Abstract
Pigeaonpea is attacked by various diseases, including the wilt disease of pigeonpea caused by Fusarium udum. This disease is a severe pathogen to this crop. This study aims to identify the potential biocontrol agent against wilt disease as a fungicide alternative. Forty-seven isolates were evaluated for antagonistic activity against F. udum by dual culture method. Interaction of F. udum and antagonistic bacteria was studied in potato dextrose agar (PDA) under in vitro conditions and lysis of fungal hyphae was observed by using Scanning Electron Microscope. Dry weight of F. udum mycelium was recorded after 3 days of co-inoculation with the rhizobacteria in PDB. Potential antagonistic bacterial isolates were further used for enzymatic assay in vitro conditions. Molecular characterization of bacteria was done by using primers based on hydrolytic genes like chitinase and 1,3-glucanase related genes, amplified at 402 and 750 bp, respectively. Out of forty-seven bacterial isolates used to assess their antagonistic activity, only eight isolates, viz., Bacillus amyloliquefaciens CFLB 31, Bacillus velezensis CFLB 24, Bacillus subtilis CFLB 11, Stenotrophomonas rhizophila CFLB 26, S. matalophila CFLB 47, Microbacteria sp. CFLB 28, G.nicotiana CFLB 18 and Pseudoarthrobacter sp. CFLB 36 showed the promising antagonistic activity against F. udum with 70–84% inhibition in a dual culture plate assay. Among them, three Bacillus species (B. amyloliquefaciens, B. velezensis, B. subtilis) and S. maltophilia CFLB 47 were found to be the most effective biocontrol agent against F. udum under in vitro conditions. Lysis of fungal hyphae was also noted during interaction of fungus and bacteria on PDA. These isolates were screened for production of hydrolytic enzymes activities and they showed positive for production of pectinase, protease and cellulase under in vitro conditions. These isolates amplified chitinase and β-1, 3-glucanase-related genes at 402 and 750 bp, respectively. In addition, bacterial strains reduced the mycelium weight of F. udum with the range of 58.42 − 86.84% during co-inoculation in PDB. However, B. amyloliquefaciens had the highest percentage of biomass reduction, up to 86.84%. Bacterial treatments are considered beneficial and nature-friendly. The results propose that the eight potential strains and their hydrolytic enzymatic properties made them promise to manage wilt disease of pigeonpea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeaonpea (Cajanus cajan (L.) Millsp), commonly known as “Tur dal” belongs to the Fabaceae family of the order Fabales. It is a perennial crop plant with yellowish flowers. Pigeonpea is commercially important because it is the highest-paid legume in the world. It is besides being used as a source of protein. Pigeonpea products such as dried grain, fresh (aerial portion), and green pods are used as a low-cost source of high-quality and quantity protein food and feed for the livelihoods of tropical and subtropical populations (Dukare et al., 2021). The most widely utilised pulse (legume) crop in India is the pigeonpea which is cultivated across a 72% area of 3.9 Mha.
Economically significant crops like pigeonpea need to be protected from numerous diseases, since demand for them is predicted to rise gradually, especially in emerging nations. Several pathogens like fungi, bacteria, viruses and nematodes attack the pigeonpea plant and cause different diseases leading to significant losses in yield. Among the essential fungal infections, Fusarium wilt is the most devastating and prevalent (Hashem et al. 2008) and has a significant mortality rate due to dangerous soil- and seed-borne fungus Fusarium udum Butler. According to Morrissey et al. (2004), soil-borne fungal pathogens such F. udum (FU), Rhizoctonia spp., Phytophthora sp., Pythium sp., cause severe plant diseases, reduce crop production, and degrade yield quality. Such fungal pathogen infections can infect a variety of economically important agricultural crops, causing illnesses. Fusarium and other soil pathogenic fungi are usually managed using synthetic fungicides such as thiram, benomyl, bavistin (Melent’ev et al. 2006). Unfortunately, excessive use of chemical fungicides can harm the environment, have an adverse impact on beneficial microorganisms that are not intended to be affected, cause pathogens to become resistant to fungicides, and endanger human health (Dukare et al. 2020). To manage the wilt disease, there is an urgent need to follow eco-friendly approaches to save environment from pesticide -related risks. Bacteria play an important role to enhance plant growth as well as prevent the diseases caused by may pathogens (Singh et al. 2022; Yadav et al. 2023).
Owing to their environmentally friendly nature, biological disease prevention utilising antagonistic bacteria (biocontrol) stimulates sustainable agriculture (Dukare et al. 2020). Recent days, biocontrol has focused on the use of bacteria that produce mycolytic enzymes and encourage plant development. Furthermore, the use of rhizospheric and endophytic microbes to manage crops’ abiotic and biotic stressors has a long history (Sangwan and Dukare 2018). Plant growth-promoting bacteria have the potential to minimize or avoid pathogen toxicity by various indirect mechanisms, such as sulphur oxidation and the production of bacteria-to-plant signal molecules (which include oligosaccharides and peptides), contribute to capacity of bacteria to promote plant growth, producing antibiotics, cell wall disintegrating enzymes, competing for niches, and so on (Hayat et al. 2010; Pandya et al. 2015; Saini et al. 2015; Coutinho et al. 2015; Aamir et al. 2021; Rai et al. 2023). According to study, a large number of bacterial species from the genera Lysobacter, Bacillus, Pseudomonas, Paenibacillus, Azotobacter and Streptomyces, endophytic Fusarium have been suggested as the greatest alternatives for the development of bioformulations, biopesticides, and bio stimulants to tackle the problems with agriculture and climate change (Aamir et al. 2019; Samal et al. 2022, 2023; Rai et al. 2023).
Bacterial communities produce an extensive number of enzymes. Moreover, the hydrolytic enzymes generated by bacteria can break down plant pathogenic fungal cell wall components. These hydrolytic enzymes’ primary role is to dissolve the glycosidic bonds that hold cell wall polysaccharides together. Hydrolytic enzymes include amylase, pectinase, cellulase, chitinase, and glucanase. In this way, synthesis of hydrolytic enzyme by bacteria is essential to the phytopathogen-controlling system (Mishra et al. 2020). Furthermore, by dissolving the fungal pathogen cell walls and resulting in cell death, these enzymes assist in controlling plant diseases sustainably (Susilowati et al. 2021).
This study aimed to identify, screening the antifungal efficacy of bacterial strains against F. udum and investigate their potential as a biocontrol agent against wilt pathogen. Also, biochemical characterization of hydrolytic enzymatic activity and molecular characterize the cell wall degrading enzymes like β-1, 3-glucanase, and chitinase genes of different bacterial isolates was determined to know their function in suppressing the growth of F. udum.
Materials and methods
Bacterial culture
Forty-seven isolates of bacteria including Bacillus amyloliquefaciens (ON514187), Bacillus velezensis (ON514218), Bacillus subtilis (ON753753), Stenophomonas rhizophila (ON514222), Stenotrophomonas maltophilia (OR186297), Microbacterium sp. (ON764207), Glutamicibacter nicotianae (ON763990), Pseudoarthrobacter sp. (ON764802) were obtained from Plant Bacteriology Laboratory, Division of Plant Pathology, ICAR- Indian Agricultural Research, Institute, New Delhi, (Table 1) and were employed for this study and kept on nutrient agar plates for a short duration while being kept in nutrient broth (NB, HiMedia, India) modified with 50% glycerol at −80 °C for a long time.
Maintenance and growth of Fusarium udum
The virulent Fusarium udum (OR185560) isolate that causes pigeon pea wilt disease was isolated from wilt infected plant inoculated on potato dextrose broth (PDB) by following standard procedure (Barwant et al., 2020) By routinely subculturing on potato dextrose agar (PDA), the viability and pathogenicity of the culture were retained.
In vitro antagonistic activity of bacteria against Fusarium udum
The dual culture approach was used to assess antifungal activity against Fusarium udum, a soil-borne plant pathogenic fungal, was cultured on potato dextrose agar (PDA) medium. A 5.00 mm diameter clump of seven days old F. udum mycelium was taken from and placed in the centre of a Petri plate with fresh PDA (Oldenburg et al. 1996). A loop of the exponentially growing bacterial culture of each isolate was streaked in a straight line on one side of a 90 mm diameter Petri dish, with the space between the fungus and the bacterial culture kept at 2 cm, and the plates were incubated for 5days at 28ºC. The test fungus’s radial growth inhibition has been noticed regularly. Only plates containing cultures of the pathogenic fungus were cultured. In each isolate, three replicas were taken. The diameters of the colonies were assessed after five days, and the average values relative to the control were used to determine fungitoxicity. The following formula to calculate the growth inhibition (%) of the test fungus was used as described by Kumar et al. (2018).
Where R1 is the radial growth of F. udum in the control plate, and R2 is the radial growth of F. udum in the antagonist-tested plate.
Scanning electron microscopy of fungal hyphae
Hyphae of F. udum were fixed for 24 h at 4ºC temperature in 2% glutaraldehyde, then washed 4 times with phosphate buffer (0.1 M) and fixed for 2 h at 20 ºC in 1% osmium tetraoxide. In a Nanotech sputter coating system (Leica EM, ACE 6000), the hyphae were sputter coated with gold palladium after being dehydrated in a graded sequence of ethanol concentrations (20%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%) for 15 min each. They were then CO2 dried (Leica CPB 030). This information was reported by (Kang et al. 2000; Gajbhiye et al. 2010). Samples were stored in a desiccator until they were examined at 20 kV using a SEM (Zeiss).
Hydrolytic enzymatic activity of antagonistic bacterial strains
Protease activity
The protease activity of the bacterial isolates was determined using the skim milk agar medium (Skim milk powder 28 g l − 1, tryptone 5 g l− 1, yeast extract 2.5 g l − 1, glucose 1 g l− 1, agar 15 g l− 1) as described previously (Chaiharn et al. 2008). The isolates were spot inoculated on skim milk agar medium, and after two days of incubation at 30 °C, proteolytic activity was assessed by clear zone around the colonies.
Cellulase activity
The bacterial isolates were screened for cellulase production by plating them carboxymethyl cellulose (CMC) plate (Yeast extract 1 g l− 1, Mannitol 10 g l− 1, K2HPO4 0.500 g l − 1, MgSO4 0.200 g l− 1, NaCl 0.100 g l− 1, Agar 15 g l− 1, pH 7.0 ± 0.2) agar media supplemented with CMC 10 g l− 1 (Sigma-Aldrich, Germany). After 48 h incubation at 30 °C, plates were flooded with Congo red dye, and distaining with 1% NaCl. The clear halos zone formed surrounding the colonies indicated their cellulolytic activity (Hankin et al., 1977).
Amylase activity
Amylase activity was performed by inoculating the bacterial isolates on starch agar medium (soluble starch 2 g l− 1; peptone 5 g l− 1; beef extract 3 g l− 1; agar 15 g l− 1, pH 7.0 ± 0.2) and incubated for 24–48 h at 30ºC. The plates were flooded with iodine solution at the end of the incubation period, stored for a minute, and then poured out. Iodine reacts with starch to form a blue coloured complex. The colourless zones surrounding colonies showed the production of amylase (Collins et al., 1970).
Pectinase assay
1% pectin in PSMA basal medium ((NH4)2HPO4 3 g l− 1, KH2PO42 g l− 1, K2HPO4 3 g l− 1, MgSO4 0.1 g l− 1, Agar 20 g l− 1). One loopful of the bacterial cell suspension was streaked on the medium and incubated for five days. Gram’s iodine solution was poured onto the pectin agar, and a clearance zone was observed against the dark blue background (Melkamu et al. 2013).
Lipase activity
Lipase activity was determined by inoculating the actively grown culture on Tween 80 agar media (CaCl2 2 g l− 1, peptone 10 g l− 1, tween 80 4.7 mL, agar 20 g l− 1, distilled water 1 L, pH 7), and the plates were incubated at 28 ± 2 ◦C for 4 days. The formation of a halo area around the bacterial colonies demonstrates the production of lipase enzyme (Schaad et al. 2001).
Isolation of bacterial genomic DNA and identification of cell wall degrading enzyme producing antagonistic bacteria through PCR
The van Soolingen et al. (1994) approach was used to largely unmodified isolate bacterial genomic DNA. After centrifuging the culture, which had been cultured in NB for 24 h, it was used for DNA isolation. While agarose gel electrophoresis was used for qualitative estimation, and the DNA band was observed by visualizing the gel for the DNA band in UVITECH gel doc system, the quantitative analysis of the DNA was done spectrophotometrically using a bio photometer (Eppendorf, India) that is based on Beer Lambert’s law.
Using a gene-specific primer (GSP) created online by the Primer3 tool, which is accessible online at the site http://primer3.ut.ee/ and specially synthesized by ITD. PCR amplification of the β1,3-glucanase and chitinase genes responsible for biocontrol against fungal pathogens was performed from genomic DNA of eight bacterial isolates i.e. Bacillus amyloliquefaciens (ON514187), Bacillus velezensis (ON514218), Bacillus subtilis (ON753753), Stenotrophomonas rhizophila (ON514222), Stenotrophomonas maltophilia (OR186297), Microbacterium sp. (ON764207), Glutamicibacter nicotianae (ON763990), Pseudarthrobacter sp. (ON764802). The concentration of 100 ng of genomic DNA, 0.5 µl of Taq polymerase (3U/µl), 2.5 µl of 10 X buffer, 1 µl of dNTP mix (10 mM), 2 µl of MgCl2 (25 mM), and 0.5 µl of each primer (10 pmol/µl) made up the 25 µl reaction mixture. β-1, 3- glucanase gene was amplified by using primer: gbF-TGGCACACCATACGAAAGAA and gbR-AGATACTTGTCCATCACCTAAC with PCR condition: initial denaturation at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 62 °C for 45 s, extension at 72 °C for 1 min, extension at 72 °C for 10 min at 402 bp. The concentration of 100 ng of genomic DNA, 0.5 µl of Taq polymerase (3U/µl), 2.5 µls of 10 buffer, 1 µl of dNTP mix (10mM), 2 µl of MgCl2 (25mM), and 0.5 µl of each primer (10 pmol/µl) made up the 25 µl reaction mixture. The Chitinase gene was amplified at 750 bp by using primer: cbF-GAATATCCTGGCGTTGAAACGAT and cbR-GCCACGTCCGTAAAAGGGT with PCR condition: initial denaturation at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 59 °C for 30 s, extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. The 1.2% agarose gel loaded with PCR product for both genes was observed (Nagar et al. 2016) and photographed by using Gel Documentation (BIO-RAD, GEL DOCTM XR + with image Lab™ software).
Biomass of F. udum is influenced by effective biocontrol bacterial strains
According to a modified approach, the antagonistic effects of the eight most potent antifungal B. amyloliquefaciens, B. velezensis, B. subtilis, S. rhizophila, S. maltophilia, Microbacterium sp., G. nicotianae, and Pseudarthrobacter sp. were determined in liquid cultures on the biomass of F. udum (Goa et al. 2016). For this experiment, chosen bacteria (24-hour-old culture) and F. udum (cell density of 2 × 108 spores ml− 1) were inoculated simultaneously (co-inoculation) into 100 ml of conical flasks containing 25 ml of PD broth. PDB with only a pathogen inoculation was placed in the control flask. Following inoculation, the flask was incubated at 30 °C, and following filtration through pre-weighed Whatman No. 1 filter paper, the dry weight of the pathogen mycelium was determined.
Statistical analysis
The IBM SPSS programme, version 16.0, was used to analyse the data, which was recorded in three duplicates. The analysis of variance was established, and Duncan’s multiple range tests were applied to compare the mean values at P < 0.05. The graphs show the standard error of means (3 replications) data as bars.
Results
Screening of bacterial isolates antifungal activity against F. udum in vitro condition
Forty-seven bacterial isolates were evaluated for antagonistic activity against F. udum on PDA by the dual culture method and among them, eight bacteria isolate such as Glutamicibacter nictotianae CFLB18 (83.33%), B. subtilis CFLB11 (83.33%), B. velezensis CFLB24 (81%), S. rhizophilla CFLB26(81.96%), Microbacterium sp.CFLB28(83.33%), B.amyloliquefaciens CFLB 31 (83.33%), and S. maltophilia CFLB 47 (81.65%) inhibited > 80% growth of the fungus (Table 1; Fig. 1).
Effect of biocontrol agent on ultrastructure of Fusarium udum
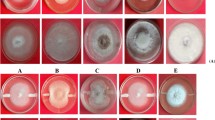
Lysis, tangled, twisting and deformation of hyphae F. udum was observed by using SEM with co-inoculation of B. amyloliquefaciens CFLB-31 (Fig. 2B), B. subtilis (Fig. 2C) and Glutamicibacter nictotianae (Fig. 2D) which inhibited the growth of the fungus. Normal hyphae of F. udum without bacterial inoculation was found intact regular shape with a smooth surface (Fig. 2A).
SEM analysis of antagonistic bacteria interacting with hyphae of pathogens on PDA medium at 5th day after incubation at 27˚C, (A) denoted normal hyphae of F. udum, (B) Co-inoculated with B. amyloliquefaciens with abnormal and lysis of hyphae, (C) Co-inoculation with B. subtilis, (D) Co-inoculation with Glutamicibacter nictotianae
Hydrolytic enzymatic
Eight bacterial isolates showing better antagonistic activity against F. udum was taken for five hydrolytic enzymatic (protease, amylase, pectinase, cellulase, and lipase) activities on different growth media under in vitro conditions (Table 2). Out of eight, seven isolates viz., CFLB11, CFLB 31, CFLB 26, CFLB 24, CFLB 28, CFLB 18, CFLB 47showed positive for amylase activity ranging from 0.89 to 1.100 cm. Maximum amylase activity was found in the isolate CFLB 31. All Eight isolates were found positive for cellulase, protease and pectinase activities. However, the isolates CFLB 28, CFLB 18 and CFLB 26 showed maximum index for cellulase (2.30 ± 0.057 cm), pectinase (1.80 ± 0.057 cm) and protease (1.03 ± 0.033 cm) enzyme activities respectively. Lipase was produced by only 3 out of 8 isolates viz., CFLB 11, CFLB 31 and CFLB 47with a lipase index 1.10–1.76 cm and the highest lipase index was found in CFLB 47 (1.76 ± 0.033 cm) (Table 2; Fig. 3).
Fungal biomass is affected by the co-inoculation of biocontrol bacteria
The effect of each co-inoculated antagonistic bacterial culture with F. udum on the mycelium dry weight (gm) was observed after 24, 48, and 72 h of inoculation in potato dextrose broth. The mycelium’s dry weight of F. udum was slightly increased in all the treatments except FU + 18 and FU + 36 (Fig. 4). Minimum mycelium dry weight was recorded in FU + 31 (B. amyloliquefaciens) after 24, 48, and 72 h, followed by F + 11 (B. subtilis) and FU + 18 (G. nicotiana) under in vitro conditions. These antagonistic bacteria drastically reduced the biomass of fungal mycelium as compared to F. udum alone (Fig. 3). In terms of Percentage of Biomass reduced concerning F. udum (Control), Maximum biomass decreased in co-inoculation with FU + 31 (86.84%) followed by 72.10% with FU + 11, FU + 18 gave 67.36%, FU + 47 gave 61.05%, FU + 24 gave 60.52%, FU + 28 gave 60%, FU + 36 gave 58.42% and minimum with FU + 26 up to 54.21%—maximum percentage of biomass reduction by FU + 31 up to 86.84%.
Effect of hydrolytic enzymatic activities of antagonistic bacteria on biomass of F. udum under broth culture. Values are means of three replications ± standard errors (SE). According to Duncan’s multiple range test, different lowercase letters that match to the treatment bars indicate a significant treatment difference at P < 0.05. FU: F. udum, B. amyloliquefaciens (CFLB 31), B. velezensis (CFLB 24), B. subtilis (CFLB 11), S. rhizophila (CFLB 26), S. maltophilia (CFLB 47), Microbacterium sp. (CFLB 28), G. nicotianae (CFLB 18), Pseudoarthrobacter sp. (CFLB 36)
Identification of cell wall degrading antagonistic bacteria using PCR
The cell wall degrading enzymes like chitinase and β -1, 3-glucanase genes-based primers were designed. These gene-specific primers were used to amplify the chitinase and β-1, 3-glucanase biocontrol genes of antagonistic bacteria B. amyloliquefaciens (CFLB 31), B. velezensis (CFLB 24), B. subtilis (CFLB 11), S. rhizophila (CFLB 26), S. maltophilia (CFLB 47), Microbacterium sp. (CFLB 28), G. nicotianae (CFLB 18), Pseudoarthrobacter sp. (CFLB 36) at 402 bp and 750 (Fig. 5).
PCR amplification of (a) chitinase (b) 1,3-glucanase on 1.2% Agarose gel showing 402 bp chitinase gene (left to right lane Marker, B. amyloliquefaciens, Microbacterium sp, B. velezensis, G. nicotianae, S. rhizophila, S. maltophilia, B. subtilis, Pseudoarthrobacter sp.) and 1,3 glucanase 750 bp amplification. (Lane 1 M for 100 bp plus ladder, Lanes 1–8(left to right): B. amyloliquefaciens, Microbacterium sp, S. maltophilia, B. subtilis, B. velezensis, G. nicotianae, Pseudarthrobacter sp.)
Discussion
The antagonistic bacteria used in biological control is considered the best alternative method for physical and chemical disease management against fungal and bacterial phytopathogens due to their aggressive colonization ability, fast growth, and simple handling. (Saraf et al. 2014; Berg and Smalla 2009; Singh et al. 2022; Yadav et al. 2023). In the present study, eight bacterial isolates viz., Glutamicibacter nictotianae CFLB 18, B. subtilis CFLB 11, B. velezensis CFLB 24, S. rhizophilla CFLB 26, Microbacterium sp. CFLB 28, B. amyloliquefaciens CFLB 31, and S. maltophilia CFLB 47 showed > 80% inhibition of F. udum growth over control on the PDA medium. The resulting finding was corroborative with earlier workers (Kumar et al. 2012; Seo et al., 2012; Mardanova et al. 2016; Lee et al. 2017; Rojas-Solíset al. 2018; Rojas-Reyes-Perez et al., 2019; Savi et al. 2019; Jin et al. 2020; Fu et al. 2021; Ham et al. 2022). They reported that Bacillus spp. has been demonstrated to possess strong antagonistic activity against numerous fungal phytopathogens, defending host plants from expanding pathogens and promoting plant growth. In this study, three Bacillus species (B. subtilis, B. velezensis and B. amyloliquefaciens) had potential to use as a biocontrol agent against F. udum.
In dual culture method to study where fungus F. udum was co-inoculated with antagonistic bacteria such as B. amyloliquefaciens, Glutamicibacter nictotianae and B. subtilis separately (Fig. 2). With the help of SEM, deformation, swelling, vacuolization, thickening and lysis of the hypha was observed in the hyphae of the F. udum and slow down the development of conidiophores of the fungus. Similarly, Chaurasia et al., (2005) reported that F. oxysporum and Pythium fertile hyphae exhibited vacuolization, granulation, and lysis. The conidia also showed thicker walls and swelling. Like our study, Bacillus sp. produced hydrolytic enzymes in response to F. udum (Dukare et al., 2021). The strain of Bacillus sp. can protect plants from root illnesses brought on by phytopathogenic fungi by producing the enzymes (chitinase, pectinase, cellulase, protease and lipase) that break down the fungal cell wall. This hampers pathogen development and activity. It has been shown that hydrolytic enzymes can control plant diseases. Cell lysis from fungal infections can occur when a hydrolytic enzyme, such as pectinase, protease, cellulase, amylase, chitinase, or glucanase, dissolves the fungal cell wall. Several cellular deformities of lyses, protoplasmic damages, mycelia deformations, modifications in cell membrane permeability, and outflow of cytoplasmic fluid have been brought on by the enzymatic collapse of pathogen hyphae in mycoparasitism (Di Francesco et al. 2016). As a result, protoplasmic loss from such enzymatic fungal wall disintegration finally results in fungal death. According to Chang et al. (2007), the biocontrol bacterial lytic enzymes may have inhibited conidial germination or lysed the fungal cell wall, decreasing pathogen mycelial biomass. By measuring a decrease in biomasses of pathogenic fungi, biocontrol bacteria’s antagonistic activities can be determined (Swain et al. 2007; Boamah et al. 2021), which was corroborative to or findings as B. amyloliquefaciens, B. subtilis G. nicotiana drastically reduced the biomass of F. udum mycelium dry weight in broth culture.
The bacteria have different mechanisms to suppress the growth of fungal pathogens by producing antimicrobial metabolites (Glare et al. 2012), including antibiotics, siderophores, hydrolytic enzymes, etc. (Leclère et al., 2005a,b; Amaria et al. 2023; Douriet-Gámez et al. 2018; Mishra et al. 2020). The synthesis of hydrolytic enzymes is a vital biocontrol agent activity that efficiently competes for available resources by breaking down the pathogen’s complex polymer structures to obtain carbon as an energy source for bacterial growth and reproduction (Kumari et al. 2022). In this study, we found a significant variation in the production of different hydrolytic enzymes, such as protease, amylase, pectinase, cellulase, and lipase, in different growth media (Table 2). Eight bacterial isolates were tested for their hydrolytic enzyme production ability and among them B. subtilis CFLB 11, B. amyloliquefaciens CFLB, 31 and S. maltophilia CFLB 47 produced amylase, pectinase, lipase, protease and cellulase enzymes to form a halo zone in medium while S. rhizophilla CFLB 26, B. velezensis CFLB 24, Microbacterium sp. CFLB 28 and G. nictotianae CFLB 18 did not produce lipase enzymes. The results of this study concur with the results reported by Kumar et al. (2012), Sarhan and Shehata (2014), and Choudhary and Sindhu (2015) that biocontrol agents’ lytic enzymes kill or restrict the growth of infections by hydrolyzing polymeric substances such as chitin, proteins, cellulose, and hemicelluloses. That interferes with the development and activity of fungal pathogens. A hydrolytic enzyme, such as cellulase, chitinase, glucanase, or protease, can break down the fungal cell wall and result in the cell lysis of fungal infections. Additionally, Abdel Monaim (2016) discovered that the strains of B. subtilis, B. amyloliquefaciens, and B. velezensis could create extracellular compounds, volatile antibiotics, siderophores, hydrogen cyanide (HCN), and indole acetic acid (IAA) in vitro conditions. Reddy et al. (2022) suggest that the lytic enzymes of biocontrol agents kill or inhibit the spread of diseases by hydrolyzing polymer compounds such as chitin, proteins, cellulose, and hemicelluloses.
In the present study, two hydrolytic enzyme-related genes, chitinase and 1, 3 ß-glucanase, were characterized in eight isolates of antagonistic bacteria (B. amyloliquefaciens, Microbacterium sp., B. velezensi, G. nicotianae, S. rhizophila, S. maltophilia, B. subtilis, and Pseudoarthrobacter sp.) by using specific primers amplified at 402 and 750 bp. It is anticipated that this chitinase and 1, 3 ß-glucanase-related genes are responsible for producing these enzymes, which inhibit the proliferation of phytopathogens (Lee et al. 2012). Similarly, Downing et al. (2000) reported that two complete genes encoding chitinases are present in B25, and these genes may be crucial in inhibiting fungal growth. Chitin and fungal lysates increase the transcript levels of B25 chitinase genes (Figueroa-Lopez et al. 2017), suggesting that these genes may play a role in controlling plant fungal infection. 1, 3 ß-glucanase hydrolyzes ß-glucan polysaccharides into glucose monomers, and chitinase hydrolyzes the ß-1,4 N acetylglucosamine bond into GlcNAc monomers, it is anticipated that these activities of bacterial biocontrol agents hindered the synthesis of R. microporus or cause damage to its mycelial wall (Nicole and Benhamou 1991; Mishra et al. 2020). Chen et al. (2004) demonstrated the importance of chitinase in antifungal activity and discovered the increased antifungal activity of the culture supernatant against Botrytis elliptica. Chitin, an insoluble linear β-1, 4-linked polymer of N-acetylglucosamine (GlcNAc) that is one of the main components of the fungal cell wall, is hydrolytically degraded by chitinases, glycosyl hydrolases (Adams 2004; Geoghegan et al. 2017; Gao et al. 2019). Chitin, an insoluble linear β-1,3-linked polymer of N-acetylglucosamine (GlcNAc) that makes up an essential part of the fungal cell wall, is hydrolyzed by chitinases, glycosyl hydrolases, which have an endolytic mode of action (Gao 2016; Goa et al., 2019;). Another enzyme that breaks down cell walls is 1, 3-glucanase, which has an endocytic mode of action and catalyzes the hydrolysis of glucans, another critical component of the fungal cell wall (Solanki et al. 2012).
Conclusions
Eight bacterial isolates were selected with potential antagonistic activity with various hydrolytic enzymes and hyphae malformation. These eight isolates were reported as compatible and could be consortium in different combinations of isolates. Among them, 8 isolates B. amyloliquefaciens (CFLB 31), B. velezensis (CFLB24), B. subtilis (CFLB11), S. rhizophila (CFLB 26), S. maltophilia (CFLB 47), Microbacterium sp. (CFLB 28), and G. nicotianae (CFLB 18). In our study, this is the first report and makes an essential contribution by giving valuable information on the bacteria of F. udum, the causal agent in the wilt disease of the pigeon pea plant. In short, the strains’ diverse fungal inhibitory properties allowed them to manage the wilt disease effectively. These bacterial strains also reduce fungal pathogen biomass when co-inoculated with bacterial isolates while considerably decreasing the frequency of wilt disease incidence. These bacterial strains can thus be employed as possible biocontrol agents for sustainably managing the Fusarium wilt disease. They can reduce the overuse of synthetic fungicides used to treat the wilt. Further research will be conducted to identify the efficiency of the potential bacteria biocontrol agent through in vivo analysis.
Abbreviations
- PDA:
-
Potato Dextrose Agar
- PDB:
-
Potato Dextrose Broth
- CMC:
-
Carboxymetyl cellulose agar
- FU:
-
Fusarium udum
- PCR:
-
Polymerase chain Reaction
- GSP:
-
Gene Specific Primer
- HCN:
-
Hydrogen Cyanide
- GlcNAc:
-
N-acetylglucosamine
- IAA:
-
Indole Acetic Acid
References
Aamir M, Rai KK, Dubey MK, Zehra A, Tripathi YN, Divyanshu K, Upadhyay RS (2019) Impact of climate change on soil carbon exchange, ecosystem dynamics, and plant–microbe interactions. Climate change and agricultural ecosystems. Woodhead Publishing, pp 379–413
Aamir M, Samal S, Rai A, Kashyap SP, Singh SK, Ahmed M, Upadhyay RS (2021) Plant microbiome: diversity, distribution, and functional relevance in crop improvement and sustainable agriculture. Microbiome stimulants for crops. Woodhead Publishing, pp 417–436
Abdel-Monaim MF (2016) Efficacy of secondary metabolites and extracellular lytic enzymes of plant growth promoting rhizobacteria (PGPR) in controlling fusarium wilt of chickpea. Egypt J Agricultural Res 94(3):573–589
Adams DJ (2004) Fungal cell wall chitinases and glucanases. Microbiology 150(7):2029–2035
Amaria W, Sinaga MS, Mutaqin KH (2023) Bacterial biocontrol potential against Rigidoporusmicroporus: hydrolytic enzyme activity and antibiotic inhibition. J Saudi Soc Agricultural Sci
Barwant M, Lavhate N (2020) Isolation and maintenance of fungal pathogens aspergillus Niger and aspergillus flavus. Int J Appl Nat Sci 9(3):47–52
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68(1):1–13
Boamah S, Zhang S, Xu B, Li T, Calderón-Urrea A (2021) Trichoderma longibrachiatum (TG1) enhances wheat seedlings tolerance to salt stress and resistance to Fusarium pseudo graminearum. Front Plant Sci 12:741231
Chaiharn M, Chunhaleuchanon S, Kozo A, Lumyong S (2008) Screening of rhizobacteria for their plant growth promoting activities. Curr Appl Sci Technol 8(1):18–23
Chang WT, Chen YC, Jao CL (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Technol 98(6):1224–1230
Chaurasia B, Pandey A, Palni LMS, TrivediP, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiological research, 160(1), 75–81
Chen CY, Wang YH, Huang CJ (2004) Enhancement of the antifungal activity of Bacillus subtilis F29-3 by the chitinase encoded by Bacillus circulans chiA gene. Can J Microbiol 50(6):451–454
Choudhary SR, Sindhu SS (2015) Suppression of Rhizoctonia solani root rot disease of cluster bean (Cyamopsis tetragonoloba) and plant growth promotion by rhizosphere bacteria. Plant Pathol J (Faisalabad) 14(2):48–57
Collins CH, Lyne PM (1970) Microbiological methods. Microbiological Methods, (3rd. Edition)
Coutinho BG, Licastro D, Mendonça-Previato L, Cámara M, Venturi V (2015) Plant-influenced gene expression in the rice endophyte Burkholderiakururiensis M130. Mol Plant Microbe Interact 28(1):10–21
Di Francesco A, Martini C, Mari M (2016) Biological control of postharvest diseases by microbial antagonists: how many mechanisms of action? Eur J Plant Pathol 145:711–717
Douriet-Gámez NR, Maldonado-Mendoza IE, Ibarra-Laclette E, Blom J, Calderón-Vázquez CL (2018) Genomic analysis of Bacillus sp. strain B25, a biocontrol agent of maize pathogen Fusarium verticillioides. Curr Microbiol 75:247–255
Downing KJ, Leslie G, Thomson JA (2000) Biocontrol of the sugarcane borer Eldana saccharina by expression of the Bacillus thuringiensis cry1Ac7 and Serratia marcescens chiA genes in sugarcane-associated bacteria. Appl Environ Microbiol 66(7):2804–2810
Dukare A, Paul S (2021) Biological control of Fusarium wilt and growth promotion in pigeon pea (Cajanus cajan) by antagonistic rhizobacteria, displaying multiple modes of pathogen inhibition. Rhizosphere 17:100278
Dukare A, Paul S, Arambam A (2020) Isolation and efficacy of native chitinolytic rhizobacteria for biocontrol activities against Fusarium wilt and plant growth promotion in pigeon pea (Cajanus cajan L). Egypt J Biol Pest Control 30(1):1–12
Figueroa-Lopez AM, Leyva-Madrigal KY, Cervantes-Gamez RG, Beltran-Arredondo LI, Douriet-Gamez NR, Castro-Martinez C, Maldonado-Mendoza IE (2017) Induction of Bacillus cereus chitinases as a response to lysates of Fusarium verticillioides. Rom Biotechnol Lett, 22(4)
Fu B, Olawole O, Beattie GA (2021) Biological control and microbial ecology draft genome sequence data of glutamicibacter sp. FBE-19, a bacterium antagonistic to the plant pathogen Erwinia tracheiphila. Phytopathology® 111(4):765–768
Gajbhiye A, Rai AR, Meshram SU, Dongre AB (2010) Isolation, evaluation and characterization of Bacillus subtilis from cotton rhizospheric soil with biocontrol activity against Fusarium oxysporum. World J Microbiol Biotechnol 26:1187–1194
Gao L (2016) An improved method to optimize the culture conditions for biomass and sporulation of mycoparasitic fungus Trichoderma viride TV-1. J Yeast Fungal Res 7(1):1–6
Gao F, Zhang BS, Zhao JH, Huang JF, Jia PS, Wang S, Guo HS (2019) Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat Plants 5(11):1167–1176
Geoghegan I, Steinberg G, Gurr S (2017) The role of the fungal cell wall in the infection of plants. Trends Microbiol 25(12):957–967
Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J, Stewart A (2012) Have biopesticides come of age? Trends Biotechnol 30(5):250–258
Ham SH, Yoon AR, Oh HE, Park YG (2022) Plant growth-promoting microorganism pseudarthrobacter sp. NIBRBAC000502770 enhances the growth and flavonoid content of Geumaleppicum. Microorganisms 10(6):1241
Hankin L, Anagnostakis SL (1977) Solid media containing carboxy methyl cellulose to detect cx cellulase activity of micro-organisms. Microbiology 98(1):109–115
Hashem EA, Abdalla HE, Hussein YA, Abd-Elnabi MA (2008) In vitro selection of soybean callus resistant to Fusarium oxysporum metabolites. In Proceedings Third Environment Conference, Faculty of Sci., ZagazigUniv (pp. 1–19)
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Jin P, Wang H, Tan Z, Xuan Z, Dahar GY, Li QX, Liu W (2020) Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic Biochem Physiol 163:102–107
Kang Z, Buchenauer H (2000) Cytology and ultrastructure of the infection of wheat spikes by Fusarium Culmorum. Mycol Res 104(9):1083–1093
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167(8):493–499
Kumar V, Anal AKD, Nath V (2018) Biocontrol fitness of an indigenous Trichoderma viride, isolate NRCL T-01 against Fusarium solani and Alternaria alternata causing diseases in Litchi (Litchi chinensis)
Kumari S, Khanna V, Singh A (2022) Characterization and evaluation of extracellular hydrolytic proteins from rhizobacterial antagonists isolated from Fusarium oxysporum f. sp. ciceris infected chickpea fields. Indian Phytopathol 75(1):165–177
Leclère V, Béchet M, Adam A, Guez JS, Wathelet B, Ongena M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Applied and Environmental Microbiology, 71(8), 4577–4584
Leclère V, Béchet M, Adam A, Guez JS, Wathelet B, Ongena M, Jacques P (2005b) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71(8):4577–4584
Lee KJ, Oh BT, Seralathan KK (2012) Advances in plant growth promoting rhizobacteria for biological control of plant diseases. Bacteria in agrobiology: disease management. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–13
Lee T, Park D, Kim K, Lim SM, Yu NH, Kim S, Kim JC (2017) Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol J 33(5):499
Mardanova AM, Hadieva GF, Lutfullin MT, Khilyas IVE, Minnullina LF, Gilyazeva AG, Sharipova MR (2016) Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agricultural Sci 8(1):1–20
Melent’ev AI, Helisto P, Kuz’mina LY, Galimzyanova NF, Aktuganov GE, Korpela T (2006) Use of antagonistic bacilli for biocontrol of fungi degrading fresh wood. Appl Biochem Microbiol 42:62–66
Melkamu T, Diriba M, Gezahegn B, Girma A (2013) Antagonistic effects of rhizobacteria against Coffee Wilt Disease caused by Gibberellaxylarioides. Asian J Plant Pathol 7(3):109–122
Mishra P, Mishra J, Dwivedi SK, Arora NK (2020) Microbial enzymes in biocontrol of phytopathogens. Microb Enzymes: Roles Appl Industries, 259–285
Morrissey JP, Dow JM, Mark GL, O’Gara F (2004) Are microbes at the root of a solution to world food production? Rational exploitation of interactions between microbes and plants can help to transform agriculture. EMBO Rep 5(10):922–926
Nagar GB (2016) Molecular characterization of chitinase and β-1, 3-glucanase gene of soybean plant growth promoting bacterium Bacillus sp. SJ-5
Nicole MR, Benhamou N (1991) Cytochemical aspects of cellulose breakdown during the infection process of rubber tree roots by Rigidoporus Lignosus. Phytopathology 81(11):1412–1420
Oldenburg KR, Vo KT, Ruhland B, Schatz PJ, Yuan Z (1996) A dual culture assay for detection of antimicrobial activity. J BioMol Screen 1(3):123–130
Pandya M, Rajput M, Rajkumar S (2015) Exploring plant growth promoting potential of non rhizobial root nodules endophytes of Vigna radiata. Microbiology 84:80–89
Rai S, Solanki MK, Solanki AC, Samal S (2023) Microbial endophytes as probiotics for the plant health: an overview. Microb Endophytes Plant Growth, 269–281
Reddy EC, Reddy GS, Goudar V, Sriramula A, Swarnalatha GV, Tawaha A, A. R. M., Sayyed RZ (2022) Hydrolytic enzyme producing plant growth-promoting rhizobacteria (PGPR) in plant growth promotion and biocontrol. Secondary metabolites and volatiles of PGPR in plant-growth promotion. Springer International Publishing, Cham, pp 303–312
Reyes-Perez JJ, Hernandez-Montiel LG, Vero S, Noa-Carrazana JC, Quiñones-Aguilar EE, Rincón-Enríquez G (2019) Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. J Food Sci Technol 56:4992–4999
Rojas-Solís D, Zetter-Salmón E, Contreras-Pérez M, del Carmen Rocha-Granados M, Macías-Rodríguez L, Santoyo G (2018) Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal Agric Biotechnol 13:46–52
Saini R, Dudeja SS, Giri R, Kumar V (2015) Isolation, characterization, and evaluation of bacterial root and nodule endophytes from chickpea cultivated in Northern India. J Basic Microbiol 55(1):74–81
Samal, S (2022). Role of plant transcription factors in abiotic stress tolerance. Acta Sci Agric 6.4:58–69.
Samal S, Rai S, Upadhaya RS (2023) Endophytic fusarium and their association with plant growth. Microbial endophytes and Plant Growth. Academic, pp 259–268
Sangwan S, Dukare A (2018) Microbe-mediated bioremediation: an eco-friendly sustainable approach for environmental clean-up. Adv Soil Microbiology: Recent Trends Future Prospects: 1: Soil-Microbe Interact, 145–163
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169(1):18–29
Sarhan EA, Shehata HS (2014) Potential plant growth-promoting activity of Pseudomonas spp. and Bacillus spp. as biocontrol agents against damping-off in alfalfa. Plant Pathol J (Faisalabad) 13(1):8–17
Savi DC, Shaaban KA, Gos FM, Thorson JS, Glienke C, Rohr J (2019) Secondary metabolites produced by Microbacterium sp. LGMB471 with antifungal activity against the phytopathogen phyllostictacitricarpa. Folia Microbiol 64:453–460
Schaad NW, Jones JB, Chun W (2001) Laboratory guide for the identification of plant pathogenic bacteria (no. Ed. 3). American phytopathological society. APS
Seo S, Matthews KR (2012) Influence of the plant defense response to Escherichia coli O157: H7 cell surface structures on survival of that enteric pathogen on plant surfaces. Appl Environ Microbiol 78(16):5882–5889
Singh D, Devappa V, Yadav DK (2022) Suppression of tomato bacterial wilt incited by Ralstonia pseudosolanacearum using polyketide antibiotic-producing Bacillus spp. isolated from rhizospheric soil. Agriculture, 12(12), 2009
Solanki MK, Robert AS, Singh RK, Kumar S, Pandey AK, Srivastava AK, Arora DK (2012) Characterization of mycolytic enzymes of Bacillus strains and their bio-protection role against Rhizoctonia solani in tomato. Curr Microbiol 65:330–336
Susilowati DN, Rahayuningsih S, Sofiana I, Radiastuti N (2021) The potential of nutmeg’s microbes (Myristica fragrans Houtt.) As antagonistic agents against Rigidoporusmicroporus. Jurnal Lahan Suboptimal: J Suboptimal Lands 10(1):1–13
Swain MR, Naskar SK, Ray RC (2007) Indole-3-acetic acid production and effect on sprouting of yam (Dioscorearotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol J Microbiol 56(2):103
van Soolingen D, De Haas PE, Hermans PW, Van Embden JD (1994) DNA fingerprinting of mycobacterium tuberculosis. Methods Enzymol 235:196–205
Yadav DK, Devappa V, Kashyap AS, Kumar N, Rana VS, Sunita K, Singh D (2023) Boosting the biocontrol efficacy of Bacillus amyloliquefaciens DSBA-11 through physical and chemical mutagens to control bacterial wilt disease of tomato caused by Ralstonia solanacearum. Microorganisms 11(7):1790
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samal, S., Singh, D., Upadhyay, R.S. et al. Hydrolytic genes of antagonistic rhizobacteria strains on Fusarium udum causing wilt disease in pigeonpea. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01641-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01641-z