Abstract
The study aimed to evaluate the effectiveness of three plant extracts (cumin (Cuminum cyminum L.), peel fruit of pomegranate (Punica granatum), and fruit of black pepper (Piper nigrum L.)), against Pectobacterium carotovorum subsp. carotovorum (Pcc), a bacterium that causes potato tuber soft rot disease. This disease can result in significant losses to potato production and affects the quality of potatoes during storage, transit and shipment. To conduct the study, five isolates of the pathogenic bacterium were obtained from naturally infected potato tubers. According to the in vitro screening, the most virulence isolate Pcc2 was molecularly identified using 16S rRNA gene partial sequencing. Three plant extracts (cumin, pomegranate, and black pepper), were tested for their antibacterial activity against the bacterium using in vitro experiments. The results showed that all the three plants extract exhibited inhibition of the bacterial growth. Among the three-plant extract, pomegranate was found to have the best inhibitory effect on the bacterium (0.92 cm inhibition zone). Based on the findings of the in vitro experiments, the use of all extract at a concentration of 50 mg was recommended for controlling the soft rot disease in potato tubers during storage conditions. The data demonstrated that pomegranate extract was on the first ranking with bacterial growth reduction percentage estimated (1.4 mm), followed by cumin extract with growth reduction estimated (0.92). The data revealed that all of the tested plant extracts were able to reduce the severity of the disease. Of all the extracts, pomegranate extract showed the highest reduction in disease severity (91.3%). It is evident that the treatments with pomegranate, black pepper, and cumin consistently led to an increase in total phenol content over the course of 21 days. Treatments with methanolic extract of pomegranate, black pepper, and cumin lead to varying degrees of increased peroxidase (PO), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) activities over the course of the experiment. The data shows that the effectiveness of these treatments generally increases with time. In conclusion, the study showed that all plants extract tested herein has the potential to control potato tuber soft rot disease, which is a major problem affecting potato production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum) is one of the world’s most important staple crops, serving as a valuable source of nutrition and sustenance for billions of people (Rabia et al. 2018). The reduced rate of potato production may occur within different conditions such as field, transit, storage and marketing. These factors, along with favorable environmental conditions and infected tuber seeds, make the production susceptible to huge losses (Doolotkeldieva et al. 2016). The cultivation of potatoes is frequently affected by various pathogens, among which Pectobacterium carotovorum subsp. carotovorum (Pcc) stands out as a notorious bacterial pathogen responsible for causing bacterial soft rot disease (Abd-Elgahny et al. 2022). This pathogen poses a significant threat to potato production worldwide, resulting in substantial economic losses and food security concerns (Sulaiman et al. 2020).
P. carotovorum subsp. carotovorum is a highly destructive pathogen known for its ability to rapidly break down plant cell walls, leading to the characteristic soft rot symptoms in potato tubers. The bacterium’s capacity to survive in soil and on plant debris, as well as its wide host range, make it a challenging target for disease management strategies (Doolotkeldieva et al. 2016). Soft rot disease of potato tubers caused by Pcc causes great reduction in yield, resulting in economic losses in the field during transit and storage causing losses up to 60% (Mantsebo et al. 2014).
In recent years, there has been a growing interest in exploring eco-friendly and sustainable approaches to combat plant pathogens, with a particular focus on the use of plant extracts with antimicrobial properties (Abo-Elyousr et al. 2020; Hossain et al. 2019). Natural plant compounds have gained attention due to their potential to control various plant diseases while minimizing the negative environmental impacts associated with synthetic chemical pesticides.
Various plant extracts derived from different parts of plants, such as roots, barks, seeds, shoots, leaves, and fruits, have been the subject of investigation regarding their antibacterial properties against plant pathogenic bacteria, as highlighted in studies conducted by da Silva et al. (2016); Purushotham and Anupama (2018) and Abo-Elyousr and Bagy (2019). There has been a notable upsurge in the examination of these plant extracts’ antimicrobial potential, particularly for the purpose of controlling plant diseases, with a special focus on bacterial infections, as observed in the research by Mangalagiri et al. (2021).
The utilization of plant extracts to induce phenol content and enhance antioxidant enzyme activity holds promise in the realm of plant disease management. When plants are subjected to stressors such as pathogenic infections, they often respond by increasing their phenol content as a defense mechanism (Ibrahim and Abo-Elyousr 2023). Plant extracts, derived from various botanical sources, can amplify this natural response by providing a supplementary source of phenolic compounds. These phenols act as phytoalexins, inhibiting the proliferation of pathogens and protecting plants from further damage (Sallam et al. 2021). Furthermore, the plant extracts can also encourage the activity of antioxidant enzymes within the infected tissues, thereby mitigating oxidative stress and limiting the extent of cellular damage caused by the disease. This dual approach not only aids in disease resistance but also contributes to the overall health and vitality of plants, offering a sustainable and eco-friendly means of managing plant diseases in agriculture and horticulture.
This research aims to address the urgent need for effective and environmentally friendly solutions to control bacterial soft rot in potatoes. We present an evaluation of several plant extracts, chosen for their known antimicrobial properties, as potential alternatives for managing P. carotovorum subsp. carotovorum infections.
Materials and methods
Isolation of the causal pathogen
Naturally infected potato tubers with soft rot symptoms were collected from different localities inside Assiut, Egypt. For soft rot bacterial causal pathogen isolation, the infected potato tubers washed several times with running tap water (H2O) and soaked in sodium hypochlorite (NaOCl) solution 1% for 2 min, then washed twice using sterilized water surface sterilized of the tubers that could interfere with isolation process. After that, they cut into small portions including both healthy and infected tissues; small portion of diseased tissues homogenized in sterilized water in clean and sterile petri dish to make sample suspension. Then a loopful of the resultant suspension streaked over plates supplemented with solid sterilized medium “nutrient sucrose agar” (NSA) as recommended by Dowson (1957). The plates incubated at 27–29 °C for 48 h and examined for bacterial growth development. A single colony from each streaking was selected for pure culture maintenance onto sterilized slants supplemented with the mentioned medium and stored in refrigerator at 4 °C for subsequent studies.
Pathogenicity tests
Preparation of bacterial suspension
Bacterial suspension of each tested isolate was prepared by growing the pure bacterial culture in 100 ml sterilized liquid medium nutrient sucrose broth (NSB), then incubation at 27 ± 2 C for 48 h on a rotary shaker at 150 rpm. The resultant bacterial suspension was centrifuged at 7000 rpm for 3 min. After that, the pellet was resuspended in sterilized water and the bacterial suspension was adjusted to be 1 × 108 cfu/ml using spectrophotometer (Milton Roy company–Spectronic 20D) / at wavelength 620 nm as followed by McGuire and Kelman (1984).
Test for soft rotting
Health potato tubers (Cara cultivar) underwent surface sterilization using a 1% solution of sodium hypochlorite (NaOCl) to eliminate any potential interference from other microorganisms that could compromise the pectinolytic activity of the tested isolates. A sterile cork-borer was then employed to create a hole in each tuber, measuring 1 cm in depth and 0.5 cm in width. Subsequently, approximately 200 µl of a exactly adjusted suspension (1 × 108 cfu/ml) derived from 48-hour-old bacterial cultures was introduced into the base of each hole. The openings were then sealed with the potato plugs that were initially removed. The treated tubers were carefully placed in sterile plastic containers supplemented with sterile moist cotton and subjected to incubation at a constant temperature of 27 ± 2 °C for 96 h. Following the incubation period, the treated tubers were halved to facilitate an examination of any rotting by the method outlined by DeBoer and Kelman (1978). In control tubers, the bacterial suspension was replaced with sterilized distilled H2O. This experimental procedure was repeated twice, with five replicates for each tested isolate, ensuring robustness and reliability in evaluating the isolates’ impact.
Disease severity assessment
Disease severity of the soft rot disease (weight loss) was estimated mathematically using the equation of Yaganza et al. (2004), as follow:
Where: Tw1 = Total weight of tuber.
Tw2 = Total weight of tuber without rotting tissue.
Identification of the pathogenic bacteria
The most effective pathogenic bacterial isolate was chosen for identification by 16s rRNA sequencing, based on a previous experiment (pathogenicity test).
In vitro study, control of potato soft rot disease by plant extract
The purpose of that experiment is to make a comparative assessment of the antibacterial activity made by different plant extract (cumin, pomegranate, and black pepper) against bacterial soft rot pathogen P. carotovorum subsp. carotovorum in vitro. Various concentrations of the tested plant extract (0, 10, 20, 30, 40 and 50 µg/ml) and the antibacterial activity was evaluated.
Preparation of bacterial suspension
The bacterial suspension of P. carotovorum subsp. carotovorum isolate Pcc2 with the highest value of disease severity prepared as mentioned before.
Preparation of plant extracts
Three plant species, namely fruit cumin (Cuminum cyminum L.), peel fruit of pomegranate (Punica granatum), and fruit of black pepper (Piper nigrum L.), were collected from various locations within Assiut, Egypt and Jeddeh city, Saudi Aribia. Fruit of Cumin and Black pepper and the fruit peel of pomegranate were also utilized. To prepare the methanolic extracts, the collected plant materials were first air-dried at room temperature for duration of 15 days. Subsequently, they were finely powdered using a grinder. The maceration process involved mixing these powdered plant materials with an 80:20 methanol: water solution, maintaining a sample-to-solvent ratio of 1:10 w/v. The mixture was subjected to continuous shaking for three days at room temperature. Following maceration, the materials were filtered through two layers of cheesecloth and Whatman No. 1 filter paper. The resulting filtrates were combined, concentrated using a rotary evaporator (Hidolph VV2000), and then subjected to freeze-drying using a Telstar-LyoQuest plus-55 lyophilizer. The yield of the extract was calculated, and the final product was stored in opaque glass tubes at −20 °C, as described by Somda et al. (2007).
Kirby-Bauer disc diffusion method
The Kirby-Bauer disc diffusion method Hafez et al. (2014) was used to assess the antibacterial activity efficacy of three plant extracts fruit cumin, peel fruit of pomegranate, and fruit of black pepper. In this method, 200 µl of adjusted suspension (1 × 108cfu/ml) 48 h-old cultures of tested isolate Pcc2 were spread over 9 cm in width sterile Petri plates supplemented with sterilized nutrient sucrose agar and after drying different concentration of tested plant extract (0, 10, 20, 30, 40 and 50) were pipetted into 9 mm punched. After 2 days from cultivation at 27 °C, inhibition zones in cm were measured. Four replicates were used for each treatment. The experiment was carried out four times for each concentration and repeated twice for accuracy.
Storage conditions against P. carotovorum subsp. carotovorum
This experiment applies to evaluate the efficacy of three plant species, cumin, peel fruit of pomegranate, and fruit of black pepper, to suppress soft rot disease caused by P. carotovorum subsp. carotovorum (Pcc2). The method was carried out in 2 parts, treated tubers, and control tubers. Firstly, treated potato tubers were immersed in 10 µg/ml of each plant extract for 15 min, and then left until complete moisture dryness. Secondly, control tubers were submerged in sterilized distilled H2O. Treated potato tubers were inoculated with 200 µl of adjusted suspension (1 × 108cfu/ml) at 27–29 °C of P. carotovorum subsp. carotovorum Pcc2, unlike control tubers inoculated with 200 µl sterilized distilled water. Both treated and control tubers kept in clean sterilized plastic containers supplemented with sterile moist cotton with incubation at optimum temperature 27–29 °C for 96 h for soft rot evolution. Disease assessment was recorded as demonstrated by Saleh et al. (1996).
Induction of enzymes and phenol contents in potato tuber
The effect of three plant extracts on biochemical changes of inoculated potato plants by Pcc3 were studied under storage conditions. Samples were collected, at zero time and at 7, 15, and 21 days after treatment for determined of total phenol contents, flavonoid and enzyme activities.
Determination of total phenol contents
Preparation of samples
To prepare the sample, one gram of potato plant tuber was first homogenized using liquid nitrogen and then thoroughly mixed with 10 ml of 80% methanol. This mixture was subsequently subjected to centrifugation at 1000×g for 30 min at a temperature of 4 °C. After centrifugation, the resulting pellet was discarded following the addition of ascorbic acid at a concentration of 0.1 g/5 ml. The homogenate was then evaporated utilizing a rotary evaporator, maintaining a temperature of 65 °C, with this process being repeated three times, each iteration lasting 5 min. The residues obtained were dissolved in 5 ml of 80% methanol. It is important to note that each treatment was conducted in quadruplicate, following the methodology described by Rapp and Ziegler (1973).
Total phenols content
The quantification of phenolic content was conducted according to the protocol outlined by Sahin et al. (2004). To prepare the reaction mixture, 0.02 ml of the methanol extract was combined with 0.5 ml of Folin reagent, 0.75 ml of a 20% Na2CO3 solution, and 8 ml of water. This mixture was then incubated for 60 min at a temperature of 37 °C in a water bath. A negative control was established using methanol. The determination of total phenolic content was carried out spectrophotometrically at 767 nm, expressed as milligrams per gram of plant fresh weight, with gallic acid serving as the standard for calibration. Total phenols content = mg gallic acid/g plant material.
Enzymes activity
To assess the activity of enzymes, including peroxidase (Po), polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL), one gram of fresh potato plant leaves was subjected to cryogenic treatment using liquid nitrogen. The resulting frozen material was homogenized in 10 ml of 0.1 M Na-acetate buffer with a pH of 5.2. Following homogenization, the mixture was subjected to centrifugation at 1000×g for duration of 30 min at a temperature of 4 °C, and the enzyme activities were subsequently determined in the supernatants. Each treatment was conducted in quadruplicate to ensure the accuracy and reliability of the results.
Total protein assays
The total protein content of potato plants was determined according to the method described by Bradford (1976) using Bradford reagent prepared as follows: 100 mg Coomassie Brilliant Blue G-250 was gently dissolved in 50 ml ethanol (95%), 100 ml of 85% H3PO4 were added and the mixture was completed to 1000 ml by distilled water. The reagent was filtered and preserved at 4 °C until use.
100 µl sample was mixed gently with 900 µl Bradford reagent and incubated for 15 min at room temperature. Protein content was assayed spectrophotometrically at 595 nm using bovine serum albumin as standard.
Peroxidase activity (PO)
Peroxidase activity was determined following the method described by Putter (1974). Spectrophotometric analysis was employed, utilizing guaiacol as the substrate. The reaction mixture consisted of 0.2 ml of the supernatant, 1 ml of 0.1 M Na-acetate buffer with a pH of 5.2, 0.2 ml of 1% guaiacol, and 0.2 ml of 1% H2O2. This mixture was incubated at a temperature of 25 °C for duration of 5 min and then measured at 436 nm. The extraction buffer served as the blank for calibration purposes. Enzyme activity was subsequently calculated based on the change in absorbance and expressed as units of enzyme activity per 1 mg of protein.
Polyphenol oxidase (PPO) activity
Polyphenol oxidase (PPO) activity was assessed according to the method outlined by Batra and Kuhn (1975). The reaction mixture consisted of 0.5 ml of the supernatant, 2 ml of 50 mM Sorensen phosphate buffer with a pH of 6.5, and 0.5 ml of the substrate Bren catechol from Sigma Aldrich. This reaction mixture was placed in a water bath, maintained at a temperature of 37 °C, and allowed to incubate for a period of 2 h. After incubation, the absorbance of the reaction mixture was measured at 410 nm to determine PPO activity. This assay provides valuable insights into the enzymatic activity of PPO in the sample. Activity of PPO = OD at 410 nm/mg protein.
Phenylalanine ammonia-lyase (PAL) activity
Phenylalanine ammonia-lyase (PAL) activity was assessed following the method described by Silva et al. (2004). The reaction mixture consisted of 0.5 ml of the supernatant, 2 ml of 50 mM Na-borate/HCl buffer with a pH of 8.8, and mercaptoethanol, along with 1 ml of 60 mM phenylalanine. This mixture was then incubated at a temperature of 37 °C for a duration of 2 h. The determination of PAL activity was carried out spectrophotometrically using a Unican UV spectrophotometer, with optical density measured at 290 nm. Cinnamic acid was employed as the standard for calibration, allowing for the quantification of PAL activity in the sample. This assay provides valuable insights into the enzymatic activity of PAL, an important enzyme in the phenylpropanoid pathway. PAL activity = mM cinnamic acid/mg protein.
Statistical analysis
Data were subjected to statistical analysis using analysis of variance using the Statistical Analysis System, (SAS Institute Inc. 1996), and means were compared using LSD test according to Gomez and Gomez (1984).
Results
Pathogenicity test on potato slices
Five isolates were obtained from naturally diseased potato tubers and were used to identify their pathogenic ability on healthy potato slices. The results of repeated pathogenicity tests showed that all of these isolates were pathogenic and able to produce typical soft rot symptoms with a range of severity from strong to weak. Isolates No. 2 and 5 showed highest diseases followed by isolate No. 1. The lowest disease was recorded by isolate No. 3 and 4. The results of the pathogenicity test on the tubers are presented in Table 1. From this results isolates No. 2 (Pcc2) was selected to complete the following experiments.
Identification of pathogens
According to the in vitro screening, the most virulence isolate Pcc2 was molecularly identified using 16S rRNA gene partial sequencing. The obtained sequence was submitted to GenBank under accession number MT510006. A phylogenetic analysis was performed using the maximum likelihood method in BLAST pairwise alignments. The isolate was identified as Pectobacterium carotovorum with 100% identity and 99% query coverage (Fig. 1).
Control of potato soft rot causal pathogen by different plant extracts (in vitro study)
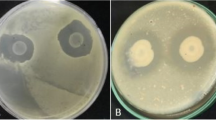
Results showed that the tested pomegranate extract revealed more bactericidal activity on soft rot causal pathogen Pcc2 than other extract. The data demonstrated that pomegranate extract was on the first ranking with bacterial growth reduction percentage estimated (1.4 mm), followed by Cumin extract with growth reduction estimated (0.92 mm). The result of this experiment was recorded in Fig. 2; Table 2.
Evaluation of cumin, pomegranate, and black pepper extracts on soft rot disease under storage conditions
Table 3 presented data that showed the ability of various plant extracts to reduce the severity of the potato tuber disease. The data revealed that all of the tested plant extracts were able to reduce the severity of the disease. Of all the extracts, pomegranate extract showed the highest reduction in disease severity (91.3%). This indicates that pomegranate extract was particularly effective in reducing the impact of the disease on potato tubers. Following pomegranate extract, the cumin and black pepper extracts also showed a reduction in disease severity (89.2 and 76.2% respectively).
Effect of certain plant extracts on total phenol contents, peroxidase activity, polyphenol oxidase and phenylalanine ammonia-lyase activities in inoculated potato plants with Pectobacterium carotovorum subsp. carotovorum
Total phenol contents
Figure 3 presents a comprehensive dataset on the total phenol contents in different plant treatments at various time points, which is valuable for understanding the impact of these treatments on phenolic compound production. It is evident that the treatments with P. granatum (pomegranate), P. nigrum (black pepper), and C. cyminum (cumin) consistently led to an increase in total phenol content over the course of 21 days. P. granatum gradually increases total phenol contents, peaking at 15 days with a value of 4.3 mg Gallic acid/g fresh weight. This suggests a positive influence of the treatment on phenolic compounds over time. Similarly, P. nigrum and C. cyminum show an increasing trend, reaching maximum values of 3.76 and 3.03 mg Gallic acid/g fresh weight, respectively, at 21 days.
Peroxidase activity
The data presented in Fig. 4 highlights the impact of specific plant extracts on peroxidase activity in potato plants that have been inoculated with P. carotovorum subsp. carotovorum. Observing the data, we discern distinct dynamics in peroxidase activity within the treatments. C. cyminum, P. nigrum, and P. granatum exhibit a progressive increase in peroxidase activity over the experimental period. C. cyminum, for instance, peaks at 15 days with a value of 4 units/mg protein.
Polyphenol oxidase activity
Figure 5 presents data on the polyphenol oxidase activity in potato plants inoculated with P. carotovorum subsp. carotovorum (Pcc2) and treated with different plant extracts. Polyphenol oxidase activity is measured in units per milligram of protein at four different time points: 0 days, 7 days, 15 days, and 21 days after treatment application. Analyzing the data reveals distinct trends in polyphenol oxidase activity across treatments and time points. C. cyminum, P. nigrum, and P. granatum show variations in polyphenol oxidase activity, with P. granatum exhibiting the highest mean activity of 0.3375 units/mg protein. Notably, P. nigrum significantly increases activity at 21 days, reaching 0.41 units/mg protein.
Phenylalanine ammonia-lyase activity
Figure 6 includes data for three different plant extracts (P. granatum, P. nigrum, C. cyminum) as well as infected and healthy control groups. PAL activity is observed to vary significantly in response to treatments and infection. The values are provided for each time point, measured in an appropriate unit. Upon examination of the data, distinct patterns in PAL activity emerge across treatments and time points. C. cyminum, P. nigrum, and P. granatum show substantial variations in PAL activity, with P. granatum exhibiting the highest mean activity of 92.3425 mg cinnamic acid/mg protein. C. cyminum and P. nigrum also display noteworthy increases, particularly at 15 and 21 days.
Discussion
In an effort to address this issue, a study was conducted to evaluate the pathogenicity and virulence of different bacterial isolates in relation to soft rot in potato tubers. The results showed that only 15 isolates were identified as pathogenic, capable of causing soft rot symptoms, and demonstrated varied levels of virulence. These results aligned with those found by previous researchers in the field, including Ismail and Moustafa (2012), Ashmawy et al. (2015), Czajkowski et al. (2015), Shmas et al. (2016), and Azaiez et al. (2018).
The identity of the most pathogenic isolate was confirmed through 16S rRNA gene sequence. These methods were found to be effective in identifying Pectobacterium ssp., which was consistent with the results of previous studies such as those by Ngadze et al. (2012), Ashmawy et al. (2015), Shmas et al. (2016), and Benada et al. (2018).
Additionally, the study evaluated the effect of different plant extracts, including P. nigrum (black pepper), P. granatum (pomegranate) and C. cyminum (cumin), on the suppression of soft rot in potato tubers during storage conditions. The results showed that these plant extracts exhibited antibacterial activity against P. carotovorum subsp. carotovorum, with pomegranate extract causing complete suppression of the disease. These results were consistent with those found by Abo-Elyousr and Bagy (2019).
The results of the study on the antibacterial activity of different plant extract on P. carotovorum subsp. carotovorum showed that each extract had a different impact on bacterial growth reduction. This is in line with the findings reported by Abo-Elyousr and Bagy (2019). The researchers specifically tested the effect of pomegranate, black pepper, and cumin at a concentration of 50 µg/ml on the suppression of potato tuber soft rot during storage conditions.
In conclusion, plant extracts have been proven to be effective in controlling soft rot disease. These natural remedies have been shown to have antibacterial properties that can effectively inhibit the growth and spread of soft rot-causing pathogens.
One notable trend observed in the data is the consistent increase in total phenol content in plants treated with P. granatum (pomegranate), P. nigrum (black pepper), and C. cyminum (cumin). This suggests that these specific treatments have a positive effect on stimulating the production of phenolic compounds in the plants. Interestingly, the infected control group also exhibited an increase in phenol content, albeit at a lower rate compared to the treated groups. This observation indicates a natural response to infection, suggesting that plants may activate their phenolic compound production as a defense mechanism against pathogens (Tuladhar et al. 2021). This finding aligns with existing literature on the role of phenols in plant defense mechanisms, highlighting their importance in the response to stressors such as infections (Rahman et al. 2022). The observed increase in phenol content in response to specific treatments suggests potential avenues for enhancing the production of these bioactive compounds in plants. Additionally, the natural response of the infected control group and the baseline provided by the healthy control group contribute to a comprehensive understanding of the factors influencing phenolic compound production in plants (Rashid et al. 2023).
Understanding the dynamics of PPO activity in inoculated potato plants provides insights into the molecular and biochemical mechanisms employed by the plant to counteract the effects of P. carotovorum subsp. carotovorum. Monitoring changes in PPO activity over time and in different treatments can contribute to a more comprehensive understanding of the plant’s defense strategies and aid in the development of strategies to enhance plant resistance to bacterial pathogens (Ujjainkar et al. 2022; Sulman et al. 2001; Silva et al. 2004).
PAL activity is a crucial indicator of the plant’s defense mechanisms, specifically in response to infection by P. carotovorum subsp. carotovorum. The significant variability in PAL activity is across the different treatments and control groups. PAL is an enzyme involved in the phenylpropanoid pathway, and its increased activity is often associated with the synthesis of phenolic compounds, which play a vital role in plant defense against pathogens (Solekha et al. 2020). The cumulative effect of the treatments is highlighted by the more pronounced increase in PAL activity at later time points. This implies that the impact of these plant extracts on PAL activity is not immediate but unfolds progressively, potentially suggesting a sustained and cumulative enhancement of the plant’s defense mechanisms (You et al. 2020). The temporal dynamics observed in PAL activity underscore the importance of considering the duration of treatments in understanding their full effects on the plant’s biochemistry.
Overall, this study highlights the importance of addressing the issue of soft rot in potato production, as it negatively impacts the quality and quantity of this crucial crop in KSA. The results provide valuable insights into the pathogenicity and virulence of different bacterial isolates, as well as the potential for using plant extract as a means of suppressing the disease.
Data availability
The dataset generated during the current study is available from the corresponding author on reasonable request.Author contributions.
References
Abd-Elghany WA, Mohamedin AH, Abo-Elyousr KAM, Hussein MAM (2022) Control of bacterial soft rot disease of Potato caused by Pectobacterium carotovorum subsp. Carotovorum using different synthetic nanoparticles. Arch Phytopathol Plant Protect 55(14):1638–1660. https://doi.org/10.1080/03235408.2022.2111247
Abo-Elyousr KAM, Bagy HMMK (2019) Control of tomato bacterial wilt using certain of plant ethanol extracts. J Phytopathol Pest Manag 5(3):77–84
Abo-Elyousr KAM, Almasoudi NM, Abdelmagid AWM, Roberto SR, Youssef K (2020) Plant extracts treatments induce resistance to bacterial spot on tomato plants for a sustainable system. Horticulturae. 6:36. https://doi.org/10.3390/horticulturae6020036. Khamis Youssef
Ashmawy NA, Jadalla NM, Shoeib AA, El-Bebany AF (2015) Identification and genetic characterization of Pectobacterium spp. and related Enterobacteriaceae causing potato soft rot diseases in Egypt. Pure Appl Microbiol 9(3):1847–1858
Azaiez S, Ben-Slimenea I, Karkoucha I, Essida R, Jalloulia S, Djebalia N, Elkahouia S, Limama F, Tabbenea O (2018) Biological control of the soft rot bacterium Pectobacterium carotovorum by Bacillus amyloliquefaciens strain Ar10 producing glycolipid-like compounds. Microbiolog Res 217:23–33. https://doi.org/10.1016/j.micres.2018.08.013
Batra GK, Kuhn CW (1975) Polyphenoloxidase and peroxidase activities associated with acquired resistance and it inhibition by 2-thiouracil in virus infected soybean. Physiol Plant Pathol 5:239–248. https://doi.org/10.1016/0048-4059(75)90090-9
Benada M, Boumaaza B, Boudalia S, Khaladi O, Guessas B (2018) Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Inter J Plant Biol 9(1):52–56. https://doi.org/10.4081/pb.2018.7568
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–250. https://doi.org/10.1006/abio.1976.9999
Czajkowski R, Pérombelon MC, Jafra S, Lojkowska E, Potrykus M, van der Wolf JM, Sledz W (2015) Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot. Ann Appl Biol 166(1):18–38. https://doi.org/10.1111/aab.12166
da Silva CMA, da Silva Costa BM, da Silva AG, de Souza Elineide B (2016) Antimicrobial activity of several Brazilian medicinal plants against phytopathogenic bacteria. Afr J Microbiol Res 10:578–583. https://doi.org/10.5897/AJMR2014.6999
DeBoer SH, Kelman A (1978). Influence of oxygen concentration and storage factors on susceptibility of potato tubers to bacterial soft rot (Erwinia carotovora). Potato Research 21(2): 65–80. https://doi.org/10.1007/BF02362262
Doolotkeldieva T, Bobusheva S, Suleymankisi A (2016) Biological control of Erwinia carotovora ssp. carotovora by streptomyces species. Advan Microbiol 6(2):104–114. https://doi.org/10.4236/aim.2016.62011
Dowson WJ (1957) Plant disease due to bacteria, 2nd edn. Cambridge University press, p 231
Gomez KA, Gomez AA (1984) Statically procedures for agricultural research, 2nd edn. John Wiley and Sons Inc., New York, USA, p 680
Hafez EE, Hassan HS, Elkady MF, Salama E (2014) Assessment of antibacterial activity for synthesized zinc oxide nanorods against plant pathogenic strains. Int J Sci Technol Educ Res 3(9):318–324
Hossain A, Abdallah Y, Ali MA, Masum MMI, Li B, Sun G, Meng Y, Wang Y, An Q (2019) Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules 9(12):1–14. https://doi.org/10.3390/biom9120863
Ibrahim OHM, Abo-Elyousr KAM (2023) Potential antimicrobial activity of various botanical extracts against the causal pathogen of the blue mold of citrus fruits. Plant Pathol J 105(2):527–538. https://doi.org/10.1007/s42161-023-01339-8
Ismail ME, Moustafa YMM (2012) Identification and pathogenicity of phytopathogenic bacteria associated with soft rot disease of girasole tubers in Egypt. J Stored Prod Posthart Res 3(6):67–74. https://doi.org/10.5897/JSPPR11.047
Mangalagiri NP, Panditi SK, Jeevigunta NLL (2021) Antimicrobial activity of essential plant oils and their major components. Heliyon 7:e06835. https://doi.org/10.1016/j.heliyon.2021.e06835
Mantsebo CC, Mazarura U, Goss M, Ngadze E (2014) The epidemiology of Pectobacterium and Dickeya species and the role of calcium in postharvest soft rot infection of potato (Solanum tuberosum) caused by the pathogens. Afri J Agr Res 9(19):1509–1515. https://doi.org/10.5897/AJAR2013.8558
McGuire RG, Kelman A (1984) Reduced severity of Erwinia soft rot in potato tubers with increased calcium content. Phytopathology 74(10):1250–1256. https://doi.org/10.1094/Phyto-74-1250
Ngadze E, Brady CL, Coutinho TA, van der Waals JE (2012) Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. Eur J Plant Pathol 134(3):533–549. https://doi.org/10.1007/s10658-012-0036-z
Purushotham SP, Anupama N (2018) In vitro antimicrobial screening of medicinal plants against clinical and phytopathogenic bacteria and fungi. Int J Pharm Sci Res 9:3005–3014
Putter J (1974) Peroxidase. In: Bergmeyer HU (ed) Methoden Der enzymatischen analyses. Verlag Chemie, Weinheim, p 725
Rabia AH, Yacout DMM, Shahin SF, Mohamed AAA, Abdelaty EF (2018) Towards sustainable production of potato under climate change conditions. Curr Appl Sci Technol 18(3):200–207
Rahman MM, Rahaman MS, Islam MR, Rahman F, Mithi FM, Alqahtani T, Almikhlafi MA, Alghamdi SQ, Alruwaili AS, Hossain MS (2022) Role of Phenolic compounds in human disease: current knowledge and future prospects. Molecules 27:233. https://doi.org/10.3390/molecules27010233
Rapp A, Ziegler A (1973) Bestimmung Der Phenolcarbonsaure in Rebblattern Weintraube Und Wein mittels Polamyid-Dunnschicht Chromatographie. Vitis 12:226–236
Rashid MO, Akter M, Uddin J, Islam S, Rahman M, Jahan K, Rahman M, Sarker R, Sadik G (2023) Antioxidant, cytotoxic, antibacterial and thrombolytic activities of Centella asiatica L.: possible role of phenolics and flavonoids. Clin Phytosci 9:1. https://doi.org/10.1186/s40816-023-00353-8
Sahin F, Gulluce M, Daferera D, Sokmen A, Sokmen M, Polissiou M, Agar G, Ozer H (2004) Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. Vulgare in the Eastern Anatolia region of Turkey. Food Control 15:549–557. https://doi.org/10.1016/j.foodcont.2003.08.009
Saleh OI, Huang PY, Huang JS (1996) Bacterial vascular necrosis and root rot disease of sugar beet in Egypt. J Phytopathol 144(5):225–230. https://doi.org/10.1111/j.1439-0434.1996.tb01520.x
Sallam NMA, Esmat FA, Abo-Elyousr KAM, Mohamed FFB, Mohamed AA, Seleim (2021) Thyme oil treatment controls the bacterial wilt disease symptoms by inducing antioxidant enzymes activity in Solanum tuberosum J. Plant Pathol 103:563–572. https://doi.org/10.1007/s42161-021-00808-2
SAS Institute Inc (1996) SAS Institute Inc., SAS Version 9.0, Cary, NC, USA
Shmas AHM, Ashmawy NA, El-Bebany AF, Shoeib AA (2016) Identification and pathogenicity of phytopathogenic bacteria associated with soft rot disease on some potato cultivars. Alexa J Agric Res 61(6):541–550
Silva HSA, Romeiro RS, Macagnan D, Halfeld-Vieira BA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants non-specific protection and increase in enzyme activities. Biol Control 29:288–295. https://doi.org/10.1016/S1049-9644(03)00163-4
Solekha R, Susanto FA, Joko T, Tri Rini Nuringtyas Yekti AP (2020) Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae Pv. Oryzae. J Plant Pathol 102:359–365. https://doi.org/10.1007/s42161-019-00426-z
Somda I, Leth V, Seeme P (2007) Evaluation of Lemongrass, Eucalyptus and neem aqueous extracts for controlling seed-borne fungi of sorghum grown in Burkina Faso. World J Agric Sci 3:218–223
Sulaiman MM, Yass STA, Aish AA, Yasir LB, Abdullah SJ, Youssef AS (2020) Activity of Trichoderma spp. against Erwinia carotovora causal agent of potato tuber soft rot. Plant Archives 20(1)
Sulman M, Fox G, Osman A, Inkerman A, Williams P, Michalowitz M (2001) Relationship between total peroxidase activity and susceptibility to black point in mature grain of some barley cultivars. In: Proceeding of the 10th Australian Barley Technical Symposium. Canberra, ACT, Australia, 16–20 Sep. 2001
Tuladhar P, Santanu S, Prakash S (2021) Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In: Jogaiah S (ed) Biocontrol agents and secondary metabolites. Woodhead Publishing, pp 419–441. https://doi.org/10.1016/B978-0-12-822919-4.00017-X
Ujjainkar V, Patil V, Mane S, Moharil M (2022) Biochemical evaluation of cotton genotypes using soluble protein, esterase (EST), Peroxidase (POX) and Polyphenol Oxidase (PPO) and their role in plant disease resistance. J Res Appl Sci Biotechnol 1(3):229–237. https://doi.org/10.55544/jrasb.1.3.30
Yaganza ES, Arul J, Tweddell RJ (2004) Effect of prestorage application of different organic and inorganic salts on stored potato quality. Potato Res 46(3):167–178. https://doi.org/10.1007/BF02736086
You X, Fang H, Wang R, Wang GL, Ning Y (2020) Phenylalanine ammonia lyases mediate broad-spectrum resistance to pathogens and insect pests in plants. Sci Bull 65:1425–1427. https://doi.org/10.1016/j.scib.2020.05.014
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Data curation, KAMA, ADA and NMA; Formal analysis, KAMA; Investigation, ADA; Methodology, Project administration, NMA; Resources, KAMA; Software, KAMA; Supervision, NMA; Visualization, ADA; Writing—original draft, KAMA, and NMA; Writing—review and editing, ADA.
Corresponding author
Ethics declarations
Institutional review board
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almasoudi, N.M., Al-Qurashi, A.D. & Abo-Elyousr, K.A.M. Assessment of certain plant extracts for controlling potato tuber soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01673-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01673-5