible fishes (O. microlepis, C. gariepinus, O.niloticus, R. moorii,B. meridionalis) from Lake Nyasa. The results indicated that gills contained higher amounts of heavy metals compared to muscles. The levels of Fe and Zn were higher in the gills of C. gariepinus and O.niloticus than O. microlepis, R. moorii, and B. meridionalis. Similarly, amounts of Cu and Cr were higher in the gills of C. gariepinus and O. microlepis. The general trend of heavy metal concentrations accumulated in selected fish species was Fe > Zn > Ni > Cu > Cr. The amounts of heavy metals found in the fish samples were within acceptable limits set by FAO and WHO. Furthermore, the risk assessments indicated that the current exposure levels are unlikely to cause serious health risks. However, the progressive monitoring of levels of heavy metals in various aquatic organisms from Lake Nyasa is important for the continued protection of human health against hazardous health effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal contamination is regarded as a serious global concern due to the non-biodegradability and environmental persistence of these metals [1]. Most heavy metals cause environmental pollution when mixed with different environmental components such as soil, water, and organisms. Thus, the exposure to this type of environmental pollution is mainly through the food chain. Heavy metals namely, cadmium (Cd), lead (Pb), nickel (Ni), chromium (Cr), copper (Cu), and zinc (Zn) are classified as potentially toxic elements and the most common environmental pollutants [2]. In a polluted environment, the concentration of metals may exceed the permissible levels suitable for human consumption and cause serious health effects. For example, Fe might can cause irregular heartbeat, cirrhosis, enlarged liver, and also damage to joints [3, 4]. Zn might cause nausea, vomiting, skin changes, liver damage, decrease levels of lipoprotein cholesterol and inhibit growth in humans [5]. Acute symptoms of Cu contamination by ingestion include vomiting and gastrointestinal distress, while long-term accumulation can damage the liver and kidney [5]. Ingestion of a significant amount of Ni may cause immediate health problems like lung cancer, respiratory failure, and birth defects [6]. Excessive amounts of Cr in the body can cause occasional irregular heartbeats, allergic reactions, kidney failure, and liver damage [7].
These heavy metals can be introduced into aquatic systems by various natural and human activities. The later has a potential for greater pollution effect because larger amounts of metals in more mobile forms could be released [8]. Anthropogenic activities such as domestic, industrial, and agricultural activities produce and use significant amounts of these metals, with aquatic systems, such as lakes and rivers, serving as the main receptors [9]. Fish can accumulate considerable amounts of heavy metals in their tissues by direct absorption through gills, skin, or ingestion of contaminated food or particles [10]. Thus, concentrations of the heavy metals in fish can reflect the amounts present in sediments and water from which they are sourced [11, 12]. However, the accumulation levels depend on exposure time, fish species, and tissue types [13]. Thus, fish are useful indicators of heavy metal pollution in aquatic environments.
Fish is an important part of human diet because of its nutritional qualities such as high protein content, omega-3 fatty acids, and vitamins. However, the consumption of heavy metal contaminated fish can be a major source of human exposure to heavy metals. Thus, the accumulation of heavy metals in fish tissues may cause considerable potential health risks to humans. The international organizations Food and Agriculture Organization (FAO) and world health organization (WHO) have limits on the amount of heavy metals in fish (Table 1). Renal failure, damage to the liver, heart disease, and even death are among the outcomes of heavy metal accumulation in human bodies [14, 15]. Areas with increased human settlement, traffic, and agricultural activities have been reported to experience elevated concentrations of heavy metals in aquatic systems [16, 17]. Consequently, fish from aquatic systems receiving industrial effluents are known to be unsuitable for human consumption [18, 19]. Therefore, the assessment of heavy metal contamination in various fish species is of significance.
Lake Nyasa is estimated to contain about 5% of the world's freshwater fish species with new species being regularly discovered [20]. Consequently, the lake has gained international recognition because it contains more varieties of fish species compared to any other freshwater lakes in the world. However, Lake Nyasa is vulnerable to environmental pollution due to increased anthropogenic activities, such as deforestation, land degradation, agriculture, and coal mining, that are conducted near the Lake or along the rivers that discharge their effluents into the lake [21]. As a result, it is estimated that nine percent of fish species in Lake Nyasa are at high risk of extinction [22]. This causes significant threats to the loss of biodiversity and local food security. Fish species that depend on the river water for spawning such as Labeo mesops and Opsaridium microlepis are critically endangered due to increased pollution [23]. Despite increased anthropogenic activities that are likely to cause heavy metal pollution, there is limited information on potential health risks associated with the consumption of fish from Lake Nyasa. Thus, in this study, we report the levels of heavy metals in selected highly edible fish species from Lake Nyasa and the potential for associated health risks.
2 Materials and Method
2.1 Study Area
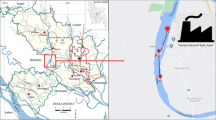
Lake Nyasa (Fig. 1) is the third deepest freshwater lake in the world. It has a north–south length of 363 km, width of 16 to 18 km, and a total size of 2604 km2. The lake region experiences a dry season between May and November and a wet season between December and April with the temperature ranging between. 22 °C and 27 °C [26]. The lake serves as an important source of protein for more than 1.6 million people, in Tanzania, Malawi, and Mozambique [20].
2.2 Collection of Fish Samples
Fish samples (n = 150) consisting of five different species, Clarias gariepinus (C. gariepinus), Opsaridium microlepis (O. microlepis), Raiamas moorii (R. moorii), Bagrus meridionalis (B. meridionalis), Oreochromis niloticus (O. niloticus), (Table 2) were collected from local fishermen at identified sampling sites that serve as major sources of fish from Lake Nyasa. Samples were collected during a wet season (March 2022) in which most of the fish species were readily available in the sampling areas. Collected fish samples were preserved in a cool box at 4 ℃ while being transported to the laboratory for heavy metal analysis.
2.3 Sample Preparation and Analysis
The thawed fish samples were dissected using a stainless-steel knife. Muscles and gills from the fish were dried in an oven at 80 ℃ for 72 h. Dried gill and muscle samples were ground using a pestle and mortar to produce a powder. Sample powder (0.5 g) was weighed and transferred into a conical flask containing a 3:1 mixture of concentrated nitric acid (68%) and hydrogen peroxide (30% w/w) [27]. The resulting mixture was slowly boiled at 100 ℃ in a water bath placed in a fume chamber for 1 h until the solution became clear. The mixture was allowed to cool to room temperature (30 ℃), then filtered through filter paper (Whatman Grade 1) and diluted with deionized water to 25 mL in a volumetric flask. The resultant solutions were analyzed for Fe, Zn, Ni, Cu, and Cr using an iCE 3000 Series Atomic Absorption Spectrophotometer (Thermo Fisher Scientific USA). Standard solutions for each metal were used to prepare calibration curves. Linear calibration curves were obtained with linear regression values of R2 > 0.989 ± 0.10. The limit of detection (LOD) for each metal were: Fe 0.05 mg/kg, Zn 0.03 mg/kg, Ni 0.0016 mg/kg, Cu 0.065 mg/kg, and Cr 0.025 mg/kg. Concentration of heavy metals is expressed in mg/kg dry weight.
2.4 Quality Assurance
To avoid potential contamination, all of the glassware used in the experiments were thoroughly cleaned by soaking in 10% nitric acid for 24 h and then rinsing with deionized water (18.2 M cm) before use. Throughout the analysis, analytical grade reagents from Sigma-Aldrich, (UK), were used. The reagent blank was analyzed for each batch of samples. The analysis was performed in triplicate and average values were reported for each sample. The analytical method's validity was checked using certified reference material acquired from (Sigma-Aldrich).
2.5 Risk Assessments
2.5.1 Target Hazard Quotient
Target hazard quotients (THQs) are simple ratios of exposure to the toxic and trace elements being assessed (reference dose) which is the highest level at which no adverse health effects are expected [28]. The THQ describes the non-carcinogenic health risk likely to be caused by exposure to a toxic element and is calculated using Eq. 1. No non-carcinogenic health impacts are expected for a THQ < 1. However, there is a possibility that the exposed population could experience health problems if the THQ > 1.
where THQ is the target hazard quotient, EF is the exposure frequency (365 days/year), ED is the exposure duration (average lifetime) of 62 years for the Tanzanian population for a non-cancer risk [29], FIR is the fish ingestion rate (7.6 kg/person/year) which makes up 19.7% of the country’s protein intake [30], CF is the conversion factor to convert fresh weight into dry weight, CM is fish heavy metal concentration in (mg/kg dw), WAB is the average body weight (55.9 kg) for Tanzanian adults [29], and ATn is the average exposure time for non-carcinogenic (EF x ED), RfD is the reference dose for the metal.
2.5.2 Estimated Daily Intake (EDI)
Estimated daily intake (EDI) is the daily exposure or consumption of fish that depends on the metal concentration contained in a food and daily food consumption. The EDI is normally calculated using Eq. 2:
where, CM is the concentration of heavy metal in fish muscles (mg/kg). DC is the daily fish consumption (g/day) per capital of the Tanzanian population (7.6 g/day), and BW is the average Tanzanian adult body weight (55.9 kg).
2.5.3 Hazard Index (HI)
A hazard index (HI) is the sum of more than one target hazard quotient (THQ) for multiple substances and multiple exposures. The hazard index can be separately calculated for chronic, sub-chronic, and shorter-duration exposures using Eq. 3. HI values higher than unity (HI > 1) indicate an alarm for public health concerns.
where HI is the sum of target hazard quotients for all investigated metals.
2.6 Statistical Analysis
The statistical analysis used in the current study includes calculations of mean values and standard deviations performed using Microsoft Excel Software. The data were analyzed for homogeneity of variance, normality, and statistical differences among the samples. Normal data distribution was analyzed by the Shapiro–Wilk test, whereas the homogeneity of variance was analyzed by Levene’s test as implemented in the software R (version 3.1.2). Due to the non-fulfillment of the requirements for the normal distribution of data and variance homogeneity, all data were analyzed by the non-parametric Wilcoxon signed-rank test statistic (V) as implemented in R (version 3.1.2). The statistical significance was set at p < 0.05.
3 Results and Discussion
3.1 Heavy Metal Concentration
The fish gills and muscle tissues are among the fish parts that accumulate significant amounts of heavy metals. As a result, the heavy metal concentration accumulated in these fish tissues is most often reported [31]. Therefore, the same fish tissues were analyzed for heavy metals in the current study. The results (Fig. 2) indicate that Fe, Zn, Cu, and Ni were present in both the gills and muscles of all fish species. However, chromium concentrations were above the detection limit (BDL) (0.0054 ppm) for only two fish species (C. gariepinus and O. microlepis). Concentrations of the heavy metals were relatively higher in gills than in muscles for all fish species. Significant differences of heavy metals between the fish tissues were noted for Fe in two species (C. gariepinus and R. moorii), Zn in three species (C. gariepinus, B. meridionalis, and R. moorii), Cu in one species (R. moorii), and Ni in one species (O. niloticus). However, C. gariepinus was found to have higher Zn in the muscles than gills. Gills generally accumulate the highest amounts of heavy metals because they are metabolically active organs [32]. Additionally, heavy metals have the ability to adsorb onto the surface of gills because it is a primary target organ exposed to aqueous pollution [33]. Similar findings of lower concentrations of heavy metals in muscles compared to gills are known [34, 35]. Studies indicate that heavy metals will accumulate in the muscle of fish only after exceeding the body's defense barriers [36]. Therefore, the current results of lower concentrations of heavy metals in muscles than in gills indicate that the heavy metal concentrations have not exceeded the body defense barriers of the investigated fish species.
The distribution of heavy metals among the fish species indicated that C. gariepinus contained the highest concentration of Fe in gills (19.95 ± 11.53 mg/kg), while the lowest value was found in gills of R. moorii (1.82 ± 0.38 mg/kg). Thus, the observed trend of total Fe accumulation in gills and muscles was in the order: C. gariepinus > O. nilotiucus > B. meridionali > O. microlepis > R. moorii (Table 3). The average Fe concentration in all studied fish species in the present study (9.36 ± 2.523 mg/kg) were lower than for other fish species collected from Rasalgan, India, (39.0 ± 1.09 mg/kg) and similar to an earlier study in Hurghada, Egypt (19.95 ± 3.10 mg/kg) [37, 38]. According to WHO [39, 40], the maximum permissible concentration for Fe is 100 mg/kg. Based on the concentrations obtained in the current study, Fe concentrations in the gills and muscles of fish species from Lake Nyasa are well below the threshold limit.
The highest concentration of Zn was found in the gills of R. moorii (10.91 ± 2.34 mg/kg), while the lowest concentration was observed in the muscles of B. meridionalis (0.92 ± 0.63 mg/kg). Total concentration of Zn in the gills and muscles of fish species were in the order: R. moorii > O. niloticus > O. microlepis > C. gariepinus > B. meridionalis (Table 3). The concentration of Zn in the present study is higher than levels in other fish species collected from the South West Malaysian coast (9.854 ± 2.317 mg/kg) [41]. However, the current Zn levels are lower compared to those from Masing Reservoir Kenya, India, and Hermosillo in Mexico [42, 43]. Zinc is an important component of the human body and a large source of nutrients for almost all plants. Algae growing in streams and lakes can absorb a large amount of zinc dissolved in water and serves as an important source of energy for fish [44]. Thus, the presence of algae in Lake Nyasa can influence the concentration of Zn in the fish species. A previous study of Lake Mugla, Turkey reported the use of fertilizers, sewage sludge, industrial wastes, and mining activities as the main potential sources of Zn pollution [45]. Similarly, the presence of agricultural and coal mining activities taking place near the current study area could be a potential source of Zn contamination in the fish species. Fortunately, the concentration of Zn found in the current study does not exceed the maximum permissible amount recommended by WHO/FAO [39, 46].
In the present study, the two highest concentrations of Cu were found in muscles of B. meridionalis (7.24 ± 0.47 mg/kg) and gills of C. gariepinus (5.59 ± 1.49 mg/kg), while the lowest concentration was observed in muscles of O. microlepis (0.59 ± 0.23 mg/kg) (Table 3). The total concentration of Cu in gills and muscles were in the order: B. meridionalis > C. gariepinus > R. moorii > O. niloticus > O. microlepis. The total concentration of Cu in the present study was higher than for a similar study in Sinaloa, Mexico (2.44 ± 0.73 mg/kg) [43]. The concentrations of Cu in the current study were within the permissible limits proposed by WHO/FAO [39, 47].
The highest concentration of Ni was observed in the muscles of O. microlepis and the lowest concentration was found in gills of B. meridionalis (1.77 ± 0.40 mg/kg). The total concentration of Ni in gills and muscles was in the order: O. microlepis > C. gariepinus > R. moorii > O. niloticus > B. meridionalis. A previous study conducted on the Coast of Andhra, India reported a higher accumulation of Ni (10.4 ± 1.4 mg/kg) in fish muscle due to the influence of mining activities [48]. Coal mining activities nearby to Lake Nyasa could make a significant contribution to the amount of Ni found in the current fish samples. Detectable levels of Cr were only observed for two fish species, O. microlepis and C. gariepinus. The highest concentration of Cr (1.42 ± 0.18 mg/kg) was recorded in muscles of O. microlepis, which also showed the highest total Cr. The gills of both fish returned similar levels of Cr (1.34 mg/kg). Worryingly, levels of Cr in the present study are an order of magnitude greater than data reported for fish species from Zhejiang, China (0.114 mg/kg) [49]. A study conducted in the Lower Meghna River reported a higher concentration of heavy metals in carnivorous fish species compared to herbivores and omnivores [50]. Both O. microlepis and C. gariepinus are carnivorous and, therefore, their feeding habits that result in accumulation of heavy metals may account for the higher levels of Ni and Cr. Despite the levels in O. microlepis and C. gariepinus, all the studied fish species were within the permissible levels for Ni and Cr as per WHO and FAO recommendations [39, 47]. This indicates that the fish species from Lake Nyasa are likely to be safe for human consumption. The average concentrations of heavy metals in the studied fish species (Table 3) were in the order: Fe > Zn > Cu > Ni > Cr. A similar trend is known for other fish species in areas affected by industrial and agricultural activities [51,52,53]. This is an indicator that anthropogenic activities, such as agriculture and coal mining, taking place near Lake Nyasa are contributing to heavy metal contamination. Thus, the management of these activities in the region could help to reduce the impact of heavy metal contamination to Lake Nyasa and surrounding ecosystems.
3.2 Target Hazard Quotient (THQ), Hazard Index (HI) and Estimated Daily Intake (EDI)
To assess the public health risk associated with the consumption of selected fish species from Lake Nyasa, target hazard quotient (THQ), hazard index (HI), and estimated daily intake (EDI) were used. THQ and HI are parameters used for risk assessments that compare the ingested amount of pollutants with the standard reference dose [54]. Values of THQ and HI below 1 indicate the exposed population is unlikely to experience obvious adverse health effects, whereas values above 1 indicate the exposed population is likely to experience health effects during an individual’s lifetime [55]. Thus, values of THQ and HI due to the ingestion of Fe, Zn, Cu, Ni, and Cr from fish species sourced from Lake Nyasa are useful long-term health indicators of the surrounding population. The findings (Table 4) show that except for Fe from gills of C. gariepinus with THQ = 1.233, all other tissue samples from fish species had values of THQ < 1 for the heavy metals. This indicates that the consumption of a variety of fish species is unlikely to cause carcinogenic risks. Although, the THQ value of Fe by consumption of C. gariepinus exceeded the non-carcinogenic threshold it was not exceedingly high. Small quantities of Fe are essential for various metabolic processes in the body, but when excessively accumulated can cause several serious health problems in humans [50]. The long-term consumption of C. gariepinus has the potential to cause health effects. Thus, ongoing monitoring of the concentration of Fe in Lake Nyasa is important for the protection of human health. In the present study, the results also show values of HI < 1 for tissue samples from all fish species except for gills and muscles from C. gariepinus. The HI values also indicate that the long-term consumption of C. gariepinus is concerning with the potential to cause an adverse health impact to the exposed population. Consequently, an ongoing program to monitor the Fe levels in C. gariepinus and the consumption of this fish species from Lake Nyasa is needed to protect the health of the local population.
The daily human exposure to a particular metal is indicated by an EDI value. It serves as a useful indicator of harmful health outcomes from the over or under consumption of a metal. Thus, an EDI value is useful to healthcare professionals to determine whether an average person's intake of a particular nutrient is adequate, so as to advise on nutritional recommendations or medical treatments [56]. Values of EDI are usually compared to the RfD for the same nutrient by calculating the ratio of EDI-to-RfD. An EDI/RfD ratio for heavy metals that is equal to or less than the RfD indicates the risk is minimum, however, if the ratio is greater than the RfD then a high health risk is indicated [51]. The factors influencing the EDI of a heavy metal from eating fish are the amount consumed and mean body weight of the person. The EDI of each heavy metal was evaluated for adults and children, and compared with the recommended dose (RfD) as proposed by the EPA [57]. The results shown in Table 5 indicate that the most EDI values were below reference doses for the most of investigated metals. However, the EDI values for Fe indicate that there are likely potential negative effects may be associated with the consumption of C. gariepinus to both adults and children. Additionally, the EDI values for Cr in C. gariepinus and O. microlepis were higher than the reference dose, which indicates the possibility of causing negative impacts in adults while the EDI value for Cu in gills of O. niloticus suggests the possibility of causing harm in children. The observations indicate that the consumption of most fish species is unlikely to cause serious harm to the consumers due to exposure to the heavy metals. However, the consumption of some fish species may associate with negative health effects due to exposure to metals Fe, Cr and Cu. This indicates the possible contribution of anthropogenic activities to heavy metals in the lake. Thus, the anthropogenic activities around Lake Nyasa need to be carefully managed to ensure that large amounts of heavy metals are not released in the basin that would cause negative impacts on human health.
4 Conclusion
This study has shown that five heavy metals, namely Fe, Zn, Cu, Ni, and Cr, are present in five selected fish species collected from Lake Nyasa. High levels of accumulated Fe were found compared to the other metals. However, the levels of heavy metals were within the acceptable ranges as proposed by WHO and FAO. The health risk indicators THQ and HI of the heavy metals were within threshold limits and indicated that no metals posed a carcinogenic health risk. However, the values for EDI suggest there are potential health effects may be associated with long term consumption of C. gariepinus and O. microlepis and O. niloticus due to continued exposures to Fe, Cr and Cu. Thus, the monitoring of human activities that may contribute to heavy metal pollution is highly required to protect fish consumers against potential negative health effects. The current study has provided preliminary information on the accumulation of heavy metals in the edible fish species. However, more investigations are required to evaluate variations of heavy metal accumulation with the age of fish species. Similarly, studies on the influence of metal accumulation on the genetic diversity of fish species in Lake Nyasa would provide useful information on the effects of heavy metals on the fish population.
Data availability
Data associated with the manuscript are available on request from the authors.
References
Agarwal SK (2009) Heavy metal pollution. APH Publishing Corporation, New Delhi
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence toxicity, and bioaccumulation. J Chem 2019:6730305. https://doi.org/10.1155/2019/6730305
Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR (2005) Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352(17):1769–1778
Lönnerdal B (2017) Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr 106(Suppl 6):1681s–1687s. https://doi.org/10.3945/ajcn.117.156042
Shah AI (2017) Heavy metal impact on aquatic life and human health–an over view. In: IAIA17 conference proceedings| IA’s contribution in addressing climate change 37th annual conference of the international association for impact assessment, pp 4–7
Taira M, Toguchi MS, Hamada Y, Takahashi J, Itou R, Toyosawa S, Ijyuin N, Okazaki M (2001) Studies on cytotoxic effect of nickel ions on three cultured fibroblasts. J Mater Sci Mater Med 12(5):373–376
Achmad RT, Auerkari EI (2017) Effects of chromium on human body. Ann Res Rev Biol 13:1–8
Vareda JP, Valente AJM, Durães L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manag. 246:101–118. https://doi.org/10.1016/j.jenvman.2019.05.126
Ouma KO, Shane A, Syampungani S (2022) Aquatic ecological risk of heavy-metal pollution associated with degraded mining landscapes of the southern africa river basins: a review. Minerals 12(2):225
Ribeiro CAO, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve France. Aquat Toxicol 74(1):53–69. https://doi.org/10.1016/j.aquatox.2005.04.008
Nhiwatiwa T, Barson M, Harrison AP, Utete B, Cooper RG (2011) Metal concentrations in water, sediment and sharptooth catfish Clarias gariepinus from three peri-urban rivers in the upper Manyame catchment Zimbabwe. Afr J Aquat Sci 36(3):243–252. https://doi.org/10.2989/16085914.2011.636906
Annabi A, Said K, Messaoudi I (2013) Cadmium: bioaccumulation, histopathology and detoxifying mechanisms in fish. Am J Res Commun 1(4):62
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake. China. Toxicol Rep. 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J (2022) Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. 34(3):101865. https://doi.org/10.1016/j.jksus.2022.101865
Witkowska D, Słowik J, Chilicka K (2021) Heavy metals and human health: possible exposure pathways and the competition for protein binding sites. Molecules 26(19):6060
Mokhtar MB, Aris AZ, Munusamy V, Praveena SM (2009) Assessment level of heavy metals in Penaeus monodon and Oreochromis spp. in selected aquaculture ponds of high densities development area. Eur J Sci Res 30(3):348–360
Nasir M, Muchlisin ZA, Saiful S, Suhendrayatna S, Munira M, Iqhrammullah M (2021) Heavy metals in the water, sediment, and fish harvested from the Krueng Sabee River Aceh Province, Indonesia. J Ecol Eng 22(9):224–231
Okereke J, Ogidi O, Obasi K (2016) Environmental and health impact of industrial wastewater effluents in Nigeria-a review. Int J Adv Res Biol Sci 3(6):55–67
La Colla NS, Botté SE, Marcovecchio JE (2018) Metals in coastal zones impacted with urban and industrial wastes: Insights on the metal accumulation pattern in fish species. J Mar Syst 181:53–62
H. Bootsma, S.E. Jorgensen, Lake Malawi/Nyasa, 36 pp, Managing Lake Basins; Practical Approaches for Sustainable Use. <http://www.worldlakes.org/uploads/ELLB% 20Malawi-NyasaDraftFinal. 14Nov2004. pdf (2004).
Branchu P, Bergonzini L, Ambrosi JP, Cardinal D, Delalande M, Pons-Branchu E, Benedetti M (2010) Hydrochemistry (major and trace elements) of Lake Malawi (Nyasa), Tanzanian Northern Basin: local versus global considerations. Hydrol Earth Syst Sci Discuss 2010:4371–4409. https://doi.org/10.5194/hessd-7-4371-2010
Gebremedhin S, Bruneel S, Getahun A, Anteneh W, Goethals P (2021) Scientific methods to understand fish population dynamics and support sustainable fisheries management. Water 13(4):574
Daud K, Wisdom C, Hamad S, Wilson J, Emmanuel K (2017) Morphological and genetic variability among Mpasa (Opsaridium microlepis Günther, 1864) populations from the inflow rivers of Lake Malawi. Int J Fish Aquac 9(5):52–58
Nauen CE (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular No. 764, p 102
Temmink JHM (1989) Environmental health criteria 86: mercury-Environmental aspects. In: IPCS international programme chemical safety. WHO, p 115
Eccles DH (1974) An outline of the physical limnology of Lake Malawi (Lake Nyasa). Limnol Oceanogr 19(5):730–742
Baharom ZS, Ishak MY (2015) Determination of heavy metal accumulation in fish species in Galas River, Kelantan and Beranang mining pool. Selangor. Proc Environ Sci. 30:320–325. https://doi.org/10.1016/j.proenv.2015.10.057
MiranzadehMahabadi H, Ramroudi M, Asgharipour M, Rahmani H, Afyuni M (2020) Assessment of heavy metals contamination and the risk of target hazard quotient in some vegetables in Isfahan. Pollution 6(1):69–78
Saria J (2017) Assessment of health risks associated with concentrations of heavy metals in fish from the coast of Tanzania. Ethiop J Environ Stud Manag 10(4):555–565
Mulokozi DP, Mmanda FP, Onyango P, Lundh T, Tamatamah R, Berg H (2020) Rural aquaculture: assessment of its contribution to household income and farmers’ perception in selected districts Tanzania. Aquac Econ Manag 24(4):387–405
Hasimuna OJ, Maulu S, Chibesa M (2022) Assessment of heavy metal contamination in water and largescale yellowfish (Labeobarbus marequensis, Smith 1841) from Solwezi River, North-Western Zambia. Cogent Food Agric 8(1):2121198. https://doi.org/10.1080/23311932.2022.2121198
Marzouk M (1994) Fish and environmental pollution. Vet Med J 42(1994):51–52
Deb SC, Fukushima T (1999) Metals in aquatic ecosystems: mechanisms of uptake, accumulation and release-ecotoxicological perspectives. Int J Environ Stud. 56(3):385–417
Ambedkar G, Muniyan M (2011) Bioaccumulation of some heavy metals in the selected five freshwater fish from Kollidam River, Tamilnadu. India. Adv Appl Sci Res. 2(5):221–225
Siraj M, Shaheen M, Sthanadar AA, Khan A, Chivers DP, Yousafzai AM (2014) A comparative study of bioaccumulation of heavy metals in two fresh water species, Aorichthys seenghala and Ompok bimaculatous at River Kabul, Khyber Pakhtunkhwa. Pakistan. J Biodiv Environ Sci. 4(3):40–54
Griboff J, Wunderlin DA, Monferran MV (2017) Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J. 130:236–244
Yehia HM, Sebaee ES (2012) Bioaccumulation of heavy metals in water, sediment and fish (Oreochromis niloticus and Clarias anguillaris), in Rosetta branch of the River Nile. Egypt. Afr J Biotechnol. 11(77):14204–14216
Javed M, Usmani N (2011) Accumulation of heavy metals in fishes: a human health concern. Int J Environ Sci. 2(2):659–670
FAO/WHO guide for developing and improving national food recall systems (2012) Food and agriculture organization of the United Nations. https://www.fao.org. Accessed 4 Apr 2023
WHO, Trace elements in human nutrition and health. World Health Organization (1996)
Kamaruzzaman B, Rina Z, John BA, Jalal K (2011) Heavy metal accumulation in commercially important fishes of South West Malaysian coast. Res J Environ Sci 5(6):595–602
Nzeve JK, Njuguna SG, Kitur EC (2014) Bioaccumulation of heavy metals in clarias gariepinus and oreochromis spirulus niger from Masinga Reservoir, Kenya. IOSR J Environ Sci Toxicol Food Technol 8(10):58–63
Frías-Espericueta MG, Zamora-Sarabia FK, Osuna-López JI, Muy-Rangel MD, Rubio-Carrasco W, Aguilar-Juárez M, Voltolina D, Cadmium, copper, lead, and zinc contents of fish marketed in NW Mexico. Sci World J. (2014) 4.
Hamilton SK, Lewis WM, Sippel SJ (1992) Energy sources for aquatic animals in the Orinoco River floodplain: evidence from stable isotopes. Oecologia 89(3):324–330
Yilmaz F.: The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Koycegiz Lake-Mugla (Turkey). Turk J Sci Technol. 4(1). (2009).
World Health Organization (2016) Evaluation of certain food additives and contaminants: eightieth report of the joint FAO/WHO expert committee on food additives, vol 80. https://apps.who.int. Accessed 14 Apr 2023
Nauen CE (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular (FAO) no. 764. Accessed 14 Apr 2023
Krishna PV, Jyothirmayi V, Rao K (2014) Human health risk assessment of heavy metal accumulation through fish consumption, from Machilipatnam Coast, Andhra Pradesh, India. J Issues ISSN, 2360. pp 8803.
Han J-L, Pan X-D, Chen Q, Huang B-F (2021) Health risk assessment of heavy metals in marine fish to the population in Zhejiang, China. Sci Rep 11(1):11079
Mohammad Belal Hossain FT, Safiur Rahman M, Yu J, Akhter S (2022) Metals bioaccumulation in 15 commonly consumed fishes from the lower Meghna river and adjacent areas of Bangladesh and associated human health hazard. Toxics 10(3):139. https://doi.org/10.3390/toxics10030139
Javed M, Usmani N (2016) Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. Springerplus 5(1):1–8
Izah SC, Angaye TC (2016) Heavy metal concentration in fishes from surface water in Nigeria: potential sources of pollutants and mitigation measures. Sky J Biochem Res. 5(4):31–47
Paul D (2017) Research on heavy metal pollution of river Ganga. A review. Ann Agrar Sci. 15(2):278–286
Alipour H, Pourkhabbaz A, Hassanpour M (2015) Estimation of potential health risks for some metallic elements by consumption of fish. Water Qual Expo Health 7:179–185. https://doi.org/10.1007/s12403-014-0137-3
Usepa U (2000) Risk-based concentration table. United States Environmental Protection Agency Philadelphia, Philadelphia
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (1997) Dietary reference intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academies Press, Washington, DC, US
Phillips LJ, Moya J (2014) Exposure factors resources: contrasting EPA’s exposure factors handbook with international sources. J Expo Sci Environ Epidemiol 24(3):233–243
Acknowledgements
The authors are grateful to the mangement of Sokoine University of Agriculture for financial support through the SUA Research and Innovation Fund (SUARIS1). The authors would also like to express their gratitude to Mr. Ndabuli Mpeji of the Chemistry and Physics Department (SUA) for his support in the analysis of heavy metals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mwambene, B., Mwakalesi, A.J. & Nehemia, A.N. Contamination and Health Risk Assessment of Heavy Metals in Selected Highly Edible Fish Species from Lake Nyasa, Tanzania. Chemistry Africa 6, 2695–2703 (2023). https://doi.org/10.1007/s42250-023-00672-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00672-w