Abstract
High alumina slag is widely found in pyrometallurgical extractions of ferronickel, ferrochromium, and platinum group metals. The effects of MgO, Al2O3, and CaO/SiO2 on the sulfur distribution ratio between high-alumina CaO–SiO2–MgO–Al2O3 slag and carbon-saturated iron were investigated. The slag consisted of Al2O3 content in the range of 27.61–40.00 wt.%, CaO/SiO2 ratio of 0.8–1.1, and MgO content of 8–16 wt.%. The theoretical liquid areas of CaO–SiO2–MgO–Al2O3 slag were analyzed through the phase diagrams. The sulfur distribution ratio was measured via the slag–metal equilibrium technique at 1500 °C. It was observed that the sulfur distribution ratio increased with higher MgO content and higher CaO/SiO2 ratio largely due to the increase in free O2− ions and the decrease in activity coefficient of sulfur ion in slag, but slightly decreased with the increasing Al2O3 content because of the decrease in free O2−.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is an increasing demand for the production of low sulfur and ultra-low sulfur steel due to the competitive steel market [1,2,3]. The presence of high sulfur in steel affects the workability of steel, lowers hot ductility and toughness, and deteriorates welding properties [4,5,6]. About 90% of the metal produced in the blast furnace is desulfurized before it is sent to be processed in a steelmaking furnace [7]. Sulfide capacity (CS) proposed by Fincham and Richardson [4, 8] and defined based on gas–slag equilibrium [9] is an effective concept to quantitatively characterize the removal of sulfur from metal. Previous researchers [10,11,12] also have used sulfide capacity to evaluate the desulfurization ability of slags based on slag–metal reactions due to the sulfide capacity of slag being related to the composition and temperature.
On the other hand, the use of lean ores leads to an increase in the Al2O3 content in slag, which deteriorates the physicochemical properties of the blast furnace slag and aggravates the difficulty of blast furnace operation. It can cause various problems, including poor fluidity of slag, larger volumes of slag, slag with a low desulfurization ability, difficulties in slag–iron separation, and a high smelting temperature [13,14,15,16,17]. Besides, high alumina slag is widely found in pyrometallurgical extractions of ferronickel, ferrochromium, and platinum group metals [18,19,20]. It is a general choice to increase the CaF2 or FeO contents of high alumina slag to lower the melting point of the high alumina slag and enhance the kinetic efficiency of the desulfurization process [21,22,23]. However, these measures have been restricted and treated carefully due to the harmful effects of CaF2 on human health and the significant iron losses caused by FeO. The effects of basicity, Al2O3, MgO, or other additives such as MnO and Na2O on the properties of CaO–SiO2–MgO–Al2O3 slag and desulfurization ability have been experimentally investigated by several researchers. Shankar et al. [24] reported that increasing CaO/SiO2 ratio (C/S) from 0.70 to 1.25 and increasing MgO content from 5 to 7 wt.% can significantly improve the sulfide capacity of CaO–SiO2–MgO–Al2O3 slag with more than 20 wt.% alumina. A similar result was obtained by Ma et al. [8] for the CaO–SiO2–MgO–Al2O3 slag at fixed 15 wt.% Al2O3. Xu et al. [25] measured the melting temperature of the CaO–SiO2–MgO–Al2O3 slag at fixed 7 wt.% MgO and the result indicated that the melting temperature decreased with the increase in the basicity from 2.08 to 4.87. Taniguchi et al. [26] also studied the sulfide capacity of CaO–SiO2–MgO–Al2O3 slag and found that the desulfurization ability of slag increased with the increase in MgO content from 0 to 4 mass% and MnO content from 0 to 5 mass%. Van Niekerk and Dippenaar [27] reported that the sulfur distribution ratio (LS) between carbon-saturated iron and Na2O–SiO2–CaO slag significantly increased and the melting temperature decreased with the addition of Na2O. Park and Min [28] found that Al2O3 decreased the sulfide capacity due to its acidic behavior.
In our study, to effectively enhance the desulfurization ability of high alumina slag, the sulfur distribution ratios between CaO–SiO2–MgO–Al2O3 slag with high alumina and carbon-saturated iron were investigated. First, the effects of MgO, Al2O3, and CaO/SiO2 ratio on the liquid areas and viscosities of the CaO–SiO2–MgO–Al2O3 slag were predicted thermodynamically using FactSage 8.0 software. Then, their effects on the distribution ratio of sulfur between high alumina slag and carbon-saturated molten iron were measured via slag–metal equilibrium technique at 1500 °C. The microstructure and phases of desulfurization slag were analyzed through scanning electron microscopy-energy dispersive spectrometry (SEM–EDS) technique. Our study will provide a technical basis for the desulfurization in the high-temperature smelting process with high alumina slag.
2 Experimental materials and methods
2.1 Raw materials
The high alumina slag and cast iron used in the desulfurization experiment were provided by a steel company in China. The chemical composition of the cast iron sample is shown in Table 1. The slag samples in the experiments were half-synthetic slag. The designed amounts of analytical-grade reagents CaCO3 (≥ 98 wt.%), SiO2 (≥ 99 wt.%), MgO (≥ 98 wt.%), and Al2O3 (≥ 99 wt.%) were added to the high alumina slag to adjust the slag compositions. The chemical composition of the high alumina slag and the designed chemical compositions of the half-synthetic slags are shown in Tables 2 and 3. All reagents CaCO3, SiO2, MgO, and Al2O3 were roasted at 1000 °C for 1 h to remove carbonate and water. Each slag sample was uniformly mixed in an agate mortar for at least 30 min. The mass ratio of slag to metal was set as 0.6:1 according to industrial production.
2.2 Methods
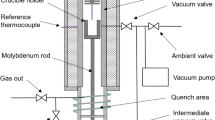
The experimental method employed is based on the equilibrium between liquid metal and liquid slag to measure the sulfur distribution ratio. Initially, 20 g powdered cast iron and 12 g half-synthetic slags were added to a graphite crucible (30 mm in diameter and 60 mm in height) which was held on a corundum brick. The furnace was heated up to a target temperature at the rate of 10 °C/min. When the temperature in the furnace rose to 1500 °C, the corundum brick and the graphite crucible containing the samples were put into the furnace as shown in Fig. 1 and kept for 6 h when the temperature in the furnace reached 1500 °C again. The N2 (> 99.99 vol.%) gas was introduced into the reaction tube to protect the samples and graphite crucibles from being oxidized in the whole heating process, with a flow rate of 3.5 L/min. When equilibrium was reached, the samples were taken out from the furnace and quenched into the cold water quickly. After being cooled, the iron was separated from the slag. The slag was dried and then crushed to less than 100 μm for chemical composition analysis. The iron was washed to remove surface residues. The sulfur contents of slag and iron were analyzed using a CS844 carbon–sulfur analyzer. The chemical composition of the slags was determined by chemical analysis methods. The sulfur distribution ratio of the slag is defined as w(S)/w[S], where w(S) is the mass percentage of sulfur in slag, and w[S] is the mass percentage of sulfur in iron.
3 Results and discussion
3.1 Liquid areas of CaO–SiO2–MgO–Al2O3 slag system
The FactSage software has been widely applied in the prediction of high-temperature processes with complex multi-component phase equilibria [29, 30]. Phase diagrams are crucial to understanding the correlation between the composition and thermodynamic properties of slags [5]. The phase diagrams were drawn by FactSage 8.0 software to predict the liquid areas of CaO–SiO2–MgO–Al2O3 slag. The liquid areas of CaO–SiO2–MgO–25 wt.% Al2O3 slag and CaO–SiO2–10 wt.% MgO–Al2O3 slag for the temperature of 1300–2000 °C are shown in Fig. 2a, b. The effects of Al2O3 and MgO contents on the liquid areas of CaO–SiO2–MgO–Al2O3 slag at 1500 °C are shown in Fig. 2c, d, respectively. As shown in Fig. 2, there are three dotted lines that represented the CaO/SiO2 ratio of 0.8, 1.0, and 1.2, respectively. In CaO–SiO2–MgO–25 wt.% Al2O3 slag system, when CaO/SiO2 is constant, the melting point of the slag first decreases and then increases with the increase in MgO content. Similarly, in CaO–SiO2–10 wt.% MgO–Al2O3 slag system, when CaO/SiO2 is constant, the melting point of the slag first decreases and then increases with the increase in Al2O3 content. This supports the conclusion that the melting point of slag increased with increasing the basicity from 0.8 to 1.1, Al2O3 content from 27.6 to 40.0 wt.%, and MgO content from 8 to 16 wt.% in CaO–SiO2–MgO–Al2O3 slag.
3.2 Reaction mechanism and sulfur distribution ratio
According to the two-film theory of the kinetic model for liquid–liquid phase reactions [31], the boundary layer characterizing the mass transfer resistance exists on both sides of the phase interface in the system consisting of molten metal and molten slag. The model of the desulfurization reaction between molten metal and molten slag is shown in Fig. 3. The desulfurization reaction can be described by the following steps:
-
(1)
Sulfur diffuses from the molten metal to the slag–metal interface, and the concentration of sulfur is decreased as it moves from the slag phase to the interface. In the following equations, where [M] is the M element in the hot metal, (O) is the oxide or ions in the slag, respectively.
$$\left[\mathrm{S}\right]\to {[\mathrm{S}]}^{*}$$(1) -
(2)
Interfacial chemical reaction takes place between sulfur and oxygen ion, and the monomeric sulfur is reduced and converted to sulfide.
$$ \left[ {\text{S}} \right] + \left( {{\text{O}}^{2 - } } \right) = \left( {{\text{S}}^{2 - } } \right) + \left[ {\text{O}} \right] $$(2) -
(3)
Generated sulfide diffuses from the interface into the slag, and the concentration of sulfide is decreased as it moves from the interface to the slag phase.
$$ \left( {{\text{S}}^{2 - } } \right)^{*} \to \left( {{\text{S}}^{2 - } } \right) $$(3)
The sulfide capacity has been defined as Eq. (5) deduced from Eq. (4):
where \({w}_{(\mathrm{S})}\) is the mass percentage of sulfur in slag; \({P}_{{\mathrm{O}}_{2}}\) and \({P}_{{\mathrm{S}}_{2}}\) are the partial pressures of O2 and S2, respectively; K1 is the equilibrium constant of reaction; \({a}_{{\mathrm{O}}^{2-}}\) is the activity of oxygen in slag; and \({\gamma }_{{\mathrm{S}}^{2-}}\) is the activity coefficient of sulfur ion in slag.
The equilibrium reaction of sulfur between slag and metal phases can be expressed as follows:
The sulfur distribution ratio between slag and metal can be related to sulfide capacity by incorporating Eqs. (4) and (6) as the following equation:
For Eq. (7),
where \({K}_{\mathrm{S}}\) is an equilibrium constant for reaction Eq. (7); \({f}_{\mathrm{S}}\) is the Henrian activity coefficient of sulfur in the hot metal; \({a}_{\mathrm{O}}\) and \({a}_{\mathrm{S}}\) are the activity of oxygen and sulfur in the hot metal, respectively; and T is temperature.
From Eqs. (8) and (9), the equilibrium sulfur partition ratio can be obtained as follows:
According to the Wagner model, the activity coefficient of sulfur or carbon in the hot metal can be obtained from Eq. (11):
where fi is the activity coefficient of species i; \({e}_{i}^{j}\) is the interaction parameter of species j on i; and \({w}_{[j]}\) is the concentration of species j in the hot metal, wt.%. The interaction parameters are given in Table 4.
The oxygen activity in the molten metal can be calculated by choosing the equilibrium of CO/C pairs, given in the following equation:
The equilibrium constant KC for reaction (12) is as follows:
where PCO is the partial pressure of CO.
The activity of carbon aC can be calculated by
The temperature dependence of the equilibrium constant is given by the following equation:
As shown in Fig. 3, the monomeric sulfur is reduced and converted to sulfide at the slag–metal reaction interface. Meanwhile, an equilibrium reaction of carbon and oxygen occurs as shown in Eq. (12). The overall atmosphere in the furnace is N2, but at the slag–metal reaction interface, there is partial pressure of CO. When the carbon-saturated metal droplet comes in contact with slag, partial pressure of CO could be taken as partial pressure of CO at the slag–metal reaction interface level and it can be taken as ∼ 101,325 Pa. Some researchers [4, 24] also supposed PCO of ∼ 101,325 Pa for similar study. Therefore, the oxygen activity can be obtained by using Eqs. (11)–(14).
Based on the above equations, the equilibrium sulfur partition ratio can be obtained as follows:
In accordance with experimental results at 1500 °C, the equation between \({L}_{\mathrm{S}}\) and \({C}_{\mathrm{S}}\) was:
The sulfur distribution ratio between slag and carbon-saturated iron is measured in the high level of Al2O3 in the range of 27.61–40.00 wt.%. The CaO/SiO2 ratio was between 0.8 and 1.1, and MgO was in the range of 8–16 wt.%. The experimental results are listed in Table 5.
3.3 Effects of CaO/SiO2 and (CaO + MgO)/SiO2 on \({{L}}_{{\varvec{S}}}\)
Figure 4a shows the effects of CaO/SiO2 on sulfur distribution ratio with 11.13 wt.% MgO and 27.61 wt.% Al2O3 at 1500 °C. It can be observed that the LS value increases from 24.29 to 65.38 with increasing the CaO/SiO2 ratio from 0.8 to 1.1. Figure 4b shows the variations in the effects of CaO/SiO2 on LS in CaO–SiO2–MgO–Al2O3 slag systems at 1500 °C. As illustrated in Fig. 4b, the results obtained by Zhang et al. [13] and Talapaneni et al. [4] are plotted for comparison. It can be seen from Fig. 5 that the effect of (CaO + MgO)/SiO2 ratio on the distribution ratio is similar to that of CaO/SiO2 ratio. It is evident that the LS increases with the increase in CaO/SiO2 or (CaO + MgO)/SiO2 at fixed Al2O3 content and MgO content. The increase in CaO/SiO2 ratio or (CaO + MgO)/SiO2 causes the disintegration of the silicate network structure, an increase in the proportions of free oxygen ions O2−, CaO, and Ca2+, and then the decrease in \({\gamma }_{{\mathrm{S}}^{2-}}\) [8, 13]. This indicated that the concentration of free O2− and \({\gamma }_{{\mathrm{S}}^{2-}}\) have strong effects on the desulfurization process, and a high concentration of free O2− and a low value of \({\gamma }_{{\mathrm{S}}^{2-}}\) promote the desulfurization performance.
3.4 Effects of Al2O3 on \({{{L}}}_{\text{S}}\)
Figure 6a shows the effects of Al2O3 on LS at a fixed CaO/SiO2 ratio of 0.9 and MgO content of 11.13 wt.%. It can be seen that the LS value slightly decreases from 24.71 to 22.31 with increasing the Al2O3 concentration from 27.61 to 40.00 wt.%. As shown in Fig. 6b, the Al2O3 content has a significant effect on the LS when the Al2O3 content in slag is below 20 wt.%. In this case, Al2O3 behaves as acidic oxides, and Al3+ ions in the slag combine with O2− ions to form AlO45− ions like a complex silicate network. As Al2O3 increases, the Ca2+ in the slag acts as a charge compensation ion and forms [Ca0.5(AlO4)]4−. This leads to the decrease in free O2− [4]. According to the CaO–SiO2–MgO–Al2O3 phase diagrams, the increasing Al2O3 content causes the formation of complex compounds with high melting points, such as spinel, thus worsening the kinetic condition for desulfurization.
3.5 Effects of MgO on \({{{L}}}_{{\varvec{S}}}\)
Figure 7a shows the dependence of LS on the MgO content in the CaO–SiO2–MgO–Al2O3 slag system at a fixed CaO/SiO2 ratio of 0.9 and Al2O3 content of 27.61 wt.% at 1500 °C. The experimental results show that the LS increases from 22.00 to 43.50 with increasing the MgO content from 8.00 to 16.00 wt.%. Correspondently, it can also be seen from Fig. 7b that the LS increases with increasing the MgO content at fixed CaO/SiO2 ratio and Al2O3 content in different CaO–SiO2–MgO–Al2O3 slag systems. This indicates that MgO has a similar effect as CaO on the desulfurization ability of slag [26]. MgO is well-known to behave as basic oxides and provide O2−, which can promote the desulfurization process. Also, it is observed from the different experimental results that CaO/SiO2 ratio has a greater effect on LS than MgO content. This is due to the fact that CaO has a higher optical basicity value (1.00) than MgO (0.78), and CaO could contribute more free O2− ions than MgO in the slag [4]. Therefore, MgO shows less effect on desulfurization ability than CaO.
3.6 Microstructure and phase analysis
Figure 8 shows the SEM–EDS images of desulfurization slag. It can be seen that the main phases contain light-gray silicate with high calcium and low magnesium, dark-gray silicate with high magnesium and low calcium, black spinel, and white ferrochrome. The sulfur is mainly distributed in the silicate phase and almost absent in the spinel phase and ferrochrome alloys. The EDS image can prove that the calcium ions and magnesium ions combine with sulfur ions to form ionic clusters, which promote the desulfurization reaction.
3.7 Comparison with sulfide capacity models
The experimentally determined sulfide capacities in the present study are compared with that calculated by different models in Refs. [32,33,34,35,36]. The sulfide capacity models for CaO–SiO2–MgO–Al2O3 slag systems are shown in Table 6. Figure 9 shows the effect of corrected optical basicity on sulfide capacities of CaO–SiO2–MgO–Al2O3 slag systems at 1500 °C. It is observed that sulfide capacity increases with increasing the corrected optical basicity of slags. The comparisons between predicted and experimentally determined sulfide capacity values for CaO–SiO2–MgO–Al2O3 slag systems at 1500 °C are shown in Fig. 10. It is evident that the sulfide capacity values predicted by Young et al. [36] are much higher than experimentally determined sulfide capacity values, which is also reported by other researchers [32, 34], while the models of Zhang et al. [32], Sosinsky and Sommerville [33], Hao and Wang [34], and Ren et al. [35] underpredict the sulfide capacity values. This may be because the above-mentioned models are based on optical basicity or corrected optical basicity and take no account of the effects of charge compensation of Ca2+ to [AlO4]5− on sulfide capacity.
4 Conclusion
The effects of MgO, Al2O3, and CaO/SiO2 ratio on the liquid areas of the CaO–SiO2–MgO–Al2O3 slag were predicted thermodynamically using FactSage 8.0 software, and then the effects of MgO, Al2O3, and CaO/SiO2 on the sulfur distribution ratio between CaO–SiO2–MgO–Al2O3 slag with high alumina and carbon-saturated iron were investigated via slag–metal equilibrium technique at 1500 °C. The liquid areas of CaO–SiO2–MgO–Al2O3 slag decreased with increasing Al2O3 content. Also, the LS increased with higher MgO content and higher CaO/SiO2 ratio largely due to the increase in free O2− icons and the decrease in \({\gamma }_{{\mathrm{S}}^{2-}}\) in slag, but slightly decreased with increasing Al2O3 content because of the decrease in free O2−. Besides, MgO showed less effect on desulfurization ability than CaO.
References
J. Yang, M. Kuwabara, T. Sakai, N. Uchida, Z. Liu, M. Sano, ISIJ Int. 47 (2007) 418–426.
D. Roy, P.C. Pistorius, R.J. Fruehan, Metall. Mater. Trans. B 44 (2013) 1086–1094.
W. Ma, H. Li, Y. Cui, B. Chen, G. Liu, J. Ji, ISIJ Int. 57 (2017) 214–219.
T. Talapaneni, N. Yedla, S. Sarkar, Metall. Res. Technol. 115 (2018) 502.
R. de Oliveira Pezzin, A.P.L. Berger, F.F. Grillo, E. Junca, H.S. Furtado, J.R. de Oliveira, J. Mater. Res. Technol. 9 (2020) 838–846.
M.Y. Mohassab-Ahmed, H.Y. Sohn, H.G. Kim, Ind. Eng. Chem. Res. 51 (2012) 3639–3645.
P.K. Iwamasa, R.J. Fruehan, Metall. Mater. Trans. B 28 (1997) 47–57.
X. Ma, M. Chen, H. Xu, J. Zhu, G. Wang, B. Zhao, ISIJ Int. 56 (2016) 2126–2131.
C.J.B. Fincham, F.D. Richardson, Proc. R. Soc. Lond. A 223 (1954) 40–62.
K. Zhang, Y. Zhang, T. Wu, Metals 8 (2018) 1068.
S.R. Simeonov, I.N. Ivanchev, A.V. Hainadjiev, ISIJ Int. 31 (1991) 1396–1399.
N.M. Anacleto, H.G. Lee, P.C. Hayes, ISIJ Int. 33 (1993) 549–555.
J. Zhang, X. Lv, Z. Yan, Y. Qin, C. Bai, Ironmak. Steelmak. 43 (2016) 378–384.
Z. Yan, X. Lv, J. Zhang, Y. Qin, C. Bai, Can. Metall. Quart. 55 (2016) 186–194.
L. Yao, S. Ren, X. Wang, Q. Liu, L. Dong, J. Yang, J. Liu, Steel Res. Int. 87 (2016) 241–249.
C.K. Du, J. Yang, X.Z. Zhao, Y.J. Shi, J.L. You, X.D. Gao, J. Iron Steel Res. 25 (2013) No. 7, 19–22.
C.Y. Xu, C. Wang, R.Z. Xu, J.L. Zhang, K.X. Jiao, Int. J. Miner. Metall. Mater. 28 (2021) 797–803.
T. Zhang, H. Zhang, S. Dai, D. Huang, W. Wang, Ceram. Int. 47 (2021) 22918–22923.
S.S. Biswal, C. Panda, S. Sahoo, T. Jena, K.C. Panda, Mater. Today Proc. 35 (2021) 97–101.
H. Zheng, Y. Ding, Q. Wen, S. Zhao, X. He, S. Zhang, C. Dong, Sci. Total Environ. 802 (2022) 149830.
L. Deng, X. Zhang, M. Zhang, X. Jia, J. Non-Cryst. Solids 500 (2018) 310–316.
K.D. Kim, W.W. Huh, D.J. Min, Metall. Mater. Trans. B 45 (2014) 889–896.
J.S. Han, J.G. Kang, J.H. Shin, Y. Chung, J.H. Park, Ceram. Int. 44 (2018) 13197–13204.
A. Shankar, M. Gornerup, S. Seetharaman, A.K. Lahiri, Metall. Mater. Trans. B 37 (2006) 941–947.
J.F. Xu, J.Y. Zhang, C. Jie, L. Tang, K. Chou, Adv. Mater. Res. 194–196 (2011) 169–174.
Y. Taniguchi, N. Sano, S. Seetharaman, ISIJ Int. 49 (2009) 156–163.
W.H. Van Niekerk, R.J. Dippenaar, ISIJ Int. 33 (1993) 59–65.
Y. Park, D.J. Min, ISIJ Int. 56 (2016) 520–526.
J. Tang, M. Chu, C. Feng, Y. Tang, Z. Liu, ISIJ Int. 56 (2016) 210–219.
T. Jiang, S. Wang, Y. Guo, F. Chen, F. Zheng, Metals 6 (2016) 107.
X.H. Huang, Principles of steel metallurgy, 4th, Metallurgical Industrial Press, Beijing, China, 2020.
G.H. Zhang, K.C. Chou, U. Pal, ISIJ Int. 53 (2013) 761–767.
D.J. Sosinsky, I.D. Sommerville, Metall. Trans. B 17 (1986) 331–337.
X. Hao, X. Wang, Steel Res. Int. 87 (2016) 359–363.
Z.S. Ren, X.J. Hu, K.C. Chou, J. Iron Steel Res. Int. 20 (2013) No. 9, 21–25.
R.W. Young, J.A. Duffy, G.J. Hassall, Z. Xu, Ironmak. Steelmak. 19 (1992) 201–219.
Acknowledgements
The authors gratefully acknowledge the support from the National Natural Science Foundation of China Project (52104345) and the Major Science and Technology Special Project of Yunnan Province (202002AB080001-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Jiang, Y., Guo, Yf. et al. Distribution ratio of sulfur between high alumina slag and carbon-saturated iron. J. Iron Steel Res. Int. 30, 1732–1742 (2023). https://doi.org/10.1007/s42243-022-00873-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-022-00873-6