Abstract
A novel and unconventional of pre-concentration method and extraction of selected HMW-PAHs (higher molecular weight polycyclic aromatic hydrocarbons) from water samples have been developed. The novel approach benefits from the high attraction between HMW-PAHs and the modified magnetic nanoparticles. In this work, milligrams of magnetic nanoparticles are initially added to the aqueous solution and dispersed in all parts of the solution by shaking. To collect the nanoparticles, a neodymium magnet is placed on the outer surface of the container, followed by discarding the solution. The analyte in the collected solid phase is then released into 1-butanol followed by gas chromatography–mass spectrometry Optimization of the experiment parameters has guided to a Gas chromatography–mass spectrometric method of detection with acceptable analytical figures of merit. The LOD (limits of detection) ranged from 0.010 (Benzo(b) fluoranthene) to 0.08 (Benzo(k) fluoranthene) µg/L. The relative standard deviations (RSD) at medium calibration concentrations differ from 3.12 (Benzo(b)fluoranthene) to 6.31% (Benzo(a)pyrene). The R% (analytical recoveries) from tap water samples of the three regulated HMW-PAHs from six locations in Baghdad city range from 63.88 (Benzo(k)fluoranthene) to 91.01 (Benzo(a)pyrene). The entire method of extraction uses lower than 150 μL of organic-based solvents(butanol) for each sample, which marks it a friendly method to the environment. The small extracting solution volume makes the modified nanoparticle a relatively in-expensive extraction procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The PAHs (polycyclic aromatic hydrocarbons) are global pollutants in the environmental system [1]. There are two sources of PAHs: natural (such as volcanos and fires in the forest) or anthropogenic (such as traffic). Incomplete Combustion of carbon-based matter at elevated temperature and with a considerably low level of O2 will release several more minor compounds such as free radicals.
Free radicals can be chemically bounded with other organic particles to produce two to three PAH rings which can undergo further chemical reactions to produce Higher molecular weight PAHs (HMW-PAHs) (Fig. 1) [2,3,4].
HMW-PAHs are compounds of significant importance for public health. HMW-PAHs consider one of the leading causes of cancer and mutagenicity. EPA (environmental protection agency) has previously reported the mutagenic effect of these compounds from data collected from animal bioassays [5,6,7,8,9].
Methods development for the analysis of HMW-PAHs is considered one of the essential aspects of the environment [10,11,12,13]. SPE (solid phase extraction) has been widely used for miner HMW-PAHs concentration detection. SPE can be linked with GC–MS, HPLC, and GC-FID, but unfortunate that HMW-PAHs analysis with SPE is not the ideal technique, and the reason is that HMW-PAHs will adsorb on the surface of the wall vessels of the extraction glassware. Hence, HMW-PAHs are not willing for multi-process extraction such as solid-phase extraction and L–L (liquid–liquid) extraction because this technique uses a large volume of organic solvents harming the environment [14,15,16,17,18].
Methods with low solvent volume must be developed to protect the environmental system.
The typical method for PAH analysis and sample preparation tracks the sequence of sample collection, PAH chromatographic analysis, and extraction. With the systematic approaches to pre-concentrate and extract PAH, the ideal methods are based on SPE (solid-phase extraction), which is usually used for sample preparation in the quantification of PAHs, mainly in drinking water [7]. The technique has also found application in determining PAH content in tar.
A packed glass column with activated silica gel and alumina was used for eluting the fractionated PAHs [8]. Otherwise, the procedure uses benzene or methanol as the eluting solvents and Sephadex as the stationary phase [10]. Though adsorption methods in low-efficiency and classical glass columns are imprecise, tedious, inaccurate, and time-consuming, they spend large volumes of organic solvents [12].
Present examples of effective adsorbent materials include fluorocarbon polymer sorbents (FPS), octadecyl-silica (ODS), multi-walled carbon nanotubes (MCN), and graphite fibers.
The standard analysis is usually performed via high-performance liquid chromatography (HPLC) with either fluorescence detection or absorption [15].
The purposes of this article were, therefore, set to synthesize modified nanoparticles as a sorbent for extraction of HMWT-PAHs from water samples coupled with GC–MS for the analysis of the HMW-PAHs [19,20,21]. Figure 2 summaries the whole purpose of the article.
2 Experimental Part
2.1 Chemicals
All chemical compounds were in high purity and was purchased from Sigma-Aldrich and Acros Organics. All HMW-PAHs used without further purification and an extreme caution during handling were taken because these compounds known to be toxic Chemicals.
2.2 Solid-Phase Extraction (SPE)
Solid phase extraction columns with capacity of three mL and has reverse stationary phase were used: type 5 × 102 mg LC18(Supelco inc.). A polypropylene type of cartridge was made that has wide entrance for the sample and narrow exit. The sorbent were conditioned with five mL alcohol (methanol) at 4.0 mL min−1 followed by 4 ml nano pure-water for equilibration at 4.0 mL min−1).
A blank solution with volume of ten mL was spiked with 50 mg L−1 HMW-PAHs followed by loading into the extraction column as 2 ml min−1. A four ml of 25% (volume/volume) methanol were used to remove the lipophilic interferences at 3.0 mL min−1 [22].
The extraction column then vacuums assisted during for 15 min followed by elution using 4 × 1.5 mL of elution solvent at 0.9 mL min−1. Then the eluted solvent was collected into vials and analysed by GC–MS.
2.3 Synthesis of Fe3O4–SiO2–Humic Acid
The preparation of Fe3O4–SiO2 nanoparticles functionalized with humic acid was First started with embedding SiO2 on the surface of Fe3O4 using the method that developed by Stöber, which include mixing of 115 mL of 0.4 (M) Na2O3Si·9H2O were mixed with 0.4 g of Fe3O4 nanoparticle suspension, followed by heating up the solution to reach 85 °C under flow of nitrogen and maintain the pH value at 6.5 using drops of Hydro-chloric acid with 1 M. The precipitate was collected after mixing for 3 h followed by washing with double-distilled water then alcoholic solvent (ethanol), then drying at 70 °C. The resulting Fe3O4–SiO2 (1.3 g) was suspended and added to a solution of humic acid followed by heating to 85 °C for 10 h. under flow of N2 flow. Both Fe3O4 nanoparticles and humic acid was chemically bonded.
2.4 GC–MS Analysis
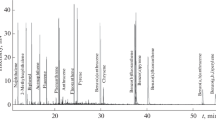
A ٍSHIMADZU GCMS model QP2020NC with mass selective detector equipped with a capillarycolumn (30 m, 0.25 mm i.d. DB-5MS, 0.25-μm flm thickness). The samples injector temperature was set to 280 °C in the pulsed splitless mode. The transfer line and ion source, temperatures were 250 150 °C, respectively. Carrier gas was helium with 1 mL min−1 flow rate of. The oven temperature was as follows: 90 °C, 3 min hold, ramp to 200 °C at 25 °C/min without any further holding time then ramp to 280. Quantifcation of the selected PAH compounds was performed by SIM (selected ion monitoring) mode.
3 Results and Discussion
3.1 Characterization of Fe3O4–SiO2–Humic Acid NPs
The synthesized of Fe3O4–SiO2–humic acid NPs were analyzed via X ray diffraction technique for the examination of the structural pattern (Fig. 3). The pattern of the X-RD of the NParticles were studied with Buker-D8-advance XR diffractometry using radiation of Ka Copper (λ = 1.5507A°) and the scanning of the samples were 10°–80° (2θ). The pattern obtained illustrate that the NParticles has a Fe3O4–SiO2–humic acid NPartilces structure. the Fig. 2C shows the occurrence of sharp peaks belong to Fe3O4 NParticless which are seen in the Fig. 2 panel A with (Δ) symbol. The broad (20–25°) peak is representing the occurrence of SiO2 (has symbol) as compared with Fig. 3 (B panel). the (∇) peak (43.4°) was belong to humic acid in the core shell structure >
The Fe-SEM images (Fig. 4) suggest the presence of Fe3O4/SiO2/humic acid NParticles with wide range of shape cluster and size. This can be explained by the creation of structures that has consider to be non-homogenous which rising from careful shielding of Fe3O4 NParticless with humic acid and SiO2.
3.2 AFOM with Extraction of Higher Molecular Weight PAHs by Applied the Solid Phase Extraction
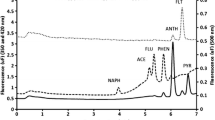
The summarization of AFOM using SPE-GC–MS. were shown in Table 1 All linear dynamic ranges (LDR) were created on average measurements of six concentrations of PAHs. Figure 5 show GC–MS chromatogram for the three selected PAHs. The measurements average of nine were prepared from aliquots of 3 collected from 3 complete experimental trials. No highest levels of concentration of the calibration Curves trials were done. The 1OSB/m represent the smallest linear concentrations related to the limit of detection, where SB represent the SD (standard/deviation) of 16 of blank measurements and slope of the calibration curve represented by “m”. The good correlation coefficients (R) show a linearity in relations in all situations. 3SB/m represent the way of calculating LODs were ranged from 0.21 (Benzo(b)fluoranthene to 0.96 ng/mL (Benzo(k)fluoranthene). LODs are above the max. contaminant level of controlled PAHs which make this method not perfect for the quantitative monitoring of PAHs. Testing the extraction efficiency. RSD value show good precision readings of concentration level of ppt.
3.3 Validation of the Method of Extraction of HMW PAHs by Applying the Synthesized Fe3O4–SiO2–Humic Acid Core/Shell NParticles
Validation of the proposed method of extraction procedure was examined by some of the analytical figures of merits such as LOD (limit of detection), LOQ (limit of quantification, linearity, accuracy, precision, and selectivity. Table 2 show that the data obtained with signal to noise equal to three and ten were used in calculating the limit of detection and quantitation, respectively. The LODs were in the range from 0.01 to 0.06 µg/L.
The plotted calibration cure was done using the analytical signal of different concentrations of solutions with an “n” value equal to 6. Excellent linearity with R2 (square correlation coefficient) more than or equal to 0.9971.
The method accuracy was evaluated by spiking water samples with the different analyte (at 350 ng/L) with 6 replicates; the deviation that was obtained from the result was less than 8%.
The method repeatability was examined by analysis of 350 ng/L of standard solution for each analyte. The obtained relative standard deviation of less than or equal to 6.31% therefore, the obtained RSD results show acceptable repeatability.
The selectivity of the method was obtained by performing the method, and it was found that there is variation in the analytical signal with less than 8%. This confirms the stability of the sample at different environment.
The whole above process was done by applying the synthesized NParticles with the selected HMW-PAHs. It mixed 1 mg of the NPartilces with 4 mL of a 4 μg/mL HMW-PAH solution. Followed by solution shaking for 8 min and then applying the magnetic field for 40 s.
The absence of fluorescence signal in the PAH solution spectrum of the supernatant is evidence of extraction because PAH was adsorbed onto the humic acid (hydrophobic surface of modified NParticles). The release of the selected PAHs was tested with several solvents (-n-hexane, butane, methanol, and butanol). It was found that butanol has astrong binding affinity for PAHs, so PAHs will preferred leaving the surface of nanoparticles rather than binding to nanoparticles. With the highest release value of around 89% for all PAHs, a minimum of 150 µl of butanol was used for the releasing process.
Table 3 shows that A wide range of detection limits values of the selected PAHs have been reported with a wide range of concentration levels. The detection limits values of current work are considerably lower than those in many previous studies by around two orders of magnitude.
Even though a straightforward LOD comparison to those indexed in the literature with different instruments is complex as different instrumental setups for mathematical and experimental achieves have been used for their calculation, LOD compares favorably. This comparison becomes applicable primarily if one deliberates the small H2O volume on 3 mm scale.
To the extent of our literature search, there are no methods efficient enough to detect these levels of PAH in 3 ml of H2O samples. This aspect makes this method suitable for regular analysis of several examples, as the small sample volume facilitates real-time sample extraction. The whole procedure of extraction consumes less than 150 µL of solvents for each sample, which makes it environmentally friendly. The small extracting solutions volume to the overall analysis cost makes SPNE a reasonably low-priced extraction approach.
3.4 Method Greenness Assessment
The method greenness was evaluated by NEMI ( National Environmental Methods Index). GAC (Green analytical chemistry) is the term that drags the attention of any analytical chemist to keep in mind environmental safety and Health matters during any given experimental work. The term greenness of any analytical experiments and procedures is a group of parameters that classify ad complicated and not easily determined quantitatively.
Numerous methods for Green analytical chemistry metrics have been established. The first described approach is NEMI, which is a metric system constructed on an easy-to-read pictogram, separated into four sections, representing reagents that are persistent, generation of waste bio accumulative or toxic, whether the conditions are corrosive, and whether reagents are hazardous).
The greenness of the suggested method representing in Fig. 6 and show an acceptable score of 65, which consider a good score and conder as acceptable. Color code; yellow, red, and green signifying medium, high and low influence on the environment, respectively.
3.5 PAH Determination in Tap Water in Baghdad city-Iraq
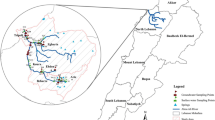
The possibility to screen HMW-PAHs in tap/water was examined with unknown water matrix samples. Every water sample was gathered at a special location inside the city Baghdad-Iraq (Fig. 7 show the sampling location) and scanned in three times. Spiking of microliter volumes of selected HMW-PAHs standard solutions with Volumes (ml) of tap water to deliver final concentrations at regulated HMW-PAHs concentration. Nanoextraction-GC–MS analysis was carried out. Table 4 outlines the regular recoveries with the standard deviations for the 6 experienced samples. Evaluation to generally recoveries in Table 4 shows no effects on the recoveries from the matrix composition of the unknown tap water samples.
References
Adeniji AO, Okoh OO, Okoh AI (2018) Analytical methods for polycyclic aromatic hydrocarbons and their global trend of distribution in water and sediment: a review. Recent Insights Pet Sci Eng. https://doi.org/10.5772/intechopen.71163
Alfarhani, B. F.; Al-Mousawi, R. R.; Alzaidy, A. S.; Aziz, R. S.; Shingar, F. K. Magnetic Nanoparticles for Hydroxy-PAHs Removal from Synthetic Urine. In IOP Conference Series: Earth and Environmental Science; 2021; Vol. 790. https://doi.org/10.1088/1755-1315/790/1/012037.
Alfarhani BF, Al-Mousawi RR, Aziz RS, Shingar FK (2021) Modified nano particles method for fluorescent dye removal from aqueous samples. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/790/1/012040
Alfarhani BF, Hammza RA, Alzaidy AS (2021) Potential effect of solvent and slit width on some properties of room temperature fluorescence of hydroxy polycyclic aromatic hydrocarbons. Chem Pap. https://doi.org/10.1007/s11696-021-01602-1
Alfarhani B, Al-Tameemi M, Goicoechea HC, Barbosa F, Campiglia AD (2018) Direct analysis of Benzo[a]Pyrene metabolites with strong overlapping in both the spectral and lifetime domains. Microchem J. https://doi.org/10.1016/j.microc.2017.09.022
Alfarhani B, Al-tameemi M, Schenone AV, Goicoechea HC, Barbosa F, Campiglia AD (2016) Room temperature fluorescence spectroscopy of Benzo[a]Pyrene metabolites on octadecyl extraction membranes. Microchem J. https://doi.org/10.1016/j.microc.2016.06.010
Andersson JT, Achten C (2015) Time to say goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for environmental purposes. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2014.991042
Awad AA, Mujbel Hasson M, Fadhel AA, Alfarhani BF (2019) Synthesis and characterization of a new Schiff base ligand type N2O2 and Their Cobalt (II), Nickel (II), Copper (II), and Zinc (II) Complexes. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/1294/5/052040
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. https://doi.org/10.1016/j.biomaterials.2004.10.012
Hayes HV, Wilson WB, Santana AM, Campiglia AD, Sander LC, Wise SA (2019) Determination of molecular mass 302 polycyclic aromatic hydrocarbons in standard reference material 1597a by reversed-phase liquid chromatography and constant energy synchronous fluorescence spectroscopy. Microchem J. https://doi.org/10.1016/j.microc.2019.104061
Han M, Zhang R, Yu K, Yan A, Li H, Zhang R et al (2022) Environmental fate and effects of PAHs in tropical mariculture ponds near the northern South China Sea: rainfall plays a key role. Sci Total Environ 847:157442
Tan H, Wu Q, Wang C, Wu D, Cui Y, Li Q, Wu C (2022) Polycyclic aromatic hydrocarbons (PAHs) in surface soils of tropical reef islands in China under the external plant and soil introduction: occurrence, sources, risks, and relationships with soil properties, vegetation cover, and soil source. Chemosphere 306:135556
Sun N, Yu S, Cai Z, Liu J, Wang T, Qi B et al (2022) Inhibition of polycyclic aromatic hydrocarbon (PAHs) released from sediments in an integrated rice and crab coculture system by rice straw biochar. J Clean Prod 367:133058
Han M, Li H, Kang Y, Liu H, Huang X, Zhang R, Yu K (2022) Bioaccumulation and trophic transfer of PAHs in tropical marine food webs from coral reef ecosystems, the South China Sea: compositional pattern, driving factors, ecological aspects, and risk assessment. Chemosphere 308:136295
Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Portier J, Pollert E, Duguet E (2006) Magnetic nanoparticle design for medical applications. Prog Solid State Chem. https://doi.org/10.1016/j.progsolidstchem.2005.11.010
Chakravarty P, Chowdhury D, Deka H (2022) Ecological risk assessment of priority PAHs pollutants in crude oil contaminated soil and its impacts on soil biological properties. J Hazard Mater 437:129325
Salam Hussein E, Bassam FAF, Salwan Ali A, Nadhir AA (2020) Modeling of trihalomethane compounds formation in Baghdad water supply network. Sci Rev Eng Environ Sci. https://doi.org/10.22630/PNIKS.2020.29.2.12
Dong L, Lin L, He J, Pan X, Wu X, Yang Y et al (2022) PAHs in the surface water and sediments of the middle and lower reaches of the Han River, China: occurrence, source, and probabilistic risk assessment. Process Saf Environ Prot 164:208–218
Schwarzenbach RP, Egli T, Hofstetter TB, Von Gunten U, Wehrli B (2010) Global water pollution and human health. Annu Rev Environ Resour. https://doi.org/10.1146/annurev-environ-100809-125342
Sun H, Chen Q, Chen W, Qu C, Mo J, Song J et al (2022) Assessment of biological community in riparian zone contaminated by PAHs: linking source apportionment to biodiversity. Sci Total Environ 851:158121
Wilson WB, Campiglia AD (2011) Determination of polycyclic aromatic hydrocarbons with molecular weight 302 in water samples by solid-phase nano-extraction and laser excited time-resolved Shpol’skii spectroscopy. Analyst. https://doi.org/10.1039/c1an15309a
Bandal HA, Kim H (2022) In situ construction of Fe3O4@ FeOOH for efficient electrocatalytic urea oxidation. J Colloid Interface Sci 627:1030–1038
Jouyban A, Ali Farajzadeh M, Nemati M, Alizadeh Nabil AA, Mogaddam MRA (2020) Preparation of ferrofluid from toner powder and deep eutectic solvent used in air-assisted liquid-liquid microextraction: application in analysis of sixteen polycyclic aromatic hydrocarbons in urine and saliva samples of tobacco smokers. Microchem J 154:104631
Fazaieli F, AfsharMogaddam MR, Farajzadeh MA, Feriduni B, Mohebbi A (2020) Development of the organic solvents-free mode of solidification of floating organic droplet-based dispersive liquid-liquid microextraction for the extraction of polycyclic aromatic hydrocarbons from honey samples before their determination by gas chromatography-mass spectrometry. J Sep Sci 43(12):2393–2400
Jouyban A, Ali Farajzadeh M, Reza AfsharMogaddam M, Nemati M, Akbar Alizadeh Nabil A (2020) Anal Methods 12:1522–1531
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animals participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alfarhani, B.F., Hammza, R.A. & Zadeh, E.H.G. Quantification of Higher Molecular Weight Polycyclic Aromatic Hydrocarbons in Water Samples by Modified Magnetic Nanoparticle and Gas Chromatography–Mass Spectrometry. Chemistry Africa 5, 2185–2191 (2022). https://doi.org/10.1007/s42250-022-00487-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00487-1