Abstract
The liquidus temperature of the Fe–C–Mn–Si–Al alloy was investigated by using an improved differential thermal analysis method, which effectively tackles down the manganese evaporation in the course of differential thermal analysis experiments for high-manganese twinning-induced plasticity (TWIP) steels at high temperature. It was found that the liquidus temperature is more strongly dependent on the silicon content than expected. By considering the high manganese content in the Fe–C–Mn–Si–Al TWIP steels, the effect of carbon content on the depression coefficient of manganese should not be ignored, which has considerable impact on the liquidus temperature. An equation was summarized to effectively predict the liquidus temperature for a wide range of high-manganese steels. Meanwhile, the prediction results of the equation are consistent with the experimental results, as well as those results acquired from ThermoCalc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-manganese transformation-induced plasticity (TRIP) and twinning-induced plasticity (TWIP) steels have showed good mechanical properties, such as high strength, excellent ductility and high energy absorption capacities. It has aroused extensive concern recently in the academic field. These promising steels are regarded as new generation automotive steels due to the advantages of weight reduction, energy saving and safety performance for automobiles.
The high-manganese TRIP/TWIP steels comprise a large amount of manganese (15–25 mass%) as well as aluminum and silicon (2–4 mass%) [1,2,3,4,5]. Several researchers [6, 7] assert that the composition is the key factor for the performance of TRIP/TWIP steels. These Fe–C–Mn–Si–Al TWIP steels can take on preferable properties [8,9,10,11,12,13,14,15,16]. To ensure the quality for the production, it is of crucial significance to characterize the solidification for these high-alloy steels, especially to measure the liquidus temperature of these alloys.
In industrial production, accurate knowledge of liquidus temperature is of great importance for the determination of casting temperature. Casting temperature counts as the critical technological parameter in production and shall evidently affect the ultimate quality. Furthermore, the liquidus temperature is also essential for modeling the solidification behavior. For measuring the liquidus temperature, thermal analysis method can be a good way to obtain the results. It provides different curves of temperature (or heat flow) in which a peak deviating from baseline may indicate a phase transformation. Accordingly, the liquidus temperature (and solidus temperature) can be acquired directly.
The differential thermal analysis (DTA) and differential scanning calorimetry (DSC) are the commonly used thermal analysis methods. In the study of liquidus temperature, several researchers [17,18,19] have reported the liquidus temperature measurement through adopting DTA. DTA records any temperature difference between sample and reference sample while the material is undergoing identical thermal cycles. The typical DTA curve can be denoted through adopting the temperature difference (∆T) plotted on the ordinate with endothermic reactions downward and temperature or time on the abscissa increasing from left to right. The liquidus information is attained through analyzing the DTA curve. Many investigators highlighted that careful DTA calibration is of significance for correcting possible instrumental impacts and shall lay a foundation for reliable thermal analysis [18].
Additionally, liquidus temperature can also be obtained by existing data. For instance, the liquidus information of binary alloys can be directly grasped from the existing phase diagram. For multicomponent alloys, the standard approach sums the depressions of each component. Because each element has imposed on the melting point of pure iron, it is on the basis of the binary phase diagram without considering the interaction between solute elements. This method can get satisfied results for dilute solution because of negligible interaction between solute elements. Empirical regression equations shall readily obtain liquidus temperature and be quite conducive to engineers' working in the production plant. However, the valid range of element content is commonly limited in these equations. Such limitation is required to be noted for a successful prediction.
In accordance with the literature [20,21,22], researchers have studied liquidus temperature of various alloys. Yet, works on these high-alloy Fe–C–Mn–Si–Al steels have been rarely issued. Meanwhile, further researches indicate another issue that TWIP steels have large manganese content. It is well known that manganese is one of the volatile elements. The manganese evaporation during the high-temperature experiment shall contaminate the instrument and affect the precision of the experiment. An improved DTA method was developed here to measure the liquidus temperature of Fe–C–Mn–Si–Al steels. Additionally, an equation has been summarized from the experimental data to calculate the liquidus temperature. In contrast with other equations reported, the equation in this paper shall attain better predicted results.
2 Experimental
2.1 Problem of manganese evaporation
In this study, it is noteworthy that high manganese steels have a big trouble that manganese evaporation will contaminate the thermocouple and equipment during the high temperature environment [23]. As exhibited in Fig. 1, the cap of a crucible was coated by manganese after the DTA measurement for high manganese TWIP steels.
To avoid the damage, an improved DTA method was developed to measure liquidus information. The key to avoid the polution of the instrument is reduction of maganese volatilization at high temperature. Previous research has shown that the volatilization of manganese is related to the content of oxygen at high temperature [24]. The lower the oxygen content, the smaller the manganese evaporation. In this study, oxygen adsorbent material was used to reduce the oxygen content in crucible and reduce the manganese volatilization of TWIP steels. Therefore, the pollution caused by manganese evaporation was avoided. The schematic diagram can be seen in Fig. 2.
In this study, Netzsch STA409PG Luxx (Simultaneous Thermal Analyzer, Selb, Germany) was employed with a platinum DTA-TG sensor and type-S thermocouples. An additional tantalum lid was installed on the crucible; this special tantalum lid, with excellent ability for gas absorption, was made of tantalum metal. Zirconium getter under the DTA sensor was adopted to clean the protective gas in the oven. The layout of DTA sensor with zirconium getter and tantalum lid is exhibited in Fig. 3.
Good result was attained by the improved DTA measurement. The level of the manganese evaporation was alleviated and controlled in the crucible effectively. Volatile manganese has never escaped from the crucible to cause any damage. Figure 4 denotes the status of the crucible cap after high-temperature DTA measurement for TWIP steels. The crucible cap was coated with thick manganese layer in dark color when using the ordinary DTA method for TWIP steels (Fig. 4a). In contrast, through adopting the improved method under the same conditions, the manganese layer on the tantalum lid got thinner and was colored as brown. No manganese was found at the edge of tantalum lid or on the cap of the crucible as presented in Fig. 4b. It bespeaks that the manganese evaporation was alleviated. It was different from the status of ordinary DTA method since some manganese remained at the edge of the cap of the crucible in ordinary DTA method, which means that the manganese escaped from the edge of the crucible. The improved DTA method showed its advantages to control the evaporation of manganese in the center field. Additionally, no manganese leaked out. For this reason, the thermocouple and instrument would not be damaged during the high temperature DTA measurement for TWIP steels.
2.2 Differential thermal analysis
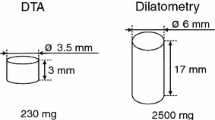
All samples for DTA were cut into a 3.0 mm × 2.1 mm × 2.1 mm cuboid. The mass of samples with these dimensions was around (100 ± 5) mg. The liquidus temperature was determined by the temperature at the endothermic peak maximum upon heating, which is a common method in the publication [25, 26].
For each measurement, the liquidus temperature was analyzed by multiple heating cycles. In this approach, the sample was heated to peak temperature following a fast heating rate. Thereupon, it cooled down 100 K to onset temperature of the melting. Before the next heating cycle, the sample was maintained isothermally for 30 s. Considering the contact efficiency between the crucible and the sample, the second heating circle with target heating rate was adopted for data extraction. An example of the DTA heating curves for the measuring liquidus temperature is shown in Fig. 5.
Each sample was measured at least three times at different heating rates under the identical conditions. Hence, at least three liquidus temperatures of one tested sample were acquired. Researches [26, 27] revealed that the liquidus temperature was intensively dependent on the heating rate. In this study, the equilibrium liquidus temperature was considered to be the final liquidus temperature due to eliminating the effect of heating rate. For accuracy, high-purity Au and Co with well-known melting points (TAu = 1337.55 K, TCo = 1766.15 K) were employed to offer a calibration. All measurements were taken under the same conditions in alumina crucibles with tantalum lids under protective gas atmosphere (argon, 99.9999%), and it had a flow of 70 cm−3 min−1 during controlled heating up to 1823.15 K.
Fifteen samples produced from a vacuum induction furnace were adopted to analyze the liquidus temperature. The compositions of the alloys are listed in Table 1.
3 Results and discussion
The liquidus temperatures of Fe–C–Mn–Si–Al steels with various compositions are listed in Table 2. Each steel sample has at least three liquidus results at different heating rates, and the equilibrium liquidus temperature of each sample is obtained through fitting the liquidus results of different heating rates, as shown in Table 2. It is noteworthy that all the experimental data were revised by the standard calibration.
The liquidus temperatures of these Fe–C–Mn–Si–Al alloys with various carbon contents range from 1619.7 and 1727.7 K. Normally, the addition of alloying elements (C, Mn, Si and Al) into the steel shall decrease the liquidus temperature. An equation can be given to explicate the relation between the selected elements and liquidus temperature.
where Tliq is the liquidus temperature; T0 is the melting temperature of the pure iron; C i is the content of element i, i = C, Si, Mn, Al, …, mass%; m, n and p are constant; f i (c) is depression coefficient function for element i, and it is related to the content of element i or may be related to the content of the other related element j; and c is the content of the related elements of depression coefficient f i (c), mass%. When the content is determined, the value of the depression coefficient function represents the slope of the liquidus of pseudobinary Fe-i phase diagram. For dilute solutions, the approach to calculate the liquidus temperature is to sum the depression of each component element based on the binary phase diagram with negligible interaction between solute elements [28]. Nevertheless, multicomponent alloys, especially high-alloy steels, are required to find out the principle relationship between component elements by elaborate experiments. The final results can be denoted as regression equations. Such method may have some restrictions, but it commonly brings a satisfactory prediction.
If a certain element in the alloy is not only high in content, but also with a large depression coefficient, it has a great influence on the liquidus temperature. For Fe–C–Mn–Si–Al steels, it has been observed that Si, C and Mn elements have a significant effect on the melting temperature. According to the results of regression analysis, fC seems roughly stable even though the content of another element varies. A pair of samples were used to calculate the unknown depression coefficient of one element through fixing that of other elements. By repeated iteration analysis, the evaluation of fSi and fMn is found to be the key factor to make the accurate prediction. Their relationship with different contents of elements Si or C is given in Table 3. The value of fSi changes in a polynomial with the increase in Si content, as presented in Fig. 6.
The other critical relationship is that the value of fMn increases with the increment in C content. The result of regression indicates that the slope of the tendency is very small, as shown in Fig. 7. But considering the considerable Mn content in the Fe–C–Mn–Si–Al steels, it has a considerable effect on the liquidus temperature.
Table 4 records all the results obtained from the regresion, and it also lists the equations of Kawawa [29] and Kagawa–Okamoto [30]. The values of depression coefficients fSi, fMn and fC in three equations are different. In Kawawa’s formula, fSi takes a value of 7.6, while in Kagawa–Okamoto’s formula, fSi takes a maximum value of 17.1 in the γ phase. In this study, however, the measured fSi has a minimum value of 18.7; in other words, with high-manganese steel, the literature suggests an underestimated fSi value, or to put it in another way, the fSi value as measured before no longer applies to this type of steel. This might be one of the reasons why the formula in the literature predicts a liquidus temperature that deviates remarkably from the measured value. For high-manganese steels, an accurate fSi value is of essential importance in order to arrive at more accurate liquidus prediction; the liquidus temperature is more strongly dependent on the silicon content than expected, and that is why more attention shall be paid to the effect of Si content.
According to the analysis results, the value of fMn has a smaller value and is correlated with carbon content. fC also indicates a smaller value except the case of γ phase in the study of Kagawa–Okamoto. As for the value of fAl, the results obtained from experimental data bespeak that fAl value is very small (fAl ≈ 0.6) in Fe–C–Mn–Si–Al alloy when the Al content is higher than 0.5%. It can be considered as zero when used for a quick prediction, and it has a very tiny impact on the liquidus temperature. The proposed equation in this study can be defined as:
Within the population of fifteen Fe–C–Mn–Si–Al alloys, the predictions obtained through adopting the current equations are compared with the experimental results in Fig. 8. The x axis is the prediction value, and the y axis is the measured value. If the slope is 1, it means that the prediction values perfectly match the measured values. It is found that the slope of the fitted regression line is 1.0017, approaching to 1, which means that the prediction values of the current equation agree fairly well with the experimental results.
The prediction results made by different authors are presented in Table 5. It is clear that the deviation is closely related to Si content. As listed in Table 5, deviation values from Kawawa’s equation are between − 20.39 and − 53.80 K when the Si contents range from 0.03 to 0.52% (TWIP1–TWIP10), whereas the deviation values become small (3.03 to − 14.72 K) when the Si contents are 2.75–4.63% (TWIP11–TWIP15). Therefore, the prediction from Kawawa’s equation can be considered as advisable when Si > 2.75%. The large error, which happens in the condition of Si < 0.52%, is the result of lacking any consideration of the relationship between Si content and fSi value.
Even though the equations of Kagawa and Okamoto take account of the alternative solidification phases, such effort can still not acquire a fine result for these high manganese steels. The large deviation values (− 7.33 to − 37.68 K) in low Si content range prove this fact. This is primarily because the interaction for Fe–C–Mn–Si–Al steels is inadequately reckoned in Kawawa and Kagawa–Okamoto’s equations. In this study, the current equation based on thermal analysis can obtain a good prediction of Fe–C–Mn–Si–Al steels, and it has small deviation values, less than 10 K.
The liquidus temperature of the alloys under investigation is also calculated with commercially available ThermoCalc (TCFE6 database) thermodynamics software. ThermoCalc thermodynamics software can basically predict the liquidus temperature of Fe–C–Mn–Si–Al steels, even though it has a worse prediction result in contrast with the current equation. Through more comparisons of a large range of Fe–C–Mn–Si–Al steels, in the overwhelming majority of cases, the gap between present equation and ThermoCalc is roughly within 10 K. It means that the current equation is suitable for a wide range of Fe–C–Mn–Si–Al steels. The minor exceptions are confirmed. It may become inappropriate for a small proportion of steels, which have Mn contents lower than 5% and high Si and Al contents (Si ≥ 2.5%, Al ≥ 3.0%). Even though the equation is limited, it also has a wide range of applicability in metallurgical engineering and can get satisfied predicted results for high manganese steels.
4 Conclusions
-
1.
To avoid the problem that manganese evaporation shall contaminate the thermocouple and equipment during the high-temperature DTA measurement for high manganese TWIP steels, an improved DTA method was developed to determine the liquidus temperature of Fe–C–Mn–Si–Al steels. It has been confirmed to be effective in tackling down the problem.
-
2.
The relationship of carbon content and fMn value is required to be highlighted for rational prediction of liquidus temperature for Fe–C–Mn–Si–Al steels, which is denoted as the value of fMn increases with the increment in C content. Si and C elements have a significant effect on the melting temperature of Fe–C–Mn–Si–Al steels. In this study, fC seems roughly stable, but the value of fSi changes in a polynomial with the increase in Si content.
-
3.
The predicted values of the current equation agree fairly well with the experimental results. The extended predictions of commercial thermodynamics software confirm that the current equation is suitable for a wide range of Fe–C–Mn–Si–Al steels and can get satisfied results. The proposed equation gives an applicative way to determine the liquidus temperature of high-manganese TWIP steels.
References
G. Frommeyer, U. Brüx, P. Neumann, ISIJ Int. 43 (2003) 438–446.
K.W. Li, C.L. Zhuang, J.H. Liu, S.B. Shen, Y.L. Ji, Z.B. Han, J. Iron Steel Res. Int. 22 (2015) No. S1, 75–79.
J. Yang, Y.N. Wang, X.M. Ruan, R.Z. Wang, K. Zhu, Z.J. Fan, Y.C. Wang, C.B. Li, X.F. Jiang, Metall. Mater. Trans. B 46 (2015) 1353–1364.
X.L. Xin, J. Yang, Y.N. Wang, R.Z. Wang, W.L. Wang, H.G. Zheng, H.T. Hu, Ironmak. Steelmak. 43 (2016) 234–242.
C. Zhuang, J. Liu, Z. Mi, H. Jiang, D. Tang, G. Wang, Steel Res. Int. 85 (2014) 1432–1439.
O. Grässel, L. Krüger, G. Frommeyer, L.W. Meyer, Int. J. Plast. 16 (2000) 1391–1409.
H. Ding, H. Ding, C.L. Qiu, Z.Y. Tang, J.M. Zeng, P. Yang, J. Iron Steel Res. Int. 18 (2011) No. 1, 36–40.
J. Yang, Y.N. Wang, X.M. Ruan, R.Z. Wang, K. Zhu, Z.J. Fan, Y.C. Wang, C.B. Li, X.F. Jiang, Metall. Mater. Trans. B 46 (2015) 1365–1375.
J.W. Cho, S. Yoo, M.S. Park, J.K. Park, K.H. Moon, Metall. Mater. Trans. B 48 (2017) 187–196.
Y.N. Wang, J. Yang, X.L. Xin, R.Z. Wang, L.Y. Xu, Metall. Mater. Trans. B 47 (2016) 1378–1389.
S.H. Wang, Z.Y. Liu, W.N. Zhang, G.D. Wang, J.L. Liu, G.F. Liang, ISIJ Int. 49 (2009) 1340–1346.
J.H. Kang, T. Ingendahl, W. Bleck, Mater. Des. 90 (2016) 340–349.
P. Lan, H. Tang, J. Zhang, Mater. Sci. Eng. A 660 (2016) 127–138.
A.E. Salas-Reyes, I. Mejía, A. Bedolla-Jacuinde, A. Boulaajaj, J. Calvo, J.M. Cabrera, Mater. Sci. Eng. A 611 (2014) 77–89.
M.H. Cai, H. Ding, Z.Y. Tang, H.Y. Lee, Y.K. Lee, Steel Res. Int. 82 (2011) 242–248.
D. Li, Y. Wei, C. Liu, L. Hou, Steel Res. Int. 84 (2013) 740–750.
W. Banda, G.A. Georgalli, C. Lang, J.J. Eksteen, J. Alloy. Compd. 461 (2008) 178–182.
R.I. Wu, J.H. Perepezko, Metall. Mater. Trans. A 31 (2000) 497–501.
M.J. Cieslak, C.R. Hills, P.F. Hlava, S.A. David, Metall. Trans. A 21 (1990) 2465–2475.
Z.B. Han, J.H. Liu, Y. He, Y.L. Ji, J. Liu, K.W. Li, J. Iron Steel Res. 28 (2016) No. 3, 40–47.
X. Wang, X. Wang, B. Wang, B. Wang, Q. Liu, Steel Res. Int. 82 (2011) 164–168.
L.I. Duarte, C. Leinenbach, J. Wang, Y. Plevachuk, V. Sklyarchuk, A. Korolyshyn, U. E. Klotz, J. F. Löffler, Int. J. Mater. Res. 102 (2011) 248–256.
C. Zhuang, J. Liu, C. Bernhard, P. Presoly, J. Iron Steel Res. Int. 22 (2015) 709–714.
B.D. You, B.W. Lee, J.J. Pak, Met. Mater. Int. 5 (1999) 497–502.
T.M. Pollock, W.H. Murphy, Metall. Mater. Trans. A 27 (1996) 1081–1094.
M.J. Cieslak, T.J. Headley, G.A. Knorovsky, A.D. Romig, T. Kollie, Metall. Trans. A 21 (1990) 479–488.
K. Gryc, B. Smetana, M. Žaludová, K. Michalek, P. Klus, M. Tkadlečková, L. Socha, J. Dobrovská, P. Machovčák, L. Válek, R. Pachlopnik, B. Chmiel, Mater. Technol. 47 (2013) 569–575.
Y.M. Won, B.G. Thomas, Metall. Mater. Trans. A 32 (2001) 1755–1767.
K. Kawakami, T. Kitagawa, K. Murakami, Y. Miyashita, Y. Tsuchida, T. Kawawa, Nippon Kokan Technical Report Overseas 36 (1982) 26–41.
A. Kagawa, T. Okamoto, Mater. Sci. Technol. 2 (1986) 997–1008.
Acknowledgements
It is gratefully acknowledged that the work presented in this paper has been supported by the Chair of Ferrous Metallurgy, Montanuniversitat Leoben, National Natural Science Foundation of China (No. 51704083), Education Department Foundation of Guizhou Province of China (No. [2017]118) and Research Foundation for Talents of Guizhou University (No. 201628). The author would like to thank Prof. Christian Bernhard, Prof. Johannes Schenk, Dr. Peter Presoly, Dr. Susanne Michelic, Bernd Lederhaas and Gerhard Wieser for their support of the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuang, Cl., Liu, Jh. Determination of liquidus temperatures in high-alloyed Fe–C–Mn–Si–Al steels using differential thermal analysis. J. Iron Steel Res. Int. 25, 546–553 (2018). https://doi.org/10.1007/s42243-018-0069-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-018-0069-4