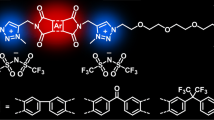
Abstract
In this work, two fluorinated poly(1,2,3-triazolium ionic liquid)s (PTILs) are accessed in two steps by copper(I)-catalyzed azide–alkyne cycloaddition AA + BB polyaddition between an α,ω-dialkyne-perfluoroether and either 1,12-dodecyl-diazide or α,ω-diazido-tetra(ethylene glycol), followed by N-alkylation of the resulting poly(1,2,3-triazole)s (PTs) by N-methyl bis(trifluoromethylsulfonyl)imide. The structure/properties correlations of PTILs and their PT intermediates are discussed based on solubility, 1H, 13C and 19F NMR spectroscopy, differential scanning calorimetry, thermogravimetric analysis, and broadband dielectric spectroscopy. Dodecyl- and tetra(ethylene glycol)-based PTILs exhibit glass transition temperatures of − 38 and − 29 °C, temperatures at 10% weight loss of ca. 340 °C and anhydrous ionic conductivities at 30 °C of 1.2 × 10−5 and 8.4 × 10−6 S cm−1, respectively. The impact of different lithium salts (i.e. LiSO3CF3, LiTFSI, and LiClO4,) on ionic conductivity of the tetra(ethylene glycol)-based PTIL was also investigated. A ca. twofold increase in ionic conductivity could be obtained by the addition of 5 wt% of LiClO4.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the past 2 decades, there has been a steadily growing interest in poly(ionic liquid)s (PILs) as they interestingly gather the unique properties of ionic liquids (e.g. enhanced thermal, chemical, electrochemical and ion conducting properties as well as switchable solubility) with those of polymer materials (e.g. tunable mechanical, viscoelastic, film-forming properties through extensive macromolecular design possibilities) [1,2,3,4,5]. PILs can be assembled from wide libraries of cationic moieties (e.g. ammonium, pyridinium, pyrrolidinium, imidazolium, phosphonium, thiazolium, and more recently 1,2,3-triazolium and 1,2,4-triazolium…) and complementary anionic moieties (e.g. halides, carboxylates, sulfonates, phosphates, inorganic fluorides, perfluorinated sulfonimides…) allowing an almost unlimited variety of combinations in addition to the broad capacities offered by advanced macromolecular engineering. The potential of PILs has been already demonstrated in many applications including catalysis [6,7,8], dye sensitized solar cells [9], thermoresponsive polyelectrolytes [10], electrochromic devices [11], gas separation membranes [6, 12,13,14], self-assembled colloids [15,16,17], sensors and actuators [18, 19], electrolyte-gated transistors [20, 21], as well as polymer electrolytes for energy production and storage [6, 22,23,24,25]. Poly(1,2,3-triazolium ionic liquid)s (PTILs) are a recent addition to broad PIL library [2, 26,27,28,29,30,31]. They are generally synthesized via copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), the most widespread example of the click chemistry philosophy [32]. Its experimental simplicity, reliability and robustness provides both ease of synthesis and broad structural design inspired from the extensive literature combining CuAAC with macromolecular engineering technologies [33,34,35,36]. Previously described PTILs include anionic polystyrene having covalently tethered TFSI anions and 1,2,3-triazolium counter cations [37], as well as cationic poly(meth)acrylates [38, 39], polyvinyl esters [40], and main chain PTILs having a broad range of counter anions [2, 26,27,28,29,30,31]. Several PTILs with enhanced properties have shown promising performances in key-enabling technologies such as gas separation membranes [41, 42], electrochromic devices [43, 44], and as antibacterial or antifungal materials [45, 46].
Fluorinated polymers such as poly(vinylidene fluoride) (PVDF) and poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) are key materials widely used in the fields of energy storage and production (e.g. as binders for electrode materials in lithium ion batteries, and polymer solar cells [47,48,49]). Although many PIL structures contain fluorine atoms assessed through the introduction of perfluorinated counter-anions (e.g. tetrafluoroborate (BF4), hexafluorophosphate (PF6), bis(trifluoromethylsulfonyl)imide (TFSI), bis(pentafluoroethylsulfonyl)imide (BETI), or bis(fluorosulfonyl)imide (FSI), etc…) [1,2,3,4,5, 28], only limited amount of studies have reported on PILs having main-chain or side-chain perfluorinated segments. Previous examples rely either on step-growth polymerization, chain-growth polymerization or post-polymerization chemical modification strategies. Améduri et al. reported the first example of fluorinated PILs by free radical statistical copolymerization of diallyldimethylammonium chloride (DADMAC) and chlorotrifluoroethylene (CTFE) [50]. Ragogna et al. reported on UV-curable formulations based on the free radial copolymerization of hexane diol diacrylate with a series of styrenic ILMs having pendent highly fluorinated phosphonium groups [51]. Detrembleur et al. developed amphiphilic fluorinated PIL diblock copolymers by cobalt-mediated radical polymerization-induced self-assembly [52]. The first polyimidazolium block is hydrophilic due to the presence of oligo(ethylene glycol) pendent groups while the second polyimidazolium block is a statistical distribution of N-vinyl-3-ethyl-imidazolium bromide and N-vinyl-3-perfluorooctyl-imidazolium bromide units. The last example of chain growth polymerization for the elaboration of fluorinated statistical and block copolymers was reported by Binder et al. relying on the radical addition fragmentation chain transfer copolymerization of an imidazolium acrylate with 2,2,2-trifluoroethyl acrylate and subsequent chain extension with pentafluorostyrene to obtain ABA fluorinated triblock copolymers having PIL outer blocks [53]. Liu et al. reported the AA + BB step growth polymerization by N-alkylation of bisimidazole and bishalides having tetrafluorobenzene units [54]. Chen et al. developed the AA + BB step growth polymerization by Suzuki coupling between 2,2′-(9,9-bis(6-bromohexyl)-9H-fluorene-2,7-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) and either 2-fluoro-1,4-diiodo-benzene or 1,2,4,5-tetrafluoro-3,6-diiodo-benzene followed by post-polymerization N-alkylation reaction with trimethylamine to afford the corresponding bistrialkylammonium-containing fluorinated conjugated PILs [55]. Mulhaupt et al. reported on a multistep post-polymerization chemical modifications strategy to obtain hyperbranched fluorine-containing PILs [56]. Hydroxy-functionalized poly(3-ethyl-3-hydroxymethyloxetane) obtained by cationic ring-opening polymerization of 3-ethyl-3-hydroxymethyloxetane was modified sequentially by (1) tosylation, and (2) N-alkylation with 1-(n-1H,1H,2H,2H-perfluorooctyl)imidazole to obtain a perfluorohexyl-containing outer shell. Hu, Chen et al. developed the synthesis of fluorinated poly(aryl ethers) containing pendent trialkylammonium groups by post-polymerization N-alkylation of trimethylamine with bromide-functionalized poly(aryl ethers) obtained by AA + BB condensation copolymerization between 9,9′-dihydroxyfluorene, 4,4′-bisphenol and decafluorobiphenyl [57]. Ho et al. have developed the post-polymerization chemical modification of PVDF-HFP to introduce different contents of pendent n-butyl imidazolium iodides and used them as electrospun membranes for quasi-solid dye-sensitized solar cells [58]. Finally, Améduri et al. prepared 1,2,3-triazolium-based fluorinated vitrimers by concomitant AA + BB step growth polyaddition and N-alkylation of poly(1,2,3-triazole) intermediates using perfluorinated derivatives [59].
Aiming at consolidating the library of PTILs with enhanced ion conducting properties by incorporating perfluoroether units and establishing a fine structure/properties relationship, we report herein the synthesis of fluorinated poly(1,2,3-triazolium)s by AA + BB CuAAC polyaddition of a dialkyne perfluoroether with two diazides followed by N-alkylation of the 1,2,3-triazole groups of the poly(1,2,3-triazole) intermediates. Discussion of their structure/properties correlations compared to previously developed PTILs relies on their characterization by 1H, 13C and 19F NMR spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and broadband dielectric spectroscopy (BDS).
2 Experimental Section
2.1 Materials
Sodium hydride (NaH, 99%), 18-crown-6 (99%), diisopropylethylamine (DIPEA, 99%), copper(I) iodide triethylphosphite (CuIP(OEt)3, 97%), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA, 99%), N-methyl bis(trifluoromethylsulfonyl)imide (CH3TFSI, 90%), lithium bis(trifluoromethylsulfonyl)imide (LiTFSI, 99.95%), lithium perchlorate (LiClO4, 99.99%), lithium hexafluorophosphate (LiPF6, 98%), lithium trifluoromethanesulfonate (LiSO3CF3, 99.995%) as well as all other reagents and solvents were purchased from Merck and used as received. 1H,1H,11H,11H-Perfluoro-3,6,9-trioxaundecane-1,11-diol (98%) was purchased from abcr and used as received. 1,12-diazido-dodecane 2 [60] and α,ω-diazido-tetra(ethylene glycol) 3 [61], were synthesized as previously described.
2.2 Characterization Methods
1H (300, 400 or 500 MHz), 13C (100 or 125 MHz) and 19F (282 or 470 MHz) NMR spectroscopy experiments were carried out with Bruker DRX 300, DRX 400 or DRX 500 spectrometers. Spectra were obtained with a 5-mm QNP probe at 363 K. Chemical shifts (δ) are given in ppm in reference to DMSO-d6 or CDCl3 for 1H and 13C NMR, and to CHCl2F for 19F NMR. Differential scanning calorimetry (DSC) was performed on a DSC Q23 (TA Instruments) using a heating rate of 10 °C/min under helium atmosphere. Tg values were measured during the second heating cycle. Thermogravimetric analysis (TGA) was performed with a TGA Q500 (TA Instruments) at 20 °C/min under helium atmosphere. Ionic conductivities (σDC) were measured using a high resolution Alpha-Analyzer (Novocontrol GmbH) assisted by a Quatro temperature controller. A solution (ca. 120 mg in 0.5 mL) of PTIL 6 was solvent cast onto a platinum electrode (Ø = 0.5″) and annealed at 110 °C/1 mmHg for ca. 24 h in order to remove solvent and water residuals. The supported polymer film was then placed over a second platinum electrode (Ø = 1″) using 200 µm thick Teflon spacers to build-up a measurement cell in a parallel plate configuration with a well-defined distance between electrodes. A further annealing under a flow of pure nitrogen during 4 h at 110 °C was performed in the cryostat of the dielectric spectrometer while monitoring the time evolution of the complex conductivity function σ*(ω,T) = σ′(ω,T) + iσ″(ω,T). Ionic conductivity measurements were started once a constant electrical response was attained, thus affording well equilibrated sample as well as reliable and reproducible experimental data. Frequency sweeps were then performed isothermally from 10 MHz to 0.1 Hz by applying a sinusoidal voltage of 0.1 V over temperatures ranging from 110 to − 70 °C, in steps of 10 °C. The temperature was controlled by heating the sample under a flow of pure nitrogen in order to prevent the presence of oxygen and humidity in the measurement chamber. The thermal stability was set to be better than 0.1 °C in absolute values with relative variations less than 0.2 °C/min. The same procedure was followed for PTIL 7, and for samples including PTIL 7 with additional lithium salts (i.e. 5 wt% LiTFSI, 5 wt% LiSO3CF3 and 5 wt% or 15 wt% LiClO4).
2.3 Synthesis of Poly(1,2,3-triazole)s 4 and 5
2.3.1 Synthesis of α,ω-Dialkyne Perfluoroether 1
Sodium hydride (2.92 g, 122 mmol) was added in small portions to a stirred solution of 1H,1H,11H,11H-perfluoro-3,6,9-trioxaundecane-1,11-diol (10.0 g, 24.4 mmol) and 18-crown-6 (100 mg, 0.40 mmol) in dry THF (350 mL) maintained at 0 °C under argon. After hydrogen was entirely emitted, a solution of propargyl bromide (10.9 mL, 90.0 mmol) in dry THF (150 mL) was added dropwise. The mixture was allowed to stir overnight at ambient temperature and after neutralization of residual NaH by methanol (10.0 mL), the mixture was extracted with dichloromethane (CH2Cl2, 2 × 150 mL). The separated organic layer was dried using MgSO4, filtered and evaporated under reduced pressure to give the crude product. The crude product was then purified by column chromatography (eluent petroleum ether) to afford the title product 1 as a yellow liquid (8.50 g, 71.7%). 1H NMR (500 MHz, CDCl3, δ): 4.31 (d, J = 2.0 Hz, CF2CH2OCH2C≡CH, 4H), 3.93 (t, J = 9.5 Hz, CF2CH2OCH2C≡CH, 4H), &2.52 (t, J = 2.0 Hz, CF2CH2OCH2C≡CH, 2H). 13C NMR (125 MHz, CDCl3, δ): 124.34-114.20 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O), 77.65 (CF2CH2OCH2C≡CH), 76.17 (CF2CH2OCH2C≡CH), 67.62 (CF2CH2OCH2C≡CH), 59.13 (CF2CH2OCH2C≡CH). 19F NMR (470 MHz, CDCl3, δ): − 77.52 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O, 4F), − 88.62 to − 88.74 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O, 8F).
2.3.2 General Procedure for the AA + BB CuAAC Polyaddition: Synthesis of 4
A solution of α,ω-dialkyne perfluoroether 1 (0.25 g, 0.51 mmol), 1,12-diazido-dodecane 2 (0.13 g, 0.51 mmol), CuIP(OEt)3 (4 mg, 0.01 mmol) and diisopropylethylamine (66 mg, 0.51 mmol) in DMF (2.0 mL) was stirred in the dark at 60 °C for 72 h. The crude reaction mixture was diluted with CH2Cl2 and precipitated three times in diethyl ether (Et2O). The product was dissolved in chloroform (CHCl3, 10 mL) and washed several times with 10 mL of a 5 mg/mL aqueous solution of PMDETA until the water phase remained colourless. The collected organic solution was dried with MgSO4, filtered and concentrated under reduced pressure to afford the title product 4 as a yellow viscous oil (329 mg, 86.7%). 1H NMR (300 MHz, DMSO-d6, δ): 8.12 (s, CF2CH2OCH2C=CH, 2H), 4.68 (bs, CF2CH2OCH2C=CH, 4H), 4.31 (t, J = 4.5 Hz, NCH2CH2(CH2)8CH2CH2N, 4H), 4.10 (bs, CF2CH2OCH2C=CH, 4H), 1.77 (bs, NCH2CH2(CH2)8CH2CH2N, 4H), 1.17 (m, NCH2(CH2)8CH2CH2N, 16H). 13C NMR (100 MHz, DMSO-d6, δ): 138.25 (CF2CH2OCH2C=CH), 130.11 (t, J = 30 Hz, CF2CH2OCH2C=CH), 121.21–118.47 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O), 68.53 (CF2CH2OCH2C=CH), 60.81 (CF2CH2OCH2C=CH), 53.98 (NCH2CH2(CH2)8CH2CH2N), 38.43–25.87 (NCH2CH2(CH2)8CH2CH2N). 19F NMR (282 MHz, DMSO-d6, δ): − 76.70 to − 78.74 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O, 4F), − 88.18 to − 89.08 (OCH2CF2 OCF2CF2OCF2CF2OCF2CH2O, 8F).
2.3.3 Synthesis of 5
The general procedure for AA + BB CuAAC polyaddition was applied to α,ω-dialkyne perfluoroether 1 (750 mg, 1.54 mmol), α,ω-diazido-tetra(ethylene glycol) 3 (376 mg, 1.54 mmol), CuIP(OEt)3 (11 mg, 0.03 mmol) and diisopropylethylamine (199 mg, 1.54 mmol) in DMF (6.0 mL) to yield PT 5 as a yellow viscous oil (997 mg, 89.1%). 1H NMR (500 MHz, DMSO-d6, δ): 8.08 (s, CF2CH2OCH2C=CH, 2H), 4.68 (bs, CF2CH2OCH2C=CH, 4H), 4.50 (t, J = 5.0 Hz, NCH2CH2OCH2CH2O, 4H), 4.08 (t, J = 9.5 Hz, CF2CH2OCH2C=CH, 4H), 3.78 (t, J = 5.0 Hz, NCH2CH2OCH2CH2O, 4H), 3.47 (t, J = 2.0 Hz, NCH2CH2OCH2CH2O, 4H), 3.41 (t, J = 3.5 Hz, NCH2CH2OCH2CH2O, 4H). 13C NMR (125 MHz, DMSO-d6, δ): 142.45 (CF2CH2OCH2C=CH), 122.76 (t, J = 278 Hz, OCH2CF2OCF2CF2OCF2CF2OCF2CH2O), 124.61 (CF2CH2OCH2C=CH), 69.45 (NCH2CH2OCH2CH2O), 69.39 (NCH2CH2OCH2CH2O), 68.52 (NCH2CH2OCH2CH2O), 67.49 (t, J = 30 Hz, CF2CH2OCH2C=CH), 64.33 (CF2CH2OCH2C=CH) 49.27 (NCH2CH2OCH2CH2O). 19F NMR (282 MHz, DMSO-d6, δ): − 76.74 to − 76.78 (OCH2CF2 OCF2CF2OCF2CF2OCF2CH2O, 4F, 4F), − 88.18 to − 88.64 (OCH2CF2 OCF2CF2OCF2CF2OCF2CH2O, 8F).
2.4 Synthesis of Poly(1,2,3-triazolium)s 6 and 7
2.4.1 General Procedure for N-alkylation of 1,2,3-Triazole Groups: Synthesis of 6
A solution of PT 4 (400 mg, 0.54 mmol of 1,2,3-triazole groups) and CH3TFSI (800 mg, 2.71 mmol) in acetonitrile (5 mL) was stirred for 48 h at 85 °C. The solution was evaporated under vacuum to give a crude product which was then dissolved in acetonitrile, precipitated three times in Et2O and evaporated under vacuum to afford PTIL 6 as a brown viscous oil (712 mg, 99.0%). 1H NMR (300 MHz, DMSO-d6, δ): 8.9 (s, CF2CH2OCH2C=CH, 2H), 4.99 (bs, CF2CH2OCH2C=CH, 4H), 4.58 (t, J = 7.5 Hz NCH2CH2(CH2)8CH2CH2N, 4H), 4.28 (bs, CF2CH2OCH2C=CH, 4H), 4.22 (s, CH3N, 6H), 1.90 (bs, NCH2CH2(CH2)8CH2CH2N, 4H), 1.23 (m, NCH2CH2(CH2)8CH2CH2N, 16H). 13C NMR (100 MHz, DMSO-d6, δ): 139.28 (CF2CH2OCH2C=CH), 130.30 (CF2CH2OCH2C=CH), 124.04,122.45,117.98 (OCH2CF2OCF2CF2OCF2CF2OCF2CH2O), 119.38 (q, J = 320 Hz, CF3SO2N), 68.40 (t, J = 30 Hz, CF2CH2OCH2C=CH), 61.15 (CF2CH2OCH2C=CH), 53.46 (NCH2CH2(CH2)8CH2CH2N), 38.28 (CH3N), 28.91–28.29 (NCH2CH2(CH2)8CH2CH2N). 19F NMR (282 MHz, DMSO-d6, δ): − 76.74 to − 76.83 (OCH2CF2 OCF2CF2OCF2CF2OCF2CH2O, 4F), − 78.75 (s, CF3SO2N, 12F), − 87.85 to − 88.40 (OCH2CF2 OCF2CF2OCF2CF2OCF2CH2O, 8F).
2.4.2 Synthesis of 7
The general procedure for N-alkylation was applied to 5 (600 mg, 0.88 mmol of 1,2,3-triazole groups) and CH3TFSI (1.21 g, 4.10 mmol) in acetonitrile (7.0 mL) to yield PTIL 7 as a dark brown viscous oil (1.06 g, 98.6%). 1H NMR (500 MHz, DMSO-d6, δ): 8.87 (s, CF2CH2OCH2C=CH, 2H), 5.01 (bs, CF2CH2OCH2C=CH, 4H), 4.79 (t, J = 5.0 Hz, NCH2CH2OCH2CH2O, 4H), 4.27 (bs, CF2CH2OCH2C=CH, 4H), 4.24 (s, CH3N, 6H), 3.90 (t, J = 4.5 Hz, NCH2CH2OCH2CH2O, 4H), 3.54 (t, J = 5.0 Hz, NCH2CH2OCH2CH2O, 4H), 3.46 (t, J = 4.0 Hz, NCH2CH2OCH2CH2O, 4H). 13C NMR (100 MHz, DMSO-d6, δ): 138.91 (CF2CH2OCH2C=CH), 130.39 (CF2CH2OCH2C=CH), 122.44, (t, J = 348 Hz, OCH2CF2OCF2CF2OCF2CF2OCF2CH2O), 119.37 (q, J = 320 Hz, CF3SO2N), 69.49 (NCH2CH2OCH2CH2O), 69.64 (NCH2CH2OCH2CH2O) 68.14 (t, J = 30 Hz, CF2CH2OCH2C=CH), 67.31 (NCH2CH2OCH2CH2O), 60.80 (CF2CH2OCH2C=CH), 53.18 (NCH2CH2OCH2CH2O), 38.01 (CH3N). 19F NMR (282 MHz, DMSO-d6, δ): − 76.86 (s, CF2CH2O, 4F), − 78.80 (s, CF3SO2N, 12F), − 87.92 (t, OCF2CF2OCF2CF2OCF2CH2O, 8F).
3 Results and Discussions
3.1 Synthesis and Physical Properties of PTs 4 and 5
α,ω-Dialkyne perfluoroether monomer 1 was first synthesized in 71.7% yield by alkylation reaction between 1H,1H,11H,11H-perfluoro-3,6,9-trioxaundecane-1,11-diol and propargyl bromide (Scheme S1). The structure and purity of 1 were confirmed by 1H, 13C and 19F NMR spectroscopy (Fig. S1–S3). PTs 4 and 5 (Scheme 1) were then synthesized by AA + BB CuAAC polyaddition accordingly to the previously optimized reaction conditions, i.e. 20 wt% of dialkyne 1 and diazide 2 or 3 in N,N-dimethylformamide (DMF) using 1 equiv. DIPEA and 0.05 equiv. CuIP(OEt)3 as catalytic system [30]. After 72 h of reaction at 60 °C, the obtained polymers were purified by precipitation in Et2O and copper catalyst was removed by liquid/liquid extraction using CHCl3 and aqueous solutions of PMDETA. Solubility study was then performed with several solvents (Table S1). PTs 4 and 5 are insoluble in water, n-heptane, Et2O and toluene (PhCH3) while they are soluble at 10 mg/mL in CHCl3, CH2Cl2, ethyl acetate (EtOAc), tetrahydrofuran (THF), acetonitrile (CH3CN), DMF, and acetone. However, only PT 5 is soluble at 1 mg/mL in methanol (MeOH) and dimethylsulfoxide (DMSO).
1H NMR spectroscopy (Fig. 1) confirmed the structure of PTs 4 and 5 by the presence of the characteristic signal of the 1,2,3-triazole protons at 8.12 and 8.08 ppm for 4 and 5, respectively. Moreover, the absence of signals corresponding to propargyl and azidomethyl chain ends proved that polymers having relatively high polymerization degrees (Xn) were obtained. DSC confirmed that PT 4 containing dodecyl segments is semi-crystalline with a melting temperature (Tm) of 68 °C and a glass transition temperature (Tg) of − 21 °C (Fig. S4). Conversely, PT 5 containing tetra(ethylene glycol) (TEG) segments is amorphous with a Tg of − 32 °C (Table 1). The presence of the perfluoroether segment affords a decrease of Tm and/or Tg values compared to previously reported perhydrogenated PT analogues having either an undecanoyl (Tm = 107 °C and Tg = − 9 °C) or a triethylene glycol (Tg = − 23 °C) spacer between 1,2,3-triazole groups [26]. However, Tg values of are higher than those reported by Habas et al. for semi-crystalline and amorphous fluorinated PTs obtained from the AA + BB CuAAC polyaddition of a longer perfluoropolyether dialkyne and different fluorinated diazides (Tg values below − 103 °C) [62, 63]. TGA results (Fig. S5) show that PTs 4 and 5 undergoes 10% weight loss (Td10) at 335 and 340 °C, respectively, which is in par with PT analogues having an undecanoyl or a triethylene glycol spacer (i.e. Td10 = 340 and 345 °C, respectively) [26].
3.2 Synthesis and Physical Properties of PTILs 6 and 7
PTILs 6 and 7 were then obtained by selective N-alkylation at the N-3 position of the 1,2,3-triazole groups of PTs 4 and 5 using CH3TFSI in CH3CN at 85 °C for 48 h. After purification by precipitation in Et2O, the structures of PTILs 6 and 7 were confirmed by careful assignment of 1H, 13C and 19F NMR spectra. 1H NMR of 6 and 7 (Fig. 2) shows the quantitative N-alkylation of 1,2,3-triazole groups with the appearance of the N-3 methyl signal at ca. 4.2 ppm with a 3:1 ratio according to the 1,2,3-triazolium signal at ca. 8.9 ppm as well as the total disappearance of the 1,2,3-triazole signal at ca. 8.1 ppm which characteristic of the PT precursors. Moreover, significant shifts were observed for the chemical displacements of most signals of the protons neighbouring the 1,2,3-triazole/1,2,3-triazolium groups (Fig. S6 and S7). 13C NMR (see e.g. Fig. S8 for PT 5 and PTIL 7) have also been used to further confirm the quantitative nature of the N-alkylation reaction: the peak corresponding to 1,2,3-triazole signal at 124.98 ppm disappeared, while new signals at 38.01, 130.39 and 119.38 ppm were ascribed to the carbon atoms of the N-3 methyl and 1,2,3-triazolium groups as well as the TFSI counter-anions, respectively. Furthermore, N-alkylation of the 1,2,3-triazole groups was also corroborated by the appearance of the trifluoromethyl signal from the TFSI counter-anions at − 78.75 ppm in the 19F NMR spectra (Fig. S9). All the above evidence a successful and quantitative N-alkylation reaction at the N-3 position of the 1,2,3-triazole groups of PTs 4 and 5.
As their neutral precursors 4 and 5, PTILs 6 and 7 are insoluble in H2O, heptane, Et2O and PhCH3 while they are soluble at 10 mg/mL in acetone, THF, CH3CN, and DMF (Table S1). However, as generally observed, several changes in solubility occur after N-alkylation of the 1,2,3-triazole groups [2]. Indeed, conversely to their neutral precursors 4 and 5, PTILs 6 and 7 are insoluble in CH2Cl2, CHCl3 and EtOAc. Finally, while PT 4 is only partially soluble at 1 mg/mL in MeOH and DMSO, corresponding PTIL 6 is fully soluble at 10 mg/mL in these solvents. Moreover, whereas dodecyl-containing PT 4 is semi-crystalline, PTIL 6 is amorphous with a significant decrease of Tg from − 21 to − 38 °C, respectively. Conversely, N-alkylation of the 1,2,3-triazole groups has only a limited impact on tetra(ethylene glycol)-containing derivatives as Tg values slightly increase from − 32 to − 29 °C for PT 5 and PTIL 7, respectively (Fig. S4). These differences are mainly due to the loss of crystallinity of PTIL 6 after N-alkylation of 4, while both PT 5 and PTIL 7 are amorphous. It is worth noting that the presence of more bulky and less flexible perfluoroether segments affords an increase in Tg compared to previously reported perhydrogenated PTIL analogues having an undecanoyl or a triethylene glycol spacer between the 1,2,3-triazolium groups (Tg = –68/− 35 °C and − 38/− 29 °C for fluorinated and hydrogenated analogues, respectively) [26]. TGA measurements (Fig. S5) demonstrate the good thermal stability of PTILs 6 and 7 that exhibit Td10 values above 340 °C (Table 1). These values are comparable to those reported for PTIL analogues having an undecanoyl or a triethylene glycol spacer between the 1,2,3-triazolium groups (Td10 = 370 and 340 °C, respectively). However, it is surprising that Td10 values of PTILs 6 and 7 are ca. 5 °C higher than for PTs 4 and 5 (Td10 = 335 and 340 °C, respectively) while thermal stability generally decreases after N-alkylation of the 1,2,3-triazole groups [2]. This is most probably a positive consequence of the presence of the perfluoroether segments.
3.3 Ionic Conductivity of PTILs 6 and 7
The temperature dependence of the ionic conductivities of PTILs 6 and 7 was investigated by broadband dielectric spectroscopy (BDS, Fig. S10). σDC values measured under anhydrous conditions are reported as a function of reciprocal temperature in Fig. 3. As generally observed for glassy materials, ionic conductivities of 6 and 7 above Tg follow a Vogel–Fulcher–Tammann (VFT) dependence. Temperature-dependent ionic conductivities of PTILs 6 and 7 were thus fitted with the VFT Eq. (1),
with σ∞ the ionic conductivity in the limit of high temperatures, B the fitting parameter related to the activation energy of ionic conduction, and T0 the Vogel temperature which generally ranges from 30 to 70 K below Tg. The parameters affording the best fittings of the experimental curves are reported in Table 1. PTILs 6 and 7 exhibit relatively high and comparable values of anhydrous ionic conductivity (σDC at 30 °C = 1.2 × 10−5 and 8.4 × 10−6 S/cm, respectively). This difference is consistent with the evolution of Tg values measured by DSC, i.e. Tg increases from − 38 to − 29 °C for 6 and 7, respectively. Obtained values are in the upper range of values previously measured for TFSI-containing PTILs which range from 1.0 × 10−7 to 6.7 × 10−5 S/cm [2, 64]. σDC of PTIL 6 (issued from dodecyl and perfluoroether units) is comparable to a previously reported triethylene glycol-based PTIL obtained by AB + AB CuAAC polyaddition and having a comparable Tg of − 35 °C [26]. However, σDC of PTIL 7 (issued from tetraethylene glycol and perfluoroether units) is lower than both PTIL 6 and above mentioned triethylene glycol-based PTIL (σDC at 30 °C = 8.4 × 10−6, 1.2 × 10−5 and 1.6 × 10−5 S/cm, respectively). This most probably means that the perfluoroether units are more compatible with dodecyl units than tetraethylene glycol ones. The impact of the addition of different lithium salts (i.e. 5 wt% of LiSO3CF3, 5 wt% of LiTFSI, or 5 and 15 wt% of LiClO4) was then investigated by BDS (Fig. S11 and S12) using PTIL 7 as ether units are known to enable a better solubilization and dissociation of lithium salts compared to e.g. aliphatic units found in PTIL 6. Indeed, the solubilization and compatibilization of an additional lithium salt is essential to afford an enhancement of ionic conductivity. While addition of 5 wt% LiSO3CF3 affords a ca. 40-fold decrease of σDC at 30 °C (σDC at 30 °C = 2.2 × 10−7 S/cm), ionic conductivity is almost unchanged by the addition of 5 wt% LiTFSI. However, addition of 5 wt% LiClO4 affords a twofold increase in σDC reaching 1.5 × 10−5 S/cm at 30 °C (Fig. S12). Further addition of 15 wt% LiClO4 induces a drastic ca. 115-fold decrease in ionic conductivity (σDC = 6.9 × 10−8 S/cm at 30 °C). This is most likely due to the aggregation and insufficient solubilization of such amount of LiClO4 in the PTIL matrix.
4 Conclusion
This paper successfully demonstrates the synthesis and characterization of two PTILs combining the attractive features of AA + BB CuAAC polyaddition and quantitative N-alkylation of 1,2,3-triazole groups together with the unique properties of perfluoroether materials. Although having Tg values above those that could be expected for fluorinated materials, obtained perfluoroether-based PTILs display high ionic conductivity with σDC at 30 °C in the 10−5 S/cm range. Moreover, thermal stability is positively impacted by the presence of fluorinated segments compared to other previously reported PTIL analogues. Both PTILs afford remarkably high ionic conductivity values although the dodecyl-based PTIL 6 affords a value ca. twice as high of tetraethylene glycol-based PTIL 7. LiClO4 proved to be the best additive as a ca. twofold increase in ionic conductivity was observed when 5 wt% were added to PTIL 7. In summary, our study provides an important insight into the structure/properties relationship of PTILs with main chain perfluoroether segments. The high level of ionic conductivity reached together with high content of fluorine atoms make these materials potential candidates for functional polymer electrolytes required for future lightweight and flexible diplays, solar cells, batteries and other electrochemical devices. The search for other fluorinated PTIL derivatives capable of more intensely improving ion transport without lithium salts is currently explored.
References
Yuan J, Mecerreyes D, Antonietti M (2013) Poly(ionic Liquid)s: an update. Prog Polym Sci 38:1009–1036
Obadia MM, Drockenmuller E (2016) Poly(1,2,3-Triazolium)s: a new class of functional polymer electrolytes. Chem Commun 52:2433–2450
Shaplov AS, Ponkratov DO, Vygodskii YS (2016) Poly(ionic Liquid)s: synthesis, properties, and application. Polym Sci Ser B 58:73–142
Qian W, Texter J, Yan F (2017) Frontiers in poly(ionic liquid)s: syntheses and applications. Chem Soc Rev 46:1124–1159
Chen M, White BT, Kasprzak CR, Long TE (2018) Advances in phosphonium-based ionic liquids and poly(ionic liquid)s as conductive materials. Eur Polym J 108:28–37
Ajjan FN, Ambrogi M, Tiruye GA, Cordella D, Fernandes AM, Grygiel K, Isik M, Patil N, Porcarelli L, Rocasalbas G, Vendramientto G, Zeglio E, Antonietti M, Mecerreyes D, Moreno M, Taton D, Solin N, Yuan J (2017) Innovative polyelectrolytes/poly(ionic liquid)s for energy and environment. Polym Int 66:1119–1128
Zhou X, Weber J, Yuan J (2018) Poly(ionic liquid)s: platform for CO2 capture and catalysis. Curr Opin Green Sustain Chem 16:39–46
Lambert R, Wirotius AL, Vignolle J, Taton D (2019) C-C couplings in water by micellar catalysis at low loadings from a recyclable polymer-supported Pd(ii)-NHC nanocatalyst. Polym Chem 10:460–466
Lee CP, Ho KC (2018) Poly(ionic liquid)s for dye-sensitized solar cells: a mini-review. Eur Polym J 108:420–428
Kohno Y, Saita S, Men Y, Yuan J, Ohno H (2015) Thermoresponsive polyelectrolytes derived from ionic liquids. Polym Chem 6:2163–2178
Shaplov AS, Ponkratov DO, Aubert PH, Lozinskaya EI, Plesse C, Maziz A, Vlasov PS, Vidal F, Vygodskii YS (2014) Truly solid state electrochromic devices constructed from polymeric ionic liquids as solid electrolytes and electrodes formulated by vapor phase polymerization of 3,4-ethylenedioxythiophene. Polymer 55:3385–3396
Zulfiqar S, Sarwar MI, Mecerreyes D (2015) Polymeric ionic liquids for CO2 capture and separation: potential, progress and challenges. Polym Chem 6:6435–6451
Cowan MG, Gin DL, Noble RD (2016) Poly(ionic liquid)/ionic liquid ion-gels with high “free” ionic liquid content: platform membrane materials for CO2/light gas separations. Acc Chem Res 49:724–732
Tomé LC, Marrucho IM (2016) Ionic liquid-based materials: a platform to design engineered CO2 separation membranes. Chem Soc Rev 45:2785–2824
Men Y, Kuzmic D, Yuan J (2014) Poly(ionic liquid) colloidal particles. Curr Opin Colloid Interface Sci 19:76–83
Zhang W, Kochovski Z, Lu Y, Schmidt BVKJ, Antonietti M, Yuan J (2016) Internal morphology-controllable self-assembly in poly(ionic liquid) nanoparticles. ACS Nano 10:7731–7737
He H, Rahimi K, Zhong M, Mourran A, Luebke DR, Nulwala HB, Moeller M, Matyjaszewski K (2017) Cubosomes from hierarchical self-assembly of poly(ionic liquid) block copolymers. Nat Commun 8:14057–14064
Margaretta E, Fahs GB, Inglefield DL, Jangu C, Wang D, Heflin JR, Moore RB, Long TE (2016) Imidazolium-containing ABA triblock copolymers as electroactive devices. ACS App Mater Interfaces 8:1280–1288
Guterman R, Ambrogi M, Yuan J (2016) Harnessing poly(ionic liquid)s for sensing applications. Macromol Rapid Commun 37:1106–1115
Moon HC, Lodge TP, Frisbie CD (2016) Electrochemiluminescent displays based on ion gels: correlation between device performance and choice of electrolyte. J Mater Chem C 4:8448–8453
Kim HJ, Chen B, Suo Z, Hayward RC (2020) Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 367:773–776
Shaplov AS, Marcilla R, Mecerreyes D (2015) Recent advances in innovative polymer electrolytes based on poly (ionic liquid)s. Electrochim Acta 175:18–34
Eshetu GG, Mecerreyes D, Forsyth M, Zhang H, Armand M (2019) Polymeric ionic liquids for lithium-based rechargeable batteries. Mol Syst Des Eng 4:294–309
Osada I, de Vries H, Scrosati B, Passerini S (2015) Ionic-liquid-based polymer electrolytes for battery applications. Angew Chem Int Ed 55:500–513
Forsyth M, Porcarelli L, Wang X, Goujon N, Mecerreyes D (2019) Innovative electrolytes based on ionic liquids and polymers for next-generation solid-state batteries. Acc Chem Res 52:686–694
Abdelhedi-Miladi I, Obadia MM, Allaoua I, Serghei A, Ben Romdhane H, Drockenmuller E (2014) 1,2,3-Triazolium-based poly(ionic liquid)s obtained through click chemistry polyaddition. Macromol Chem Phys 215:2229–2236
Obadia MM, Crépet A, Serghei A, Montarnal D, Drockenmuller E (2015) Expanding the structural variety of poly(1,2,3-triazolium)s obtained by simultaneous 1,3-dipolar Huisgen polyaddition and N-alkylation. Polymer 79:309–315
Obadia MM, Fagour S, Vygodskii YS, Vidal F, Serghei A, Shaplov AS, Drockenmuller E (2016) Probing the effect of anion structure on the physical properties of cationic 1,2,3-triazolium-based poly(ionic liquid)s. J Polym Sci Part A Polym Chem 54:2191–2199
Wang C, Li H, Zhang H, Sun R, Song W, Xie M (2018) Enhanced ionic and electronic conductivity of polyacetylene with dendritic 1,2,3-Triazolium-oligo(ethylene glycol) pendants. Macromol Chem Phys 219:1800025
Flachard D, Serghei A, Fumagalli M, Drockenmuller E (2019) Main-chain poly(1,2,3-triazolium hydroxide)s obtained through AA + BB click polyaddition anion exchange membranes. Polym Int 68:1591–1598
Anaya O, Haddane A, Drockenmuller E, Abdelhedi Miladi I, Ben Romdhane H (2019) Poly(1,2,3-triazolium imide)s obtained through AA + BB click polyaddition. Chem Afr 2:713–721
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A Stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 41:2596–2599
Hawker CJ, Wooley KL (2005) The convergence of synthetic organic and polymer chemistries. Science 309:1200–1205
Golas PL, Matyjaszewski K (2007) click chemistry and ATRP: a beneficial union for the preparation of functional materials. QSAR Comb Sci 26:1116–1134
Sumerlin BS, Vogt AP (2010) Macromolecular engineering through click chemistry and other efficient transformations. Macromolecules 43:1–13
Arslan M, Tasdelen MA (2019) Click chemistry in macromolecular design: complex architectures from functional polymers. Chem Afr 2:195–214
Obadia MM, Mudraboyina BP, Serghei A, Phan TNT, Gigmes D, Drockenmuller E (2014) Enhancing properties of anionic poly(ionic liquid)s with 1,2,3-triazolium counter cations. ACS Macro Lett 3:658–662
Sood R, Zhang B, Serghei A, Bernard J, Drockenmuller E (2015) Triethylene glycol-based poly(1,2,3-triazolium acrylate)s with enhanced ionic conductivity. Polym Chem 6:3521–3525
Kallel Elloumi A, Abdelhedi Miladi I, Serghei A, Taton D, Aissou K, Ben Romdhane H, Drockenmuller E (2018) Partially biosourced poly(1,2,3-triazolium)-based diblock copolymers derived from levulinic acid. Macromolecules 51:5820–5830
Obadia MM, Colliat-Dangus G, Debuigne A, Serghei A, Detrembleur C, Drockenmuller E (2015) Poly(vinyl ester 1,2,3-triazolium)s: a new member of the poly(ionic liquid)s family. Chem Commun 51:3332–3335
Zhou X, Obadia MM, Venna SR, Roth EA, Serghei A, Luebke DR, Nulwala HB (2016) Highly cross-linked polyether-based 1,2,3-triazolium ion conducting membranes with enhanced gas separation properties. Eur Polym J 84:65–76
Ye L, Wan L, Tang J, Li Y, Huang F (2018) Novolac-based poly(1,2,3-triazolium)s with good ionic conductivity and enhanced CO2 permeation. RSC Adv 8:8552–8557
Puguan JMC, Jadhav AR, Boton LB, Kim H (2018) Fast-switching all-solid state electrochromic device having main-chain 1,2,3-triazolium-based polyelectrolyte with extended oxyethylene spacer obtained via click chemistry. Sol Energy Mater Sol Cells 179:409–416
Puguan JMC, Kim H (2019) A switchable single-molecule electrochromic device derived from a viologen-tethered triazolium-based poly(ionic liquid). J Mater Chem A 7:21668–22167
Tejero R, López D, López-Fabal F, Gómez-Garcés JL, Fernández-García M (2015) Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym Chem 6:3449–3459
Tan W, Lia Q, Donga F, Zhanga J, Luana F, Weia L, Chena Y, Guoa Z (2018) Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: synthesis, characterization, and antifungal property. Carbohydr Polym 182:180–187
Costa CM, Silva MM, Lanceros-Méndez S (2013) Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv 3:11404–11417
Ameduri B (2009) From vinylidene fluoride (VDF) to the applications of VDF-containing polymers and copolymers: recent developments and future trends. Chem Rev 109:6632–6686
Soulestina T, Ladmiral V, Dos Santos FD, Améduri B (2017) Vinylidene fluoride- and trifluoroethylene-containing fluorinated electroactive copolymers. How does chemistry impact properties? Prog Polym Sci 72:16–60
Valade D, Boschet F, Roualdes S, Ameduri B (2009) Preparation of solid alkaline fuel cell binders based on fluorinated poly(diallyldimethylammonium chloride)s [poly(DADMAC)] or poly(chlorotrifluoroethylene-co-DADMAC) copolymers. J Polym Sci Part A Polym Chem 47:2043–2058
Guterman R, Berven BM, Corkery TC, Nie HY, Idacavage M, Gillies ER, Ragogna PJ (2013) Fluorinated polymerizable phosphonium salts from PH3: surface properties of photopolymerized films. J Polym Sci Part A Polym Chem 51:2782–2792
Cordella D, Ouhib F, Aqil A, Defize T, Jerome C, Serghei A, Drockenmuller E, Aissou K, Taton D, Detrembleur C (2017) Fluorinated poly(ionic liquid) diblock copolymers obtained by cobalt-mediated radical polymerization-induced self-assembly. ACS Macro Lett 6:121–126
Chen S, Funtan A, Gao F, Cui B, Meister A, Parkin SSP, Binder WH (2018) Synthesis and morphology of semifluorinated polymeric ionic liquids. Macromolecules 51:8620–8628
Yang ZZ, Zhao Y, Ji G, Zhang H, Yu B, Gao X, Liu Z (2014) Fluoro-functionalized polymeric ionic liquids: highly efficient catalysts for CO2 cycloaddition to cyclic carbonates under mild conditions. Green Chem 16:3724–3728
Liu H, Huang L, Cheng X, Hu A, Xu H, Chen L, Chen Y (2016) N-type Self-doping of fluorinate conjugated polyelectrolytes for polymer solar cells: modulation of dipole, morphology, and conductivity. ACS Appl Mater Interfaces 9:1145–1153
Schadt K, Kerscher B, Thomann R, Mülhaupt R (2013) Structured semifluorinated polymer ionic liquids for metal nanoparticle preparation and dispersion in fluorous compartments. Macromolecules 46:4799–4804
Gao Q, Pan X, Buregeya PI, Lu P, Zhang X, Yan X, Hu Z, Chen S (2018) Stable anion exchange membranes derived from fluorinated poly(aryl ethers) with quaternized fluorene units for fuel cell applications. J Appl Polym Sci 135:46301–46310
Pang HW, Yu HF, Huang YJ, Li CT, Ho KC (2018) Electrospun membranes of imidazole-grafted PVDF-HFP polymeric ionic liquids for highly efficient quasi-solid-state dye-sensitized solar cells. J Mater Chem A 6:14215–14223
Lopez G, Granado L, Coquil G, Lárez-Sosa A, Louvain N, Améduri B (2019) Perfluoropolyether (PFPE)-based vitrimers with ionic conductivity. Macromolecules 52:2148–2155
Neto V, Granet R, Krausz P (2010) Novel class of non-ionic monocatenary and bolaform alkylglycoside surfactants. Synthesis by microwave-assisted glycosylation and olefin cross-metathesis or by “click-chemistry”: physicochemical studies. Tetrahedron 66:4633–4646
Goswami LN, Houston ZH, Sarma SJ, Jalistgi SS, Hawthorne MF (2013) Efficient synthesis of diverse heterobifunctionalized clickable oligo(ethylene glycol) linkers: potential applications in bioconjugation and targeted drug delivery. Org Biomol Chem 11:1116–1126
Lopez G, Améduri B, Habas JP (2016) A versatile strategy to synthesize perfluoropolyether-based thermoplastic fluoropolymers by alkyne-azide step growth polymerization. Macromol Rapid Commun 37:711–717
Lopez G, Améduri B, Habas JP (2017) A perfluoropolyether-based elastomers library with on-demand thermorheological features. Eur Polym J 95:207–215
Jourdain A, Serghei A, Drockenmuller E (2016) Enhanced ionic conductivity of a 1,2,3-triazolium-based poly(siloxane ionic liquid) homopolymer. ACS Macro Lett 5:1283–1286
Acknowledgements
The authors gratefully acknowledge the financial support from the ANR through the OLIBLOCK project (ANR-17-CE08-0011-02), the Tunisian Ministry of Higher Education and Scientific Research (University of Tunis El Manar), the “Coopérations et mobilités internationales Rhône-Alpes” (CMIRA) and IDEXLYON.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anaya, O., Kallel Elloumi, A., Thankappan, H. et al. Synthesis and Structure/Properties Correlations of Fluorinated Poly(1,2,3-triazolium)s. Chemistry Africa 3, 759–768 (2020). https://doi.org/10.1007/s42250-020-00164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00164-1