Abstract
Recently, tissue engineering (TE) is one of the fast growing research fields due the accessibility of extra-molecular matrix (ECM) at cellular and molecular level with valuable potential prospective of hydrogels. The enhancement in the production of hydrogel-based cellular scaffolds with the structural composition of ECM has been accelerated with involvement of rapid prototyping techniques. Basically, the recreation of ECM has been derived from naturally existed or synthetic hydrogel-based polymers. The rapid utilization of hydrogels in TE puts forward the scope of bioprinting for the fabrication of the functional biological tissues, cartilage, skin and artificial organs. The main focus of the researchers is on biofabrication of the biomaterials with maintaining the biocompatibility, biodegradability and increasing growth efficiency. In this review, biological development in the structure and cross-linking connections of natural or synthetic hydrogels are discussed. The methods and design criteria that influence the chemical and mechanical properties and interaction of seeding cells before and after the implantations are also demonstrated. The methodology of bioprinting techniques along with recent development has also been reviewed. In the end, some capabilities and shortcomings are pointed out for further development of hydrogels-based scaffolds and selection of bioprinting technology depending on their application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tissue engineering can simply be defined as a field of science having multi-disciplinary nature of combining life sciences and engineering technology together with molecular biological attributes to improve the structural regeneration and growth of tissues for malfunctioning and clinical applications. The approach of the tissue engineering is to develop new techniques for biocompatible seed cells and tissues for different transplants directly or indirectly in the body. The materials used for TE should be biocompatible which means that they should be compatible without affecting the biological and physical nature of the living tissues. These biomaterials influence the focus of TE toward the development of reparative cells to make an efficient matrix that is biologically suitable for implantation through appropriate supportive scaffold with biocompatible nature and improve the growth of the tissue [1]. Recently, the statistics released by US department of Health and Services show that from 2012 to 2017, the number of patients of transplants raised from 28,054 to 34,770, respectively, with more than 114,000 patients who are still are on the waiting list [2]. The history of tissue engineering moves back to early 1970s, when the demand of transplantation of organs in medical field rose with less number of donor’s availability [3]. This leads to the combination of cell and organ developmental biology, implantation engineering, clinic medical and veterinary sciences, biomaterials and bio physics and mechanics [4]. Researchers started working on tissue engineering with combination of these fields for creating living tissues by utilizing the few cells from any particular body part and linking them with different biomaterials like polylactic acid [5], polyethylene glycol [6], hydrogel collagen [7] or different types of extracellular matrix [8] for covering the shortage of donors with fast recovery, improved quality and less complications after transplantation [9].
Tissue engineering includes the development of appropriate 3D scaffolds that should have the ability to provide a regenerative environment for the growth of tissues and organs. Typical biomaterial scaffolds are stuffed with bioreactors having the function of mechanical support and chemical stimuli to growing cells. These scaffolds may be directly implanted to the infected part or kept in vitro environment for synthesizing cells for further implantation [10]. The essential factors of tissue engineering are shown in Fig. 1.
The implanted cells or tissues with the supporting matrix have the ability to reconstruct within the scaffolds and regenerate the infected parts of the body. But in doing so, there are many challenges of regeneration of seeded cells, and then, transplantation has its own complications. The homogenous growth of the seeded cells into their parents’ cells within the scaffold is one of the biggest challenge. Secondly, the low-quality growth cells also affect the transplantation and badly influence the interaction of cell and supporting matrix. So to overcome these challenges of the tissue engineering, some natural or synthetic biopolymers are used in matrix interface to enhance the regeneration process [1, 11]. These matrix materials should pose the properties of reproducing themselves in different kinds of cells, and the process should be cost-effective and easily processed [12].
The main concept of this review is to explain the importance of hydrogels in the field of tissue engineering with the characteristics that enable such biomaterials to easily interact with particular tissues and can exist with normal working conditions in specific environment during direct implantations or in vivo/vitro. The stability and degradability of such materials are very important factors during tissue engineering applications. The classification of hydrogels is given on the basis of naturally and synthetically extracted biomaterials with recent development for improving the biocompatibility, biodegradability and mechanical properties. Further, additive manufacturing (AM) techniques for such biomaterials with their specific characteristics and design criteria are discussed. The characteristics of bioinks and criteria for selecting these bioinks for specific method of 3D printing with considerable limitations are also explained.
Hydrogels
Hydrogels belonged to hydrated polymeric materials having ≥ 90% of water content by weight. As hydrogels have greater pervading quality for oxygen and also no response for foreign bodies, they were applied in the field of biomedical as contact and intraocular lens from 1960s [13]. Researchers always worked on finding the materials for some replacement of extracellular matrix (ECM) for structural support of the cells, and hydrogels are one of the best materials as they have great resemblance with natural ECM. Hydrogels have the great ability to control cellular and molecular attachments with structural and functional integrity. They are also biocompatible and biodegradable in nature [9]. The faculties which hydrogels provide during tissue engineering are similar to properties of natural ECM, which helps in the attachment and migration with retaining the biochemical nature of the cells [14], enables easy and fast diffusion of the nutrients [9] with mechanical and biological support to enhance the growth of seeded cells [15]. The scaffolds can be manufactured with natural or synthetic hydrogels having resemblance with natural ECM.
The general benefits of the hydrogels are biocompatible, biodegradable and easily injected in vitro for growth after specific conditions, great mean of transportation of the nutrients during development process and can easily be modified for the usage in different places [16]. It also has some limitations: It usually provides low mechanical strength, is very difficult to handle, needs high sterilized conditions and is expensive for treatment.
Types of hydrogels
Hydrogels, on the basis of their nature, place of transplantation and requirement of support, can be classified into two main categories as naturally existed and synthetically prepared hydrogels. Both the types have their own advantages and uses. Naturally existed hydrogels possess natural ligands which provide significant adhesion to the cells and usually was obtained from natural ECM, but some are extracted from non-mammalian sources like brown seaweed alginate [17]. However, synthetically produced hydrogels can be customized chemically according to the application and usage of the material [18]. They lack the property of cell adhesion, but it can be improved through functionalization with ECM proteins.
Hydrogels with natural materials
The hydrogel polymers which originated from the national biomaterials are classified as natural hydrogels. Many hydrogels made of natural proteins such as collagen and gelatin are biodegradable, biocompatible with supportive nature during cellular attachment [16]. With these advantages, they also have some drawbacks like variation in properties during the preparation of every batch, difficult to synthesis and process and material properties are not constant [19]. In tissue engineering, the applications of naturally existed hydrogels are numerous as shown in Table 1.
Collagen
The most abundantly used protein as hydrogels is collagen, which is present almost 30% in the body of mammals [20]. In tissue engineering, collagen is the most important protein used because of its triple-standard helical structures with self-aggregating quality using covalent and hydrogen bonds. They construct different types of cartilages including fibrous or articular [4]. The quality of these proteins is the variation in their supportive linkage that can be changed according to the use in vivo [21] by utilizing their chemical [9] or physical cross-linking designs [22] or mixing with different polymers [23]. Collagen has the ability to form different kinds of gels, sponges, etc., which exist due to variation in strands of collagen and induction in the cross-linking.
It has a great potential for the production of hydrogels as it has resembling properties of natural ECM having the ability of enhancing cell functionality and adhesion properties. It can be used in the manufacturing of many types of artificial tissues like skin [24], cartilage [25], heart valves [26], breast reconstruction [27], vocal cord [9, 28] and spinal cord [29]. Recently, many researchers are working for the improvement in the use of collagen-based scaffolds. Calabrese worked a lot in the development of collagen and discussed the chondrogenesis process for converting collagen into cartilage and the full differentiation of bioactive factors during vivo stage [30]. He also worked on hydrogels bone formation with collagen/hydroxyapatite as biomaterial scaffolds in vitro and after implantation [31, 32].
Different techniques have been used for the fabrication of collagen hydrogels. The manufacturing of aligned wall composite fibers is done using electrospinning technique from collagen and other protein fibers because it became more biocompatible with the mixing with electrospun fibers [33]. Nakada et al. [34] also worked on the improving of biocompatibility of collagen with denaturizing the collagen at high temperature and low pressure to convert collagen fibers into cross-linkage structures. Some cross-linking of fibers like carbodiimide also elevate biocompatibility and stability of collagen–chitosan scaffolds [35].
Gelatin
Gelatin is another biomaterial used for the manufacturing of hydrogels. It can be obtained by partial hydrolysis of collagen which is a mixture of proteins and peptides usually obtained from skins, bones and connective tissues of the animals. Gelatin is also very important because of its degradability and compatibility nature in vitro as well as during direct implantation. It also retains its bioactive nature including MMP-sensitive sites and RGD sequences with cost-effectiveness [56].
Gelatin can easily be obtained from different by-products of the animals, forming high strength and changeable natured hydrogels. Thermally, they are less stable at high temperatures with water-soluble property. They have wide range of applications in biomedical [36]. The capability of cross-linking for the gelatin is little less, but its mechanical supportive strength has been increased with the technique of double network of photo-cross-linking of gelatin and gellan gum [37]. As the strength of the DN hydrogels was not impressive as encapsulated cells and cell-compatible conditions, the model of micro-gel-reinforced hydrogels was suggested with the same products, gelatin and gellan gum for better biological and mechanical properties as compared to DN hydrogels [57]. Some researchers worked on improving biocompatibility of the gelatin by mixing other non-toxic and biocompatible materials like dextran aldehyde [58] or chitosan [59].
Research on gelatin also reveals that they do not influence the functional and physical conditions of antigenic response toward the body, but they affect macrophages to activate them [60]. Development has also been done to improve the biocompatibility of gelatin and related biomaterials by combining sponge scaffolds of hydroxyapatite with different modified surfaces [38, 61]. The altering of soaking process by depositing the nano-hydroxyapatite helps to improve the growth of seeded cells and increase their adhesive bonding properties [39]. For the additive manufacturing techniques, a 3D scaffold has been developed with PCL placed between gelatin–chitosan hydrogels for congenital heart defects. They are biodegradable patches containing a thin PCL-layer self-assembled core which provide significant strength to the ventricular walls for proper function. This novel research helps the doctors to facilitate patients more efficiently [40].
Pullulan
Another kind of hydrogel is pullulan, usually manufactured from the yeast such as fungus Aureobasidium pullulans. In 1958, Bernier was the first scientist to extract the pullulan and tried to understand its chemical structure. Pullulan is a type of exopolysaccharide (EPS) formed as thick sledge in amorphous form on the surface of bacterial infected cells [62,63,64]. The appearance of the pullulan powder is white with no taste and odor. It is not soluble in any organic or inorganic solvents except water. Due to the properties of water-soluble and toxic nature, pullulan is used in medical application such as drugs carrier, preparation of syrups [41,42,43] and packaging material [44].
Pullulan can also be manufactured by fermentation of different waste materials [45]. Machy also worked on the extraction of pullulan and dextran which improves the rapid growth of cells located in endothelium region [46]. Similarly, Amrita et al. [47] utilized pullulan and their derivatives as scaffolds for tissue engineering by pore wall mineralization method with enhanced osteo-conductive properties. To improve the mechanical properties of the pullulan, Aschenbrenner employed cross-linking technique to combine pullulan and dextran [65]. Therefore, different types of pullulan including nanoparticles and nano-gels can be used in drug delivery systems, curing of tumor cells and supporting normal cells for toxicity of drugs.
Hyaluronic acid
Hyaluronic acid (HA) chemically known as hyaluronan naturally was found in the body of mammals having non-sulfated glycosaminoglycan and extensively found in the different body parts including neural, epithelial and connective tissues, eye and joint fluids [48, 66]. They are very essential part of different human body mechanisms such as cell growth, wound healing, embryonic and tumor development [49,50,51].
On the basis of cross-linkage variation, HA can be divided into two categories such as monophasic or biphasic [52]. Both of them are toxic-free fillers, and biphasic HA mostly is used in hyaluronidase resistance and syringeability [53]. Molecular weight is an important factor in changing the properties and application of HA with other materials. Similarly, HA is water-soluble, helps in the process of angiogenesis, biodegradability and does not provoke any immunogenic response with higher molecular weight. HA with lower molecular weight displays angiogenic, immunogenic and inflammatory response [54]. HA hydrogels are covalent bonded cross-linked manufactured by different methods including esterification [67], electrospinning cryogelation [68] and annealing [69]. The properties like biocompatibility and biodegradability of hyaluronic acid have been enhanced by photo-polymerization of UV-initiators during cross-linking process [70]. Recently, researchers are focusing on the biomedical applications of HA and its derivatives like visco-supplementation [55], wound healing [71], tissue engineering [72] and therapeutics [50].
Hydrogels with synthetic materials
Synthetic polymers have great influence for the researchers to be utilized in the field of biomedical and pharmaceutical applications because of their wide availability, biocompatibility, biodegradable nature and easy to handle. It also provides wide range of material selection with different physical and chemical behavior suitable for various applications as shown in Table 2. For the first time in 1954, Wichterle and Lim worked for the development of polymeric hydrogel biomaterial using 2-hydroxyethyl methacrylate (HEMA) and ethylene dimethacrylate (EDMA) as copolymers to use as contact lenses [73]. Generally polymeric hydrogels are bonded with covalent or ionic bonds and show less biocompatibility nature as compared to the naturally derived polymers. However, the prominent advantages of synthetic polymers are comparatively high degradation and mechanical properties [74] with simple structure and monomeric unit constituency [75].
Poly(lactic and glycolic) acid-based hydrogels
Poly(lactic and glycolic) acid (PLGA) has influence for the extraction of hydrogels because of considerable properties including biocompatibility, biodegradation rate, clinical applications at human level, highly allowable for modifications in surface properties and ease of transportation with handling [76]. PLGA and their copolymers are exclusively used in tissue engineering [75] especially for bones and cartilages due to their impressive strength and biodegradable nature [76, 77]. A rapid increase has been found for the usage of polymeric biomaterials in medical applications with the combination of tri- and multi-block copolymers of PLA for hydrogel preparation [78]. Similarly, the mixture of hydrophilic PLA polymers exhibits specific and useful properties with variable concentration ratios and possibilities.
Researchers worked for the degradation of poly(lactic and glycolic) acid polymers which have been considered as one of the main concerns for tissue engineering because intermediate degradation leads to decrease the pH value of the implanted cells and this action reduces the mechanical properties in newly generated bone [79]. The concentrations of lactic and glycolic acid may also affect the degradation process such as higher concentration of lactic acid take more time for biodegradation that will prolong the support for tissue regeneration [80].
Recently, more work has been done on biocompatibility of PLA-based scaffolds like bovine amniotic epithelial stem cells [95], osteoblast-like human bone fibroblast (MG-63) cells [81, 96] and nano-hydroxyapatite/PLA scaffolds with human bone marrow-derived mesenchymal stem cells (hMSCs) [82]. Biocompatibility of PLA scaffolds has also been enhanced by employing the techniques like organic solvent-free extraction [97], room temperature ionic liquids (RTILs) [98] and electrospinning technique [99]. The scaffolds of such polymers (PLA, PGA and PLGA) also provide mechanical support, guidance for the growth of new tissues and help in the complete degradation in the body for pathologically altered tissue structures such as skin, ligaments, skeletal muscles and vascular tissues [74].
Polyethylene oxide- and polyethylene glycol-based hydrogels
Polyethylene oxide (PEO) and polyethylene glycol (PEG) are also abundantly used as biomaterials for extracting hydrogels [100]. The functional attributes of PEO and PEG hydrogels are photo-polymerization capability, suitable mechanical properties, easy to handle for scaffold structures and chemical composition. All these characteristics make them suitable for 3D models for tissue development. The drawback of these hydrogels is that they do not provide sufficient adhesive support to the cells due to the limited antigenicity, immunogenicity, cell adhesion and protein binding [83].
The major properties that researchers wanted to achieve with the hydrogels are biodegradability, biocompatibility, thermo-sensitivity and easy handling for biomedical applications, and polyethylene glycol (PEG)-based copolymers are perfect biomaterial for such attributes. PEG and PEO are also permitted by FDA for pharmacological applications [84]. Combination of copolymers of PEO [101] and PEG [102] with PLLA produced polycaprolactone hydrogels with thermal reversibility. Degradability of these hydrogels has also been enhanced by combining them with oligopeptides [103], hydrolytically degradable PLA [104] and carboxymethyl cellulose [105] and PLLA for the reduction of tumor growth factor (TGF-β) [78]. PLGA showed great biocompatibility with both PEO and PEG as compared to other biomaterials like poloxamer [84]. Hou et al. [85] employed different bioactive and compatible PEG-scaffolds for tissue engineering applications with new designs and manufacturing techniques.
PEG has great compatibility nature which helps to reduce the growth of thrombus and damage caused to the tissues in vitro/vivo because PEG suppresses the platelet adhesion process. Secondly, it is also used as coating material for drugs carrier delivery purposes [83]. Incorporation of RGD sequences into PEG enhances the cell growth and attachment [86]. Similarly, Acr–PEG–RGD has also helped to improve the mineral distribution and osteoblast attachment for rat caldaria [106]. The mixture of PEG–RGD leads to the improvement of implanted seed cells in their growth and reduces the danger of thrombus formation by non-specific absorbing of fibrinogen and proteins from plasma and serum, respectively [87]. Recently, researchers have also worked for more biocompatible synthetic PEG, PEG–chitosan, PEG–polypeptides and multi-arm PEG hydrogels [88, 89].
Polycaprolactone-based hydrogels
Polycaprolactone (PCL), biodegradable, semicrystalline and hydrophobic polyester, allowed by the Food and Drug Administration (FDA) is used as drug delivery and in medical devices [107]. The application of PCL in bone tissue engineering is an integration of bioactive glasses and calcium phosphate-based ceramics which improves its mechanical properties, bioactive nature and degradation rates [108] because PCL lacks such properties originally [109, 110]. Some other applications of the PCL-based products are correction of facial aging including long-lasting and nourishing effects, decrease in volume and enhancing the clearness of skin via tissue engineering [90, 91]. The biocompatibility response of PCL for periosteal cell culture systems and human fibroblasts is outstanding [92].
The combination of PCL and other biomaterials also provides a great degree of range for the researchers to provide more suitable environment for protein growth and other bioactivities. The blend of PCL and chitosan at a ratio of 3:1 with 8% and 1%, respectively, improves the response of seeded tissues and enhances the protein observing capacity of mixture [93]. The blend of heparin and curdlan sulfate with PCL efficiently reduces thrombus formation due to the surface engineering, and similarly, biocompatibility can also be improved by combining PCL with PEG employing the techniques of electrospinning and cross-linking [94]. However, further enhancement in biocompatibility of PCL can be done via two-step modification using air plasma and carbodiimide [67].
Overview of hydrogel bioprinting technologies
The fabrication of biological organs and artificial tissue with the help of additive manufacturing (AM) is one the greatest milestone achieved by the researchers. Three dimensional (3D) printing of biomaterials commonly known as bioinks is to manufacture 3D structures like bones and tissues. These bioinks are composed of specific biocompatible materials so that they can support cellular attachments, proper growth and their function during and after printing. Biocompatibility of these materials is very important for the bioprinting because they are supposed to interact directly during extrusion, so the main focus of the researchers is on the rheological behaviors and cross-linking methods for these materials to get precise and accurate deposition [111, 112]. The combination of biomaterials with living cells also provides a potential mixture for the bioprinting of self-supporting designs. Secondly, the biocompatible materials also support cell viability as non-cytotoxic and show appropriate swelling properties for short term [113]. Hydrogels are the best selection for bioinks as they are biocompatible, biodegradable and structurally similar to naturally existing extracellular matrix (ECM) with hydrophilic environment which promote the cell growth. Due to hydrophilic nature, hydrated structures and channels are created with help in encapsulation of cells.
Both types of hydrogels including naturally existed or synthetically prepared can be employed for biofabrication. Collagen [114], gelatin [115], alginate [116] and chitosan [117] are efficiently used for bioprinting because of their high biocompatibility. But, structurally they are naturally very weak which limits their application in biofabrication [118]. On the other hand, synthetic hydrogels have better mechanical support, but degradation may prevent their utilization for bioprinted products [118, 119]. Bioprinting is divided three main categories: inkjet bioprinting, robotic dispensing and laser-assisted bioprinting [120, 121] on the basis of printing processes as shown in Fig. 2 and further explained by rheological, biological, interfacial and degradation properties.
Along with the characteristics of the bioinks and their biocompatibility, bioprinting also depends on the different parameters which decide the printing technique and suitable optimization needed for successful fabrication of tissue constructions. Some parameters of the respective techniques are listed in Table 3.
Robotic dispensing
Robotic dispensing 3D printer with a disposable syringe (plastic or metallic depending on the bioink) extrudes the biomaterial (bioink) through the nozzle with the help of mechanical screw or piston mechanism or pneumatic system as shown in Fig. 3. The pneumatic control system allows the pressurized air to extrude the bioink which may show some delay time during the compression of air [122], but the piston-driven system provides direct interaction of pressurized surface and bioink material which provides sufficient control and smooth printing. Both of these systems are very feasible for the printing of cells and tissues [121, 122]. In mechanical driven screw system, the extrusion and feed of the bioink is controlled with the rotational motion of the screw having specialized designs [120]. High-viscosity bioinks are favorable for printing through screw control system, but the problem of pressure drop may harm the cells. Main feature of these systems is the continuous extrusion of the material in the form of filaments.
Schematic demonstration of three robotic dispensing systems [120]
The important hydrogels typically used as bioinks in robotic dispensing 3D printing are collagen [125], fibrin [126], alginate [116, 127, 128], GelMA [129, 130] and hydrogel blends [131, 132]. The application of such hydrogels for biological means is difficult because they changed into gel form so quickly and collapse due to their own weight after printing [133]. The researchers have worked to improve the supporting techniques by fast curing [134, 135] and using support baths during the printing of such biomaterials [133, 136]. Geo et al. [137] employed robo-dispensing technique to manufacture an organ with micro-channels inside it as shown in Fig. 4.
Schematic explanation of a cross-sectional view of the coaxial nozzle assembly, b manufacturing process of a 3D alginate structure with built-in micro-channels and c networks formed after fabrication [137]
The design of the nozzle is coaxial, and alginate of 2% solution and solution of CaCl2 of 4% were extruded with varying flow rates. The hollow filament was extruded due the coaxial arrangement with an average inner diameter of 892 μm. Further, the results of perfusion test explained that the printed structure provides sufficient perfusion without any blockage. Another alginate-based material prepared by adding methylcellulose (MC) in 3% alginate solution having improved viscosity characteristics is used for bioprinting via robotic dispensing 3D plotting technique [138], and these scaffolds showed high elasticity, stability, microporosity with cross-linkage. Lee et al. [139] demonstrated an innovative technique with the combination of robot-dispensing system and aerosol spraying of surface gelation to fabricate 3D alginate hydrogel scaffold with 100% connected pores in a controlled fashion as shown in Fig. 5. The alginate-based scaffold implanted with cell-laden micro-beads showed cell viability more than 90% after several days of periodic culture. Ang et al. [140] utilized the robotic dispensing technique equipped with pneumatic system to manufacture 3D scaffolds of chitosan–hydroxyapatite solution. The bioink was prepared by mixing chitosan and chitosan–hydroxyapatite solution with acetic acid and extruded through teflon-lined nozzle to fabricate the scaffold according to CAD model with layer deposition. The results showed that good adhesive forces are present between the layers which enable the chitosan matrix to create well-interconnected layering pattern.
Schematic diagram of fabrication process of micro-bead (MB) and cell-laden 3D scaffolds using a three-axis robot system supplemented with an aerosol process [139]
The mechanical strength of hydrogel scaffolds is also one of the issues which limits their applications. To improve the mechanical properties, reinforcement materials are coated on the scaffolds. Kim et al. [141] employed the robotic dispensing bioprinting method to fabricate PCL (cell-laden)/alginate scaffolds with reinforced coating of alginate-based biomaterial. The results revealed great improvement in mechanical strength with homogeneous dispersal of cells.
Inkjet bioprinting
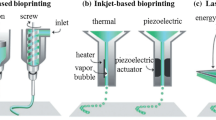
Inkjet bioprinting enables the printing of low-viscosity biomaterials in small fractions nearly 1–100 picolitres on the substrate [142]. Inkjet printing is droplet deposition-based process using piezoelectric actuators [143, 144] or thermal heaters [123] as driving force as shown in Fig. 6. Generally, these printers have two operating modes including continuous and drop on demand inkjet bioprinting [122]. In piezoelectric-based inkjet printers, the pulses generated by the piezoelectric actuators apply a voltage change according to computerized design, which extrude the biomaterial according to computerized design in drop-wise manner [145]. But in thermal-based inkjet printers, the heaters are utilized to convert the biomaterial into droplets by evaporating and then ejecting it on the printing-base platform [146]. The resolution of inkjet bioprinting ranges from 50 to 300 μm [111, 124] with the advantages of cost-effectiveness [111, 147] and high printing speeds [111, 120].
Schematic drawing of inkjet printing with two different types [120]
Recently, a lot of work has been done on inkjet technique for cell printing which allows to manufacture 3D hydrogel structures. There are several examples of such printings which provided desired mechanical and chemical properties with efficient porosity factor. Boland et al. [148] employed inkjet printing method to 3D scaffolds of alginate/gelatin hydrogels by spraying CaCl2 on to the alginic acid (un-gelled) as shown in Fig. 7. The cartridge of the printer was filled with 2% alginic acid solution to fabricate the 3D printed scaffold which was further processed for specific properties by spraying 0.25 M CaCl2 on the scaffolds. These printed scaffolds possess uniform pore size and well-adhesion of endothelial cells. Many other biomaterials can also be used to manufacture such kind of scaffolds in the form of tubes, branched or unbranched [149].
Schematic view of manufacturing of alginate-based scaffolds using modified HP DeskJet [148]
Researchers worked on commercially available inkjet printers for modifying them for the bioprinting [150]. Xu et al. [123] worked on the modification of commercial inkjet printer for printing sterile hydrogel specimen. Before using the inkjet printer, the chamber was sterilized with UV light and then washed several times with 70% ethanol. The quality of the samples proved that these modifications were acceptable after number of sterilizations. These inkjet printing techniques are very advantageous because the desired factors including deposition of living cells, growth rates after printing, right printing on exact location cross-linking and transfer of nutrients could be achievable [151] with cost-effectiveness and high efficiency. Inkjet printing technique also integrates with fields of biomedical applications like biosensors, drug screening and genomics [152]. The physical structure of these hydrogels and DNA structures is very delicate, but these could be printed directly using inkjet printers on glass slides for study and analysis purposes [153].
Laser-assisted bioprinting
The laser-assisted bioprinting employs laser beam to print biological structures [154]. The technique consists of main three components, the donor slide, pulsated laser beam source and a layer for absorbing laser energy [155] as shown in Fig. 8. The mechanism includes the deposition of the bioink on the donor slide which further converted into high-pressure bubbles through pulsed laser beam. When these micro-scale bubbles expand, they eject from the surface in the form of droplets [121]. There are two major AM techniques, stereolithography (SLA) [156] and two-photon polymerization (2PP) [122] which can be utilized for the fabrication of 3D biomaterial structures using laser assistance. SLA allows the printing of such structures range in centimeters and its resolution is very high up to 6 μm [157], while 2PP gives great resolution up to 100 nm which provides efficient interfaces between cells and printed substrate [156]. Usually two setups of light source are used in SLA including top–bottom and top–down as shown in Fig. 9.
Schematic illustration of laser-assisted bioprinting depositing bioink in the form of micro-droplets using pulsed laser source [111]
For the very first time, Odde demonstrated the 3D printing of biological structures with laser assistance using fibronectin clustering the individual cells [158]. There are many other biomaterials that can be used for such kind of printings including living cells [155], DNA [159] and peptides [160]. Karina et al. [161] fabricated the complex 3D structures of polyethylene glycol (PEG) hydrogels using stereolithography (SLA). The utilization of such cross-linked photo-active polymer PEG-dma MW 1000 up to 10% (w/v) with 0.5% of I-2959 enhanced the biofabrication attributes. These scaffolds had great importance in tissue engineering applications because SLA provides strong, smooth and precise structures with accurate placement of cells and other biological agents during fabrication. Some other biomaterials like photo-curable polymers offer controllable adhesive bonding between the cells because they can cure easily during the process of SLA [162]. PEG hydrogels also provide suitable strength for soft tissue and swelling due to their hydrophilic nature [163].
The light source of the SLA technique can also affect the cells composition and damage them. The main mutations and disorders are produced by UV light and laser lights. SLA techniques that employ visible light as a source are useful for tissue engineering application because they eliminate the dangerous effects of UV light and help in homogenous dispersion of polymers [164, 165]. Few polypeptides-based scaffolds also showed improved mechanical properties fabricated using SLA technique. Elomaa et al. [166] fabricated poly(ethylene glycol-co-depsipeptide) (PEG-co-PDP) scaffolds employing SLA with visible light source as shown in Fig. 10. The cross-linking between PEG-co-PDP and RGD-functionalized PEG acrylate enhanced the proliferation and cellular adhesive bonding. The results showed that mechanical stiffness depends on the curing time of the layer and the values range from 3 ± 1 to 38 ± 13 kPa. In another study, for semilunar cartilage structures, MeGEL is utilized to fabricate porous scaffolds [167].
Schematic demonstration of bioprinting of cross-linked cell-laden hydrogel cells [166]
Conclusion and future outlooks
The potential of the hydrogels is very positive for the future development of tissue engineering because they possess the resemblance with naturally existed ECM chemically as well as structurally. The innovation in the methodologies and conditions for providing biocompatible environments would be the key to success in future. The major focus of the researchers is to increase the biocompatibility of these hydrogels as this is the most important factor for developing new hydrogel-based scaffolds. Still, we have to understand the complexities of the process by which these hydrogel ECM seeded cells mediate their composition with the existing living cells. Exploring more information about ECM hydrogels could provide new possibilities to architecture and reprogram the chemical nature of hydrogels so that after implantation they should have the ability to repair and reconstruct the tissues if some variation happens in the actual living tissues. Secondly, the improvement in the fabrication methods of these hydrogel-based scaffolds is also developing very rapidly. These improvements could lead us to incorporate advanced manufacturing techniques like big data to optimize the process parameters [168] and designs of these hydrogel-based scaffolds to be more effective and sustainable. Some novel and innovative designs with their functional applications are reviewed above. The gradient techniques could provide more accurate and precise methods for the production of mimic ECM with enhanced biocompatibility and mechanical strength. As it is an innovative method that will change the mechanical properties of scaffolds patterns, researchers attempted to test these methods experimentally [169, 170], but still for functional applications and best approaches, they need more investigation [171]. There could be a possibility for analyzing the details about the interaction forces and cross-linking structures of hydrogels at nanoscale that can influence polymerization processes and drive the self-assembling mechanism. Multiple cells printing at one time is still a challenge for researchers and scientists which can be done through combination of materials or employing different printing techniques spontaneously. This combination of various printing methods will provide a facility for more innovative designs and structures. For example, Kim et al. [172] explained the technique for printing the scaffold with combination of electrospinning and bioplotting techniques.
Despite this development in the field of tissue engineering, including reproduction of ECM using hydrogels and implantations, there are enormous factors yet to be explored that will allow TE to broad range of biomedical and clinical applications. The properties of hydrogel-based scaffolds can be improved through combination of different natural existing or synthetic hydrogels and fabricating the structures using different 3D printing techniques.
References
De Isla N et al (2010) Introduction to tissue engineering and application for cartilage engineering. Bio-Med Mater Eng 20(3–4):127–133
Organ donation and transplantation statistics: graph data (2017). https://www.organdonor.gov/statistics-stories/statistics/data.html. Accessed 27 September 2018
Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E (2016) A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev 22(4):298–310
Lee CH, Singla A, Lee Y (2001) Biomedical applications of collagen. Int J Pharm 221(1–2):1–22
Majola A et al (1991) Absorption, biocompatibility, and fixation properties of polylactic acid in bone tissue: an experimental study in rats. Clin Orthop Relat Res 268:260–269
Zhu J (2010) Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 31(17):4639–4656
Gorgieva S, Kokol V (2011) In: Pignatello R (ed) Collagen-vs. gelatine-based biomaterials and their biocompatibility: review and perspectives. Biomaterials applications for nanomedicine. InTech, London
Sreejit P, Verma R (2013) Natural ECM as biomaterial for scaffold based cardiac regeneration using adult bone marrow derived stem cells. Stem Cell Rev Rep 9(2):158–171
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21(32–33):3307–3329
O’brien FJ (2011) Biomaterials and scaffolds for tissue engineering. Mater Today 14(3):88–95
Velema J, Kaplan D (2006) Biopolymer-based biomaterials as scaffolds for tissue engineering. In: Lee K, Kaplan D (eds) Tissue engineering I. Springer, Berlin, pp 187–238
Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8(6):457
Singh SR (2009) Principles of regenerative medicine. Ann Biomed Eng 37(12):2658–2659
Chan B, Leong K (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17(4):467–479
Anderson JM (2001) Biological responses to materials. Annu Rev Mater Res 31(1):81–110
Paleos GA (2012) What are hydrogels. Retr Oct 11:215
Li X et al (2014) 3D-printed biopolymers for tissue engineering application. Int J Polym Sci 2014:1
Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R (2014) 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16:247–276
Hutmacher DW (2006) Scaffolds in tissue engineering bone and cartilage. In: Williams D (ed) The biomaterials: silver jubilee compendium. Elsevier, Amsterdam, pp 175–189
Wang H, Yang Z (2012) Short-peptide-based molecular hydrogels: novel gelation strategies and applications for tissue engineering and drug delivery. Nanoscale 4(17):5259–5267
Gomes M et al (2013) Natural polymers in tissue engineering applications. In: Ad EA, Sin A (eds) Handbook of biopolymers and biodegradable plastics. Elsevier, Amsterdam, pp 385–425
Weadock KS, Miller EJ, Bellincampi LD, Zawadsky JP, Dunn MG (1995) Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res 29(11):1373–1379
Park S-N, Park J-C, Kim HO, Song MJ, Suh H (2002) Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials 23(4):1205–1212
Tangsadthakun C, Kanokpanont S, Sanchavanakit N, Banaprasert T, Damrongsakkul S (2017) Properties of collagen/chitosan scaffolds for skin tissue engineering. J Metals Mater Miner 16(1):37–44
DeLustro F, Condell RA, Nguyen MA, McPherson JM (1986) A comparative study of the biologic and immunologic response to medical devices derived from dermal collagen. J Biomed Mater Res 20(1):109–120
Taylor PM, Cass AE, Yacoub MH (2006) Extracellular matrix scaffolds for tissue engineering heart valves. Progr Pediatr Cardiol 21(2):219–225
Cavallo JA et al (2015) Remodeling characteristics and collagen distributions of biologic scaffold materials biopsied from postmastectomy breast reconstruction sites. Ann Plast Surg 75(1):74
Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R (2006) Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 27(7):1104–1109
Joosten E, Veldhuis W, Hamers F (2004) Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J Neurosci Res 77(1):127–142
Calabrese G et al (2017) Combination of collagen-based scaffold and bioactive factors induces adipose-derived mesenchymal stem cells chondrogenic differentiation in vitro. Front Physiol 8:50
Calabrese G et al (2016) Collagen-hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation in vitro. PLoS ONE 11(3):e0151181
Calabrese G et al (2016) Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci Rep 6:36399
Zhu B, Li W, Lewis RV, Segre CU, Wang R (2014) E-spun composite fibers of collagen and dragline silk protein: fiber mechanics, biocompatibility, and application in stem cell differentiation. Biomacromol 16(1):202–213
Nakada A et al (2013) Manufacture of a weakly denatured collagen fiber scaffold with excellent biocompatibility and space maintenance ability. Biomed Mater 8(4):045010
Chen Z et al (2016) Comparison of the properties of collagen–chitosan scaffolds after γ-ray irradiation and carbodiimide cross-linking. J Biomater Sci Polym Ed 27(10):937–953
Einerson NJ, Stevens KR, Kao WJ (2003) Synthesis and physicochemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials 24(3):509–523
Shin H, Olsen BD, Khademhosseini A (2012) The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 33(11):3143–3152
Li D et al (2014) Enhanced biocompatibility of PLGA nanofibers with gelatin/nano-hydroxyapatite bone biomimetics incorporation. ACS Appl Mater Interfaces 6(12):9402–9410
Jaiswal A, Chhabra H, Soni V, Bellare J (2013) Enhanced mechanical strength and biocompatibility of electrospun polycaprolactone-gelatin scaffold with surface deposited nano-hydroxyapatite. Mater Sci Eng, C 33(4):2376–2385
Pok S, Myers JD, Madihally SV, Jacot JG (2013) A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater 9(3):5630–5642
Singh RS, Saini GK, Kennedy JF (2010) Covalent immobilization and thermodynamic characterization of pullulanase for the hydrolysis of pullulan in batch system. Carbohyd Polym 81(2):252–259
Singh RS, Saini GK, Kennedy JF (2010) Maltotriose syrup preparation from pullulan using pullulanase. Carbohyd Polym 80(2):401–407
Singh RS, Saini GK, Kennedy JF (2011) Continuous hydrolysis of pullulan using covalently immobilized pullulanase in a packed bed reactor. Carbohyd Polym 83(2):672–675
Singh RS, Saini GK, Kennedy JF (2008) Pullulan: microbial sources, production and applications. Carbohyd Polym 73(4):515–531
Thirumavalavan K, Manikkadan T, Dhanasekar R (2009) Pullulan production from coconut by-products by Aureobasidium pullulans. Afr J Biotech 8(2):254–258
Machy D, Jozefonvicz J, Letourneur D (2006) Vascular prosthesis impregnated with crosslinked dextran, ed.: Google Patents
Arora A, Sharma P, Katti DS (2015) Pullulan-based composite scaffolds for bone tissue engineering: improved osteoconductivity by pore wall mineralization. Carbohyd Polym 123:180–189
Fraser J, Laurent T, Laurent U (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242(1):27–33
Noble PW (2002) Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol 21(1):25–29
Platt VM, Szoka FC Jr (2008) Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm 5(4):474–486
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4(7):528
Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P (2011) Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg 37(5):637–643
Park K, Kim H, Kim B (2014) Comparative study of hyaluronic acid fillers by in vitro and in vivo testing. J Eur Acad Dermatol Venereol 28(5):565–568
Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85(8):699–715
Moreland LW (2003) Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther 5(2):54
Elzoghby AO (2013) Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Controlled Release 172(3):1075–1091
Shin H, Olsen BD, Khademhosseini A (2014) Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J Mater Chem B 2(17):2508–2516
Jalaja K, Kumar PA, Dey T, Kundu SC, James NR (2014) Modified dextran cross-linked electrospun gelatin nanofibres for biomedical applications. Carbohyd Polym 114:467–475
Han F, Dong Y, Su Z, Yin R, Song A, Li S (2014) Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int J Pharm 476(1–2):124–133
Anderson JM, Miller KM (1984) Biomaterial biocompatibility and the macrophage. Biomaterials 5(1):5–10
Carpena NT, Min Y-K, Lee B-T (2015) Improved in vitro biocompatibility of surface-modified hydroxyapatite sponge scaffold with gelatin and BMP-2 in comparison against a commercial bone allograft. ASAIO J 61(1):78–86
Singh R, Saini G (2008) Pullulan-hyperproducing color variant strain of Aureobasidium pullulans FB-1 newly isolated from phylloplane of Ficus sp. Biores Technol 99(9):3896–3899
Singh RS, Saini GK (2008) Production, purification and characterization of pullulan from a novel strain of Aureobasidium pullulans FB-1. J Biotechnol 136:S506–S507
Singh RS, Saini GK, Kennedy JF (2009) Downstream processing and characterization of pullulan from a novel colour variant strain of Aureobasidium pullulans FB-1. Carbohyd Polym 78(1):89–94
Aschenbrenner E et al (2013) Using the polymeric ouzo effect for the preparation of polysaccharide-based nanoparticles. Langmuir 29(28):8845–8855
WebMD. https://www.webmd.com/vitamins/ai/ingredientmono-1062/hyaluronic-acid. Accessed 12 September 2018
Sahapaibounkit P, Prasertsung I, Mongkolnavin R, Wong CS, Damrongsakkul S (2017) A two-step method using air plasma and carbodiimide crosslinking to enhance the biocompatibility of polycaprolactone. J Biomed Mater Res B Appl Biomater 105(6):1658–1666
Kemençe N, Bölgen N (2017) Gelatin-and hydroxyapatite-based cryogels for bone tissue engineering: synthesis, characterization, in vitro and in vivo biocompatibility. J Tissue Eng Regen Med 11(1):20–33
Fujiwara J, Takahashi M, Hatakeyama T, Hatakeyama H (2000) Gelation of hyaluronic acid through annealing. Polym Int 49(12):1604–1608
Kim DH, Martin JT, Elliott DM, Smith LJ, Mauck RL (2015) Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomater 12:21–29
Jiang D, Liang J, Noble PW (2007) Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23:435–461
Collins MN, Birkinshaw C (2013) Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohyd Polym 92(2):1262–1279
Rogero SO, Malmonge SM, Lugão AB, Ikeda TI, Miyamaru L, Cruz ÁS (2003) Biocompatibility study of polymeric biomaterials. Artif Organs 27(5):424–427
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011:290602
Gunatillake PA, Adhikari R (2003) Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater 5(1):1–16
Gentile P, Chiono V, Carmagnola I, Hatton PV (2014) An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci 15(3):3640–3659
Moran JM, Pazzano D, Bonassar LJ (2003) Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tissue Eng 9(1):63–70
Ehashi T, Kakinoki S, Yamaoka T (2014) Water absorbing and quick degradable PLLA/PEG multiblock copolymers reduce the encapsulation and inflammatory cytokine production. J Artif Organs 17(4):321–328
Liu H, Slamovich EB, Webster TJ (2006) Less harmful acidic degradation of poly (lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomed 1(4):541
Rogers CM, Deehan DJ, Knuth CA, Rose FR, Shakesheff KM, Oldershaw RA (2014) Biocompatibility and enhanced osteogenic differentiation of human mesenchymal stem cells in response to surface engineered poly (d, l-lactic-co-glycolic acid) microparticles. J Biomed Mater Res, Part A 102(11):3872–3882
Nga NK, Hoai TT, Viet PH (2015) Biomimetic scaffolds based on hydroxyapatite nanorod/poly (d, l) lactic acid with their corresponding apatite-forming capability and biocompatibility for bone-tissue engineering. Colloids Surf B 128:506–514
Zong C et al (2014) Biocompatibility and bone-repairing effects: comparison between porous poly-lactic-co-glycolic acid and nano-hydroxyapatite/poly (lactic acid) scaffolds. J Biomed Nanotechnol 10(6):1091–1104
Alcantar NA, Aydil ES, Israelachvili JN (2000) Polyethylene glycol–coated biocompatible surfaces. J Biomed Mater Res 51(3):343–351
Alexander A, Khan J, Saraf S, Saraf S (2013) Poly (ethylene glycol)–poly (lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J Controlled Release 172(3):715–729
Hou Y, Schoener CA, Regan KR, Munoz-Pinto D, Hahn MS, Grunlan MA (2010) Photo-cross-linked PDMSstar-PEG hydrogels: synthesis, characterization, and potential application for tissue engineering scaffolds. Biomacromol 11(3):648–656
Yang F, Williams CG, Wang D-A, Lee H, Manson PN, Elisseeff J (2005) The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 26(30):5991–5998
Wang Y-Y, Lü L-X, Shi J-C, Wang H-F, Xiao Z-D, Huang N-P (2011) Introducing RGD peptides on PHBV films through PEG-containing cross-linkers to improve the biocompatibility. Biomacromol 12(3):551–559
Escudero-Castellanos A, Ocampo-García BE, Domínguez-García MV, Flores-Estrada J, Flores-Merino MV (2016) Hydrogels based on poly (ethylene glycol) as scaffolds for tissue engineering application: biocompatibility assessment and effect of the sterilization process. J Mater Sci - Mater Med 27(12):176
Cheng Y (2016) Poly (ethylene glycol)-polypeptide copolymer micelles for therapeutic agent delivery. Curr Pharm Biotechnol 17(3):212–226
Kim JA, Van Abel D (2015) Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J Cosmet Laser Ther 17(2):99–101
Moers-Carpi MM, Sherwood S (2013) Polycaprolactone for the correction of nasolabial folds: a 24-month, prospective, randomized, controlled clinical trial. Dermatol Surg 39(3pt1):457–463
Salgado CL, Sanchez EM, Zavaglia CA, Granja PL (2012) Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels. J Biomed Mater Res, Part A 100(1):243–251
Shalumon K, Anulekha K, Chennazhi KP, Tamura H, Nair S, Jayakumar R (2011) Fabrication of chitosan/poly (caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int J Biol Macromol 48(4):571–576
Khandwekar AP, Patil DP, Shouche Y, Doble M (2011) Surface engineering of polycaprolactone by biomacromolecules and their blood compatibility. J Biomater Appl 26(2):227–252
Russo V et al (2016) Amniotic epithelial stem cell biocompatibility for electrospun poly (lactide-co-glycolide), poly (ε-caprolactone), poly (lactic acid) scaffolds. Mater Sci Eng, C 69:321–329
Cecen B, Kozaci D, Yuksel M, Erdemli D, Bagriyanik A, Havitcioglu H (2015) Biocompatibility of MG-63 cells on collagen, poly-l-lactic acid, hydroxyapatite scaffolds with different parameters. J Appl Biomater Funct Mater 13(1):10–16
Zhao X-F, Li X-D, Kang Y-Q, Yuan Q (2011) Improved biocompatibility of novel poly (L-lactic acid)/β-tricalcium phosphate scaffolds prepared by an organic solvent-free method. Int J Nanomed 6:1385
Lee H-Y, Jin G-Z, Shin US, Kim J-H, Kim H-W (2012) Novel porous scaffolds of poly (lactic acid) produced by phase-separation using room temperature ionic liquid and the assessments of biocompatibility. J Mater Sci - Mater Med 23(5):1271–1279
Hidalgo I, Sojot F, Arvelo F, Sabino M (2013) Functional electrospun poly (lactic acid) scaffolds for biomedical applications: experimental conditions, degradation and biocompatibility study. Mol Cell Biomech MCB 10(2):85–105
Park J, Lakes RS (2007) Biomaterials: an introduction. Springer, Berlin
Saffer EM, Tew GN, Bhatia SR (2011) Poly (lactic acid)-poly (ethylene oxide) block copolymers: new directions in self-assembly and biomedical applications. Curr Med Chem 18(36):5676–5686
Jeong B, Bae YH, Lee DS, Kim SW (1997) Biodegradable block copolymers as injectable drug-delivery systems. Nature 388(6645):860
Metters A, Anseth K, Bowman C (2000) Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer 41(11):3993–4004
Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL (2001) Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials 22(22):3045–3051
Lee SY et al (2015) Synthesis and in vitro characterizations of porous carboxymethyl cellulose-poly (ethylene oxide) hydrogel film. Biomater Res 19(1):12
Burdick JA, Anseth KS (2002) Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23(22):4315–4323
Bhavsar MD, Amiji MM (2008) Development of novel biodegradable polymeric nanoparticles-in-microsphere formulation for local plasmid DNA delivery in the gastrointestinal tract. AAPS PharmSciTech 9(1):288–294
Hajiali F, Tajbakhsh S, Shojaei A (2018) Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev 58(1):164–207
Kweon H et al (2003) A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 24(5):801–808
Sun H, Mei L, Song C, Cui X, Wang P (2006) The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 27(9):1735–1740
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773
Das S et al (2015) Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater 11:233–246
Skardal A, Atala A (2015) Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng 43(3):730–746
Rhee S, Puetzer JL, Mason BN, Reinhart-King CA, Bonassar LJ (2016) 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng 2(10):1800–1805
Zehnder T, Sarker B, Boccaccini AR, Detsch R (2015) Evaluation of an alginate–gelatine crosslinked hydrogel for bioplotting. Biofabrication 7(2):025001
Leppiniemi J et al (2017) 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl Mater Interfaces 9(26):21959–21970
Xu Y, Xia D, Han J, Yuan S, Lin H, Zhao C (2017) Design and fabrication of porous chitosan scaffolds with tunable structures and mechanical properties. Carbohyd Polym 177:210–216
Donderwinkel I, van Hest JC, Cameron NR (2017) Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem 8(31):4451–4471
Kim JE, Kim SH, Jung Y (2016) Current status of three-dimensional printing inks for soft tissue regeneration. Tissue Eng Regen Med 13(6):636–646
Malda J et al (2013) 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 25(36):5011–5028
Pedde RD et al (2017) Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater 29(19):1606061
Jungst T, Smolan W, Schacht K, Scheibel T, Groll JR (2015) Strategies and molecular design criteria for 3D printable hydrogels. Chem Rev 116(3):1496–1539
Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005) Inkjet printing of viable mammalian cells. Biomaterials 26(1):93–99
Tasoglu S, Demirci U (2013) Bioprinting for stem cell research. Trends Biotechnol 31(1):10–19
Lode A et al (2016) Additive manufacturing of collagen scaffolds by three-dimensional plotting of highly viscous dispersions. Biofabrication 8(1):015015
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci 113(12):3179–3184
Jia J et al (2014) Engineering alginate as bioink for bioprinting. Acta Biomater 10(10):4323–4331
Tabriz AG, Hermida MA, Leslie NR, Shu W (2015) Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 7(4):045012
Bertassoni LE et al (2014) Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6(2):024105
Colosi C et al (2016) Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater 28(4):677–684
Ng WL, Yeong WY, Naing MW (2016) Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int J Bioprint 2(1):53–62
Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S (2016) Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep 6:24474
Hinton TJ et al (2015) Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 1(9):e1500758
Li H, Tan YJ, Leong KF, Li L (2017) 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl Mater Interfaces 9(23):20086–20097
Jin Y, Liu C, Chai W, Compaan A, Huang Y (2017) Self-supporting nanoclay as internal scaffold material for direct printing of soft hydrogel composite structures in air. ACS Appl Mater Interfaces 9(20):17456–17465
Ding H, Chang RC (2018) Printability study of bioprinted tubular structures using liquid hydrogel precursors in a support bath. Appl Sci 8(3):403
Gao Q, He Y, Fu J-Z, Liu A, Ma L (2015) Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61:203–215
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126
Lee H, Ahn S, Chun W, Kim G (2014) Enhancement of cell viability by fabrication of macroscopic 3D hydrogel scaffolds using an innovative cell-dispensing technique supplemented by preosteoblast-laden micro-beads. Carbohyd Polym 104:191–198
Ang T et al (2002) Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater Sci Eng, C 20(1–2):35–42
Kim YB et al (2016) Mechanically reinforced cell-laden scaffolds formed using alginate-based bioink printed onto the surface of a PCL/alginate mesh structure for regeneration of hard tissue. J Colloid Interface Sci 461:359–368
Calvert P (2007) Printing cells. Science 318(5848):208–209
Tse C et al (2016) Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 8(1):015017
Saunders RE, Gough JE, Derby B (2008) Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 29(2):193–203
Gudapati H, Dey M, Ozbolat I (2016) A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102:20–42
Derby B (2008) Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. J Mater Chem 18(47):5717–5721
Campbell PG, Miller ED, Fisher GW, Walker LM, Weiss LE (2005) Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials 26(33):6762–6770
Boland T et al (2007) Drop-on-demand printing of cells and materials for designer tissue constructs. Mater Sci Eng, C 27(3):372–376
Boland T, Xu T, Damon B, Cui X (2006) Application of inkjet printing to tissue engineering. Biotechnol J Healthc Nutr Technol 1(9):910–917
Pardo L, Boland T (2003) A quantitative approach to studying structures and orientation at self-assembled monolayer/fluid interfaces. J Colloid Interface Sci 257(1):116–120
Sun W, Darling A, Starly B, Nam J (2004) Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem 39(1):29–47
Lemmo AV, Fisher JT, Geysen HM, Rose DJ (1997) Characterization of an inkjet chemical microdispenser for combinatorial library synthesis. Anal Chem 69(4):543–551
Okamoto T, Suzuki T, Yamamoto N (2000) Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nat Biotechnol 18(4):438
Chrisey DB, McGill RA, Pique A (2001) Matrix assisted pulsed laser evaporation direct write, ed.: Google Patents
Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ (2006) Jet-based methods to print living cells. Biotechnol J Healthc Nutr Technol 1(9):930–948
Melchels FP, Feijen J, Grijpma DW (2010) A review on stereolithography and its applications in biomedical engineering. Biomaterials 31(24):6121–6130
Soman P, Chung PH, Zhang AP, Chen S (2013) Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol Bioeng 110(11):3038–3047
Odde DJ, Renn MJ (2000) Laser-guided direct writing of living cells. Biotechnol Bioeng 67(3):312–318
Colina M, Serra P, Fernández-Pradas JM, Sevilla L, Morenza JL (2005) DNA deposition through laser induced forward transfer. Biosens Bioelectron 20(8):1638–1642
Dinca V et al (2008) Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett 8(2):538–543
Arcaute K, Mann BK, Wicker RB (2006) Stereolithography of three-dimensional bioactive poly (ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng 34(9):1429–1441
Gobin AS, West JL (2002) Cell migration through defined, synthetic ECM analogs. FASEB J 16(7):751–753
Chan V, Zorlutuna P, Jeong JH, Kong H, Bashir R (2010) Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab Chip 10(16):2062–2070
Lin H, Cheng AW-M, Alexander PG, Beck AM, Tuan RS (2014) Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A 20(17–18):2402–2411
Lin H et al (2013) Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 34(2):331–339
Elomaa L, Pan C-C, Shanjani Y, Malkovskiy A, Seppälä JV, Yang Y (2015) Three-dimensional fabrication of cell-laden biodegradable poly (ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J Mater Chem B 3(42):8348–8358
Grogan SP et al (2013) Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater 9(7):7218–7226
Majeed A, Lv J, Peng T (2018) A framework for big data driven process analysis and optimization for additive manufacturing. Rapid Prototyp J. https://doi.org/10.1108/RPJ-04-2017-0075
Li S, Yan Y, Xiong Z, Zhang CWR, Wang X (2009) Gradient hydrogel construct based on an improved cell assembling system. J Bioact Compat Polym 24(1_suppl):84–99
Liu L, Xiong Z, Yan Y, Zhang R, Wang X, Jin L (2009) Multinozzle low-temperature deposition system for construction of gradient tissue engineering scaffolds. J Biomed Mater Res B Appl Biomater 88(1):254–263
Li S, Xiong Z, Wang X, Yan Y, Liu H, Zhang R (2009) Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J Bioact Compat Polym 24(3):249–265
Lacroix D, Planell JA, Prendergast PJ (2009) Computer-aided design and finite-element modelling of biomaterial scaffolds for bone tissue engineering. Philos Trans R Soc Lond A Math Phys Eng Sci 367(1895):1993–2009
Acknowledgements
This research was sponsored by National Natural Science Foundation of China (Grant No. 51175432), the Innovation Platform of Biofabrication (Grant No. 17SF0002), the Fundamental Research Funds for the Central Universities (Grant No. 3102014JCS05007) and the key Research and Development program of Shaanxi Province 2018 (Grant No. 2018ZDXM-GY-133).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saroia, J., Yanen, W., Wei, Q. et al. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-des. Manuf. 1, 265–279 (2018). https://doi.org/10.1007/s42242-018-0029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-018-0029-7