Abstract
Potentially useful MgO NPs were synthesized via rapid eco-friendly one-pot microwave-assisted green techniques using Taxus wallichiana leaf extract. The development, morphology, and other physicochemical properties of nanoparticles were further analyzed by UV visible spectrometer, FTIR, XRD, SEM, and EDX analysis. The developed nanoparticles were uniformly distributed having particle sizes less than 30 nm. The XRD and SEM analysis of the developed nanoparticles reveals that it is crystalline having almost spherical. Phenol red and rhodamine B are used as potential organic pollutants for the evaluation of the photocatalytic activity of the developed nanoparticles. For the screening of the antibacterial efficiency of the biogenic nanoparticles, five different bacterial pathogens were used. The nanoparticles exhibited significant antibacterial potential against the Bacillus spp., Klebsiella pneumonia, and Staphylococcus aureus with a zone of inhibition 23 mm, 20 mm, and 21 mm. The nanoparticles also show moderate antibacterial activity against Escherichia coli and Pseudomonas spp. with the zone of inhibition 14 mm and 16 mm. Furthermore, the antioxidant potential of the developed nanoparticles was investigated by using a DPPH radical scavenging assay. The present research showed that the developed nanoparticles were the potential low-cost and effective photocatalyst in the treatment of wastewater and also act as potential antibacterial and antioxidants. The use of Taxus wallichiana leaf extract in the fabrication of MgO nanoparticles was the first time reported in the present research work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a transdisciplinary area of science and technology that is involved in the synthesis application and handling of material with a size of less than 100 nm. During the last few decades, nanotechnology has become an emerging field of research interest [1]. Metal and metal oxide nanoparticles have captivated scrupulous research interest due to their extensive aptness in the diverse field of applications like the manufacturing of optical devices, sensors, pharmaceutical applications, and the field of biotechnology [2, 3]. The application of nanomaterials in various fields increases extensively and it received much priority as compared to the bulk material due to their large surface area [4]. The nanomaterials exhibit unique physical and chemical properties and the prominent reason behind their unique properties is their very high surface-to-volume ratio. Another important parameter that makes nanomaterials more useful as compared to bulk materials is their discrete energy levels. The bulk material has a continuous energy level [5]. The development of novel and sustainable methods for the manufacturing of inorganic metal oxide nanoparticles is a great dynamic area of research. Methods involved in the fabrication of nanomaterial are largely classified into two main categories, physical and chemical methods, but these methods are not very suitable for the synthesis of the materials utilized in the field of biological and medicinal applications [6, 7]. Recently, green technology has drawn more attention to the development of potentially useful ceramic nanoparticles during the last few decades due to certain advantages over the available ancient methods [8].
Green technology uses various kind of plant material and microorganism in the fabrication of different kind of potentially useful nanomaterials and the use of these materials significantly reduce the use of harmful toxic chemical [9]. Hence, green route for the fabrication of nanoparticles is one of the most convenient and eco-friendly approaches for the development of nanoparticles. Different phytochemicals like polyphenols, flavonoids, vitamins, amino acids, and polysaccharides play a prominent role in the development of nanoparticles via the green route. These phytochemicals have remarkable medicinal value and have eco-friendly behavior. They are good replacements for harmful reducing agents like formaldehyde and sodium borohydride. There is an immense scope to develop new green technology methods for the development of different kinds of nanomaterials having adequate antibacterial antifungal and photocatalytic activity [10,11,12]. The use of the green synthesis method in the manufacturing of nanomaterials enhances the biomedical application of these materials [13].
The phytoconstituents play a prominent role in the development of nanomaterials. These phytoconstituents not only play as a capping, stabilizing, and reducing agent but also play a crucial role in the modification of the shape and size of the nanoparticles [14].
Clean and safe water is one of the most essential parts of the entire living organism. Water contamination of the present water resources has become one of the most serious issues due to rapid industrialization. Heavy metal ions, dyes, and residues of pharmaceutical and agricultural waste are the major sources of water contamination [15]. Nanomaterials especially metal oxide nanoparticles receive much attention in the treatment waster by the advanced oxidation process. In the advanced oxidation process, highly reactive reaction intermediates such as hydroxyl free radical play a prominent role in the treatment of wastewater and the metal oxide nanoparticles can efficiently produce these reactive species on exposure to solar and UV light [16].
The ceramic nanoparticles have noticeable optical, physical, chemical, mechanical, and thermo-mechanical properties due to their very high surface-to-volume ratio. Among them, particularly magnesium oxide nanoparticles have notable properties and show excellent photocatalytic biomedical and pharmaceutical applications. It acts as an efficient photocatalyst in the treatment of wastewater due to its wide band energy gap, high thermal conductivity, and good mechanical strength. Tremendous application of magnesium oxide nanoparticles in the field of sensing, wastewater treatment, as an antimicrobial, in the electronics in clothing, and photography increased its market value enormously [17].
The use of microwaves in the field of synthesis of materials has been increasing rapidly due to their rapid heating and consequently reduces the time of synthesis by increasing the rate of reaction [18]. Not very much significant work has been reported on the use of microwaves in the development of magnesium oxide nanoparticles via the green route. Hence, in this manuscript, we highlight a simple low-cost eco-friendly robust method for the fabrication of MgO NPs by utilizing the aqueous leaf extract of the Taxus wallichiana.
2 Experimental
2.1 Materials
The materials used in this study are ethanol (99%), magnesium nitrate (99%), NaOH (99%), phenol red (90%), and rhodamine B (90%). All these chemicals are purchased from the Sisco Research Laboratory Pvt., Ltd. The plant materials are collected from different parts of Uttarakhand, India. The plant materials are authenticated by the Botany Department of the FRI Dehradun. The bacterial strains used in this study are received from the Microbiology Department of the university.
2.2 Isolation of leaf extract
The isolated leaf was washed properly with double distilled and it was kept in the room for 4–5 days to remove the moisture. The dried leaf is cut into fine pieces and then converted into fine powder by using an electric blender. Twenty-five milligrams of fine powder of leaf along with 250 mL of ethanol as a solvent was placed into the Soxhlet extraction assembly. The process of extraction is completed in approximately 4–5 h. When the extraction was completed the isolated leaf extract was stored in an airtight container [19].
2.3 Microwave-assisted green synthesis of MgO NPs
For the microwave-assisted green synthesis of MgO NPs, 10 mL isolated leaf extract of Taxus wallichiana was mixed in the 50 mL (0.2 M) of freshly prepared Mg (NO3)2 6H2O. The prepared mixture was now subjected to microwave irradiation for about 40–50 s. The rapid change from colorless to brown of the reaction mixture was observed. The color change initially indicates the development of MgO NPs. The developed nanoparticles were collected by centrifugation of the test solution at 20,000 rpm for about 30 min [20].
2.4 Characterization
The UV spectroscopic analysis of the developed nanoparticles was performed by using UV visible spectrophotometer (Systonics double-beam spectrophotometer) in the range of 200–800 nm. To appraise the presence of different functional groups on the surface of the developed nanoparticles as reducing and stabilizing agents, FTIR analysis was performed by using Thermo Scientific Nicolet Summit LITE iD1 FTIR. The crystallographic behavior of the developed nanoparticles was explored by using a Rigaku X-ray diffractometer in this analysis; the wavelength of the radiation is 1.5406 Å and operated at 40 kV and 40 mA (2θ = 10–80). The particle size distribution and the morphological behavior of the developed nanoparticles were estimated by using FE SEM (Zeiss evo-18). The metallic compositions of the developed nanoparticles were examined by using (EDX) energy-dispersive X-ray analyzer.
2.5 Photocatalytic activity
The photocatalytic potential of the developed nanoparticles were examined against the phenol red dye and rhodamine B dye. The 0.25 g of the biologically synthesized MgO NPs was dispersed in the 100 mL aqueous solution of the phenol red and rhodamine B dye. The prepared dye solution was stirred for about 18 h in the dark to establish adsorption and desorption equilibrium between the dye and the prepared nanoparticles. Now the solution was placed in the solar light and UV light (310 nm) and observed any color variation. The photocatalytic potential of the fabricated nanoparticles was evaluated by measuring the absorbance of the centrifuged aliquot (2 mL) of the test solution at a regular interval of 10 min. The maximum absorbance (λmax) of phenol red and rhodamine B dye was measured by UV spectrophotometer. The maximum absorbance for phenol red and rhodamine was observed at 560 nm and 530 nm, respectively [21].
2.6 Antibacterial study
The antibacterial efficacy of the biologically synthesized MgO NPs was examined by a well-diffusion method. For an antibacterial study, the bacterial microorganisms Bacillus spp., Pseudomonas, Klebsiella pneumonia, Staphylococcus aureus, and Escherichia coli were obtained from the Microbiology Department of the university. The E coli is a rod-shaped gram-positive human pathogenic bacteria. The Bacillus spp. and Staphylococcus aureus are gram-positive bacteria. The Pseudomonas and Klebsiella pneumonia (anaerobic, rod-shaped bacteria that causes nosocomial infection) are also gram-negative human pathogenic bacteria. The bacterial culture spread on the Mueller–Hinton agar medium at 35 °C. After the solidification of the agar medium, 6-mm-diameter four wells were formed by punching with a borer. In the three wells, 30 µL of the different concentrations (20%, 40%, and 60%) of green synthesized MgO NPs were inoculated with the help of a micropipette, and in one well gentamycin (50 µg/L) was inoculated as a positive control. The developed plates were incubated at 37 °C for about 24 h and the diameter of the zone of inhibition was measured in mm. The diameter of the zone of inhibition was measured by using the transparent ruler from the back of the bacterial plate [22].
2.7 Antioxidant study
The antioxidant activity of the developed nanoparticles was investigated by utilizing the 2,2-diphenyl picrylhydrazyl (DPPH) radical scavenging assay. To perform the antioxidant activity first prepare the different concentration (20, 30, 40, 50, 60, 70 μg/mL) of the MgO nanoparticles. Now, 1 mL (0.1 mM) of the DDPH solution was mixed in to test the solution of the MgO NPs, and the test tube was kept in the dark for about 30 min. After 30-min incubation, the absorbance of the test solution was evaluated at 517 nm. Ascorbic acid is used as a reference compound in this study [23]. The following equation (Eq. 1) was used in the evaluation of the radical scavenging activity of the developed MgO NPs.
where A0 represents the absorbance of control and At represents the absorbance of the sample.
3 Results and discussion
3.1 Characterization
The UV visible spectral analysis is one of the simplest methods to evaluate the optical properties of microwave-assisted biologically developed MgO NPs. The UV spectra of the developed MgO NPs can be examined in the range of 200–800 nm. The figure shows a strong absorption peak at 330 nm [24, 25]. The appearance of an absorption peak at 330 nm indicates the formation of MgO nanoparticles (Fig. 1).
3.2 Characterization of green synthesized MgO NPs by XRD analysis
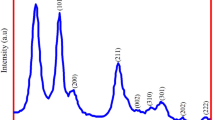
The XRD analysis of the developed MgO NPs is depicted in Fig. 2. The developed nanoparticles were found to be nanocrystalline in the nature on the basis of their XRD pattern. The different intense diffraction peak appears at 2Ɵ value of 37.86°, 43.13°, 64.87°, 74.86°, and 78.95°. These 2Ɵ values are assigned to the (111), (200), (220), (311), and (222) cubic plane of the developed MgO NPs [26]. The average crystalline size of the biologically developed MgO NPs is 22.5, which is evaluated by Debye Scherrer’s formula (Fig. 2).
3.3 FTIR
The bonding and the chemical behavior of the fabricated nanoparticles were evaluated by using FTIR analysis. The FTIR analysis is shown in the figure. The peaks found at 3425 cm−1 ascribed to the presence of the OH group which is probably due to the presence of the phenolic group (Fig. 3). The peak appears at 2410 cm−1 indicating the presence of the alkynes. The band observed at 1640 cm−1 is probably due to the C = C stretching of alkenes. A band that appears at 1380 cm−1 is probably due to the aromatic rings. Moreover, the band at 2840 cm−1 is assigned to the stretching vibration of the C–H bond of the C = C. The band that appears at 863 cm−1 is due to the sulfur compound. The band appears at 685 cm−1 associated with the stretching vibration of the Mg–O–Mg. Further, the peak that appears at 493 cm−1 could be attributed to the stretching vibration of the C–Cl bond of the alkyne group [27,28,29].
3.4 SEM and EDX studies
The detailed morphological analysis of the biogenic developed MgO NPs was carried out by using SEM analysis and it indicates that the particles are round with partially agglomerated (Fig. 4). The agglomerations of the fabricated nanoparticles are basically due to the van der Waal forces. The developed nanoparticles have different particle sizes and the size was found closer to 24 nm. The SEM analysis pictures of the fabricated nanoparticles are shown in the figure. The surface morphological studies of the fabricated nanoparticles are helpful in the identification of their photocatalytic and medical applications [30].
The fabrication of MgO NPs was further confirmed by EDX analysis. EDX profile of the developed MgO NPs shows strong absorption peak for Mg and O. The other peaks for different elements are most probably present to the phytoconstituents used in the fabrication of MgO NPs (Fig. 5).
3.5 The antibacterial study
The dose-dependent manner is utilized to evaluate the antibacterial efficacy of the developed nanoparticles against the selected bacterial pathogen. The antibacterial potential of newly synthesized MgO NPs is demonstrated in Table 1.
The microwave-assisted green synthesized MgO NPs significantly lethal to the selected microbial organism. The newly synthesized MgO NPs show great antibacterial activity against Bacillus spp., Klebsiella pneumonia, and Staphylococcus aureus and further show less activity against E. coli and Pseudomonas spp. The result of the antibacterial study also established that the antibacterial potential against all the selected pathogens increased as the concentration of the synthesized nanoparticles was increased (Fig. 6).
3.6 Mechanistic study of the antibacterial activity
The synthesized nanoparticles show antibacterial behavior in three well-defined ways: (i) the synthesized nanoparticles are lethal against the bacterial strain because of their small size they readily rapture the cell wall of the bacterial strain, (ii) the entered nanoparticles damage the intercellular structure like mitochondrial vacuoles and ribosomes of the bacterial strain, and (iii) another way which was generally involved in the antibacterial phenomenon of the developed nanoparticles was the production of reactive oxygen species; these species were responsible for cellular toxicity and oxidative stress [31]. This ultimately causes the death of the bacterial strain (Fig. 7).
3.7 The photocatalytic activity
The photocatalytic efficacy of the developed MgO NPs was attributed to exploring their ability to remove organic dyes like phenol red and rhodamine B from the wastewater released from various industries. The maximum absorbance of the phenol red and rhodamine B was measured as a time interval. The measurement was carried out at its maximum absorbance. The degradation efficiency of the developed nanoparticles against the phenol red and rhodamine in the presence of solar light and UV light (310 nm) was demonstrated in Figs. 8 and 9. The result of the photocatalytic activity indicates that the maximum absorption band of both dyes decreases subsequently with time. According to Lambert–Beer law, concentration is directly proportional to absorbance; hence, the degradation potential can be evaluated by the following equation (Eq. 2).
where C0 and C represent the concentration of dye at time t = 0 and t = t.
3.8 Mechanistic study of the photocatalytic behavior of the developed MgO NPs
The mechanism behind the photocatalytic degradation of dye in solar light and in UV light in the presence of green synthesized MgO NPs is almost similar. The main reaction involved in the photodegradation of dye is the photooxidation of dye by the reactive oxygen species. These reactive oxygen species are generated by electron transfer. When the green synthesized MgO NPs irradiated with light (UV or solar light) electron–hole pair is generated in the MgO nanostructure (Fig. 10). The electron interacts with the molecular oxygen and gives a reactive oxygen species superoxide radical anion and the generated hole interacts with a water molecule to produce hydroxyl free radical species [32]. These reactive species are responsible for the photodegradation potential of the developed MgO NPs (Fig. 11).
3.9 Antioxidant activity
Figure 11 demonstrates the antioxidant potential of the developed nanoparticles. The oxygen donating substance present on the surface morphology of the developed MgO NPs is responsible for the antioxidant behavior of the MgO NPs. The color DPPH solution is changed in this protocol which is the primary indication of the antioxidant behavior of the component (Fig. 12). The synthesized nanoparticles show low antioxidant activity as compared to the ascorbic acid that is used as a positive control in this experiment. The microwave-assisted green synthesized MgO NPs show DPPH radical scavenging activity in the range of 31.41 to 80.13% [33].
4 Conclusion
In summary, the present research highlights a very efficient eco-friendly low-cost method to develop MgO NPs. The structural characterization of the developed nanoparticles was monitored by standard analytical tools like UV, XRD, FTIR, and SEM analysis. With the help of XRD and SEM analysis, it was stabilized that the particle size of the developed nanoparticles was found to be less than 30 nm and has a spherical shape. The developed MgO NPs have good antibacterial activity against the selected pathogen with the zone of inhibition 23 mm, 21 mm, and 20 mm, respectively, for Bacillus spp., Staphylococcus aureus, and Klebsiella pneumonia. The synthesized nanoparticles also exhibited moderate antibacterial phenomenon against the E. coli and Pseudomonas spp. The catalytic potential of the synthesized nanoparticles was examined by the degradation of the two carcinogenic dyes phenol red and rhodamine B. The microwave-assisted green synthesized MgO nanoparticles show great antioxidant potential in the DPPH assay. The finding of our study established that the synthesized nanoparticles have significant applications in pharmaceutical industries.
Data availability
The data made available on reasonable request.
References
H. Kaur, S. Sing, J. Rawat, Expanding horizon: green synthesis of TiO2 nanoparticles using Carica papaya leaves for photocatalysis application. Mater Res Expr 6, 095034 (2019). https://doi.org/10.1088/2053-1591/ab2ec5
W. Ahmad, A. Pandey, V. Rajput, V. Kumar, M. Verma, H. Kim, Plant extract mediated cost-effective tin oxide nanoparticles: a review on synthesis, properties, and potential applications. Curr Res Green Sustain Chem 4, 100211 (2021). https://doi.org/10.1016/j.crgsc.2021.100211
W. Ahmad, B.S. Chandra, M. Verma, V. Kumar, H. Kim, A review on current trends in the green synthesis of nickel oxide nanoparticles, characterizations, and their applications. Environ Nanotechnol Monitor Manage 18, 100674 (2022). https://doi.org/10.1016/j.enmm.2022.100674
Y.K. Mohanta, S.K. Panda, R. Jayabalan, N. Sharma, Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front Mol Biosci 4, 1–9 (2017)
A. Rajan, A.R. Rajan, D. Philip, Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. Open Nano 2, 1–8 (2017)
S. Navalón, H. García, Nanoparticles for catalysis. Nanomaterials 6, 123 (2016)
L. Dykman, N. Khlebtsov, Gold nanoparticles in biomedical applications: recent advances and perspectives to biological and medical. Chem Soc Rev 41, 2256–2282 (2012)
N. Kaur, A. Singh, W. Ahmad, Microwave assisted green synthesis of silver nanoparticles and its application: a review. J Inorg Organomet Polym 33, 663–672 (2023). https://doi.org/10.1007/s10904-022-02470-2
N.M. Noah, P.M. Ndangili, Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sensors International 3, 100166 (2022)
W. Ahmad, V. Singh, S. Ahmed, M. Nur-e-Alam, A comprehensive study on antibacterial antioxidant and photocatalytic activity of Achyranthes aspera mediated biosynthesized Fe2O3 nanoparticles. Results Eng 14, 100450 (2022). https://doi.org/10.1016/j.rineng.2022.100450
W. Ahmad, K.K. Jaiswal, A. Bajetha, N. Naresh, R. Verma, I. Banerjee, Microwave-irradiated bio-fabrication of TiO2 nanoparticles stabilized by phytoconstituents from Phyllanthus emblica seeds and its antibacterial activities. Inorg Nano-Metal Chem (2023). https://doi.org/10.1080/24701556.2023.2184385
S. Jadoun, R. Arif, N.K. Jangid, R.K. Meena, Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19, 355–374 (2021). https://doi.org/10.1007/s10311-020-01074-x
E.E. Hossam, M.K. El-Bisi, Merely Ag nanoparticles using different cellulose fibers as removable reductant. Cellulose 21, 4219–4230 (2014)
A.B. Hanan, M.K. Zahran, E.E. Hossam, Heatless synthesis of well dispersible Au nanoparticles using pectin biopolymer. Int J Boil Macromol 91, 208–219 (2016)
T. Naseem, T. Durrani, The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: a review. Environ Chem Ecotoxicol 3, 59–75 (2021)
H. Kumari, S. Sonia, A review on photocatalysis used for wastewater treatment: dye degradation. Water Air Soil Pollut. 234, 349 (2023). https://doi.org/10.1007/s11270-023-06359-9
W. Ahmad, A. Singh, K.K. Jaiswal, P. Gupta, Green synthesis of photocatalytic TiO2 nanoparticles for potential application in photochemical degradation of ornidazole. J Inorg Organomet Polym Mater 31, 614–623 (2021). https://doi.org/10.1007/s10904-020-01703-6
M.E. Khan, A. Mohammad, W. Ali, A.U. Khan, W. Hazmi, T. Yoo, Excellent visible-light photocatalytic activity towards the degradation of tetracycline antibiotic and electrochemical sensing of hydrazine by SnO2–CdS nanostructures. J Clean Prod 349(15), 131249 (2022)
W. Ahmad, J.K. Kumar, M. Amjad, Euphorbia herita leaf extract as a reducing agent in a facile green synthesis of iron oxide nanoparticles and antimicrobial activity evaluation. Inorg Nano-Met Chem 51, 1147–1154 (2021). https://doi.org/10.1080/24701556.2020.1815062
K. Seku, B.R. Gangapuram, B. Pejjai, Microwave-assisted synthesis of silver nanoparticles and their application in catalytic, antibacterial and antioxidant activities. J Nanostruct Chem. 8, 179–188 (2018). https://doi.org/10.1007/s40097-018-0264-7
G.B. Reddy, A. Madhusudhan, D. Ramakrishna, D. Ayodhya, M. Venkatesham, G. Veerabhadram, Green chemistry approach for the synthesis of gold nanoparticles with gum kondagogu: characterization, catalytic and antibacterial activity. J Nanostruct Chem 5, 185–193 (2015). https://doi.org/10.1007/s40097-015-0149-y
M. Amina, N.M. Al Musayeib, N.A. Alarfaj, M.F. El- Tohamy, H.F. Oraby, G.A. Al Hamoud, Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS ONE 15(8), e0237567 (2020). https://doi.org/10.1371/journal.pone.0237567
J. Flieger, W. Franus, R. Panek, M. Szymańska-Chargot, W. Flieger, M. Flieger, P. Kołodziej, Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 26(16), 4986 (2021). https://doi.org/10.3390/molecules26164986
M.A. Ammulu, K. Vinay Viswanath, A.K. Giduturi, Phytoassisted synthesis of magnesium oxide nanoparticles from Pterocarpus marsupium Roxb heartwood extract and its biomedical applications. J Genet Eng Biotechnol 19, 21 (2021). https://doi.org/10.1186/s43141-021-00119-0
J. Safaei-Ghomia, S. Zahedia, M. Javida, M.A. Ghasemzadeh, MgO nanoparticles: an efficient, green and reusable catalyst for the one-pot syntheses of 2,6-dicyanoanilines and 1,3-diarylpropyl malononitriles under different conditions. J Nanostruct 5, 153–160 (2015)
R. Dobrucka, Synthesis of MgO nanoparticles using Artemisia abrotanum herbal extract and heir antioxidant and photocatalytic properties. Iran J Sci Technol Trans Sci 42, 547–555 (2018). https://doi.org/10.1007/s40995-016-0076-x
T. Somanathan, T. Krishna, V.M. Saravanan, V. Kumar, R. Kumar, MgO nanoparticles for effective uptake and release of doxorubicin drug: pH sensitive controlled drug release. J Nanosci Nanotechnol 16, 9421–9431 (2016)
M. Vergheese, S. Kiran-Vishal, Green synthesis of magnesium oxide nanoparticles using Trigonella foenum-graecum leaf extract and its antibacterial activity. J Pharmacogn Phytochem 7, 1193–1200 (2018)
E.R. Essien, V.N. Atasie, T.O. Oyebanji, Biomimetic synthesis of magnesium oxide nanoparticles using Chromolaena odorata (L.) leaf extract. Chem Pap 74, 2101–2109 (2020). https://doi.org/10.1007/s11696-020-01056-x
S.K. Moorthy, C.H. Ashok, K.V. Rao, C. Viswanathana, Synthesis and characterization of MgO nanoparticles by neem leaves through green method. Mater Today Proc 2, 4360–4368 (2015)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, E.L. Vander, R.N. Muller, Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 110, 2574 (2010)
S. Narendhran, M. Manikandan, P. Baby Shakila, Antibacterial, antioxidant properties of Solanum trilobatum and sodium hydroxide-mediated magnesium oxide nanoparticles: a green chemistry approach. Bull Mater Sci 42, 133 (2019). https://doi.org/10.1007/s12034-019-1811-7
R. Essien, V.N. Astasie, A.O. Okeafor, D.O. Nwude, Biogenic synthesis of magnesium oxide nanoparticles using Manihot esculenta (Crantz) leaf extract. Int Nano Lett 10, 43–48 (2020). https://doi.org/10.1007/s40089-019-00290-w
Author information
Authors and Affiliations
Contributions
Waseem Ahmad: conception and design, material preparation, data collection, and analysis.
Sanjay Kumar: resources.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, W., Kumar, S. Taxus wallichiana leaf extract-mediated microwave-assisted one-pot biosynthesis of MgO NPs for biomedical and photocatalytic applications. emergent mater. 7, 1081–1090 (2024). https://doi.org/10.1007/s42247-023-00612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00612-x