Abstract
We investigated the anti-phytopathogen potential of silver nanoparticles synthesized by the bioreduction of AgNO3 in Bacillus subtilis culture supernatant against Cercospora canescens, causing leaf spot disease in Vigna radiata (mungbean). The biosynthesized AgNPs were characterized using UV–visible spectroscopy that showed a broad Surface Plasmon resonance (SPR) peak at 410 nm, dynamic light scattering (DLS) measurement for size distribution and intensity, polydispersity index, and zeta potential for determining their stability. Atomic Force Microscopy determined that they were spherical with an average particle size of 8 nm. In vitro study and tripartite assay under greenhouse conditions were carried out to evaluate the nanoparticles application as a management strategy to control and/ protection from the Cercospora leaf spot disease in mungbean. The inhibitory effect of eight AgNPs concentrations was investigated for in vitro antifungal potential assessment; biosynthesized AgNPs were found to decrease the fungal growth and increase the mycelial inhibition. Their antagonistic effect was also recorded on conidial germination. The conidia under AgNPs exposure showed germination repression. The maximum mycelial inhibition (94.00 ± 0.5) was observed for 800 ppm at 96 h. The greenhouse experimentations revealed the antifungal efficiency of AgNPs against Cercospora Leaf spot disease in mungbean. The 800 ppm AgNPs concentration gave a statistically significant result (46.87 ± 3.74) and proved best in In planta experiment with maximum disease reduction. Cercospora leaf spot-induced biochemical alterations were recorded to be reversed towards normal levels after biosynthesized AgNPs application on challenged mungbean plants. Our data unravelled the potential of biosynthesized AgNPs against Cercospora canescens challenge in mungbean, pointing towards its application in plant disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal phytopathogens constitute a significant cause of various lethal diseases of economically important crops (Agrios 2009). Over the period, phytopathogens have evolved themselves, developed numerous ways of attacking plants, and overcoming plant defence mechanisms (Zvereva and Pooggin 2012). This attribute of pathogens caused devastating effects on crop physiology, homeostasis, and production that ultimately resulted in systemic damage (Agrios 2005). World widely, approximately 19,000 fungi are identified with pathogenic effects in crops. These pathogens can survive on living and dead plants, and they can endure extreme environmental conditions by becoming dormant. About 80% of plant diseases are caused by fungi (El Hussein et al. 2014). The fungal pathogens are the source of significant threats for human health due to altered crop quality, decreased crop production, and per annum yield, causing substantial economic losses for farmers (Ellis et al. 2008; Singh et al. 2012). The economic losses and crop damage can be lessened through various crop management and disease control strategies.
Different agrochemicals products are being developed and used by farmers on a large scale. Most of these chemicals have toxic effects on biologically important insects and microbes and human beings. Due to their longer active period in soil, these agrochemicals leach down into the water table, making it toxic for crops and human use. Instead of chemically manufactured pesticides, nanoparticles, especially silver ones, as antimicrobial agents are more technological and economical. It is used in medicinal treatments as an antimicrobial-agents against various plant pathogens was not realized till the nineteenth century (Jo et al. 2009). Silver exhibits various inhibitory actions against microorganisms. It is investigated that silver nanoparticles are most active against fungi, bacteria, and other microorganisms at lower concentrations and are non-toxic to humans without having any side effects (Savithramma et al. 2011). Therefore, its antimicrobial characteristics have been studied and implemented more often than any other inorganic antimicrobial agent (Kim et al. 2012). Especially silver ions are very reactive like they cause resistance in microbial respiration and metabolism, resulting in cell damage. Modification in cell membrane structure and functions is also associated with it (Pal et al. 2007). Hence, it may be used with a certain level of safety to limit the growth of fungal pathogens contrary to synthetic fungicides (Park et al. 2006).
The effectiveness of silver particles as antimicrobial agents has been amplified many folds with the help of Nanotechnology. Reduction in the particle size of a material is an efficient and reliable tool for improving its biocompatibility (Aggarwal et al. 2009; Adabi et al. 2017). Nanotechnology has helped to overcome size limitations and changed its outlook as a genetic tool. Nanoparticles having extensive surface areas increase their contact with fungi and bacteria, thus improving their fungicidal and bacterial efficiency. A more extensive surface area to volume ratio of silver nanoparticles (AgNPs) causes enhancement in their contact with microorganisms and efficiency in proliferating into their cells (Oluwatoyin et al. 2020). Resultantly, when in contact with the fungus, bacteria, and other microbes, they take over cellular metabolism and restrict their growth. AgNPs disintegrate the fungal cell wall and damage cellular proteins and DNA by breaking sulphur and phosphorus groups, respectively, leading to microbial cell death (Morones et al. 2005). Silver nanoparticles reduce respiration, electron transfer system metabolism, and transfer of substrates in the cell membrane of that microbe (Du et al. 2012). Diverse plant disease management systems are used to control the damage caused by plant pathogens like fungi, bacteria, and other microorganisms. However, the broad spectrum of the host of these microbes makes it challenging to control the damage as they have developed resistance against these chemical controls like pesticides which also cause environmental degradation. The development of nanotechnology with silver nanoparticles (AgNPs) being the very reactive ones with its decisive antimicrobial actions mentioned above have provided a very safe and economical solution in this matter (Kim et al. 2012). Hence, in the present study, silver nanoparticles (AgNPs) were biosynthesized using B subtilis, and its antifungal potential was investigated against Cercospora canescens, a fungal pathogen that causes Cercospora leaf spot disease in Vigna radiata.
Material and method
Culturing of Bacillus subtilis strain
Bacillus subtilis is a typical Gram-positive bacterium that appears rod-shaped and gives fuzzy white to a yellowish, rough appearance on growth media. The B. subtilis strain Q3 was cultured overnight on liquid Luria–Bertani (LB) medium at 37 °C. The optical density of culture was measured at 600 nm. The culture supernatant was taken by centrifuging it at 5000 rpm for 10 min.
Biosynthesis of silver nanoparticles (AgNPs)
For optimizing the biosynthesis of silver nanoparticles (AgNPs), varying levels of silver nitrate (0.5 mM, 1 mM, 3 mM, 5 mM) were tested. Each AgNO3 solution was added to 20 ml supernatant of Bacillus subtilis culture, and pH was adjusted to 7.0 by adding hydrochloric acid (HCl) and sodium hydroxide (NaOH) solutions. The samples were incubated at 37 °C with shaking at 180 rpm under dark conditions. Synthesis of AgNPs was observed after 24, 36, 48, and 72 h by visualizing the colour change from yellow to brown or dark brown compared with LB medium and culture supernatant without AgNO3 (controls). The colour change was due to the bioreduction of silver ions of AgNO3. The samples were centrifuged at 13200 rpm for 30 min, and the supernatant was discarded, followed by pellet washing with 1 ml PBS (Phosphate buffered saline), dissolved the pellets in 1 ml PBS, and stored at -20 °C as described by Pourali and Yahyaei (2016).
Characterization of biosynthesized AgNPs
UV-spectral analysis
The UV–vis absorption spectra of all samples were measured in UV–vis BioSpectrometer (Eppendorf). All samples were diluted ten times with deionized water. The bacterial culture supernatant was used as blank.
Dynamic light scattering measurement and atomic force microscopy
The biosynthesized nanoparticles (NPs) were characterized for particles size distribution, zeta potential, and polydispersity index by Dynamic Light Scattering (DLS) measurement with ZetaSizer Nano S90 (Malvern Instruments Ltd., UK). The stability of AgNPs was determined through zeta potential. Moreover, the shape and size of lyophilized biosynthesized silver nanoparticles were observed by Atomic Force Microscopy (SHIMADZU WET-SPM 9600, JAPAN).
Fourier transform infrared spectroscopy analysis
For Fourier Transform Infrared Spectroscopy (FTIR) analysis, the AgNPs suspensions were lyophilized to make dried powder form. The biosynthesized nanoparticles were analyzed in a frequency range of 550-4000 cm−1. This composition analysis was performed to determine functional groups and molecules involved in the biosynthesis and stabilization of NPs.
Characterization of Cercospora canescens
Cercospora canescens is a fungal pathogen of the mungbean responsible for Cercospora leaf spot disease. Infected leaves of mungbean with Cercospora leaf spot disease symptoms (Greyish to brown lesions with reddish-brown margins) were collected from various mungbean growing areas of Punjab, Pakistan. For characterization, the pathogen isolation was mas from the infected tissues (leaves). The infected leaves were surface sterilized with 0.1% sodium hypochlorite solution, washed with distilled water, and then dried on blotter paper. The leaves were cut into small pieces and cultured on a Potato dextrose agar (PDA) medium. Morphological identification was made from single hyphal tips cut out under the stereoscope and transferring to fresh culture plates. The plates were then incubated for 14 days at 28 °C. For molecular characterization, fungal DNA was isolated (Plattner et al. 2009), and DNA barcoding was performed based on Internal Transcribed Spacer (ITS) region, translation elongation factor 1-alpha (TEF1-α), actin (ACT), and calmodulin (CAL) genic regions in a polymerase chain reaction (PCR). The extracted DNA of C. canescens was quantified (NanoDrop 8000 Thermo Scientific, USA). PCR analysis was performed using primers, ITS1/ITS4 (White et al. 1990), EF1-728F/EF1-986R, ACT-512F/ACT-783R, CAL-228F/CAL737R (Carbone and Kohn 1999). PCR amplified products were eluted from the gel using FavorPrep gel purification kit (FAVORGEN, BIOTECH CORP., Taiwan) and direct sequenced by Eurofins DNA sequencing services, USA. Generated sequences were trimmed using BioEdit software version 7.2. These high-quality trimmed sequences were then subjected to BLASTn (Basic Local Alignment Search Tool) for homology search.
AgNPs-fungus cultivation assay for determining the inhibitory dose of AgNPs
The in vitro assay was performed to analyze the different concentrations of biosynthesized AgNPs against Cercospora canescens to find the best inhibitory doses for in planta assay. Serial dilution of AgNPs suspensions was made at different concentrations (10 ppm, 25 ppm, 40 ppm, 50 ppm, 100 ppm, 200 ppm, 400 ppm and 800 ppm),and the inhibitory effect against Cercospora canescens was recorded after 24, 48, 72 and 96 h. For experimentation, 1 ml of each serial dilution of AgNPs was spread evenly on sterilized Petri plates containing 25 ml PDA media supplemented with an antibiotic (Enrofloxacin). A circular bit (10 mm) of 8 days old Cercospora canescens culture was cut with a sterilized cork-borer and placed at the centre of each Petri plate. The plates were incubated at 28 ± 2 °C, and mycelial growth inhibition was calculated using the formula for the mycelial growth inhibition percentage described by Bekker et al. (2006). The experiments were repeated thrice. Petri plate containing fungal growth, devoid of any AgNPs concentration, was taken as control.
In planta assay for assessing the antifungal potential of biosynthesized AgNPs
The antifungal potential of biosynthesized silver nanoparticles (AgNPs) was investigated against Cercospora leaf spot (CLS) disease caused by Cercospora canescens using Vigna radiata (mungbean) as a host plant. For experimenting, earthen pots were filled with a mixture of soil and compost in a 1:1 ratio. About 5–6 seeds of mungbean genotype, NCM251-4, were sown in each pot, and an adequate amount of moisture was maintained by spraying water twice a day for proper plants growth. The 40% dilutions of AgNPs concentrations with more than 40% fungal inhibition in AgNPs-fungus cultivation assay were used for in vivo antifungal assessment. Conidial suspension of 5 × 105 concentration per ml was made with the aid of a hemocytometer. The pot experiment was conducted in a growth chamber under 26 ± 1 temperature and 95% relative humidity (RH). The experiment layout was made under a completely randomized design (CRD) and repeated thrice. Each experimental repeat comprised five sets (7-days post-inoculation, 14-days post-inoculation, 21-days post-inoculation, 28-days post-inoculation, and 35-days post-inoculation) each set was of 40 plants. Each set was assessed for AgNPs treatments, a fungicide (Score, active ingredient Difenoconazole) treatment as a positive control, and without treatment (negative control). Inoculation of Cercospora canescens was done on one-month-old mungbean plants through the foliar spray method. The efficiency of AgNPs was assessed by applying AgNPs suspensions on plant leaves compared to the fungicidal application, and percentage reduction was recorded. Disease severity was assessed using the scale given by Altas et al. (2018), and %age disease severity was calculated according to the Townsend-Heuberger formula. Disease severity was taken as disease reduction or inhibition the other way round.
Further experimental trials were carried out in a greenhouse with the best AgNPs concentration achieved in the above-mentioned experiment to develop management strategies. Three experiments were trialled (i) AgNPs treatment post pathogen challenge, (ii) AgNPs treatment pre pathogen challenge, and (iii) simultaneous treatment of AgNPs with pathogen challenge. Each experiment was carried out with three replicates, and each replicate was of 20 plants and pathogen-challenged plants as control. After seven days of inoculation, as symptoms appeared, the disease severity was analyzed on ten randomly selected leaves by the percentage of reddish-grey leaf spots covering the leaves of inoculated plants.
Statistical analysis
The in vitro assay data for determining the inhibitory dose of AgNPs against C. canescens was recorded in terms of mycelial growth inhibition percentage. The experiment was repeated three times under Completely Randomized Design (CRD), and statistical analysis was carried out with one way repeated measure analysis of variance (ANOVA). The in planta assay was layout under two-factor factorial CRD and repeated thrice. Means were compared using Tukey's HSD (honestly significant difference) test with Bonferroni adjustment to remove Type I error, at a conservative level of significance α/s (α = 0.05; s = number of tests or number of experiments in an experimental set). The statistical analysis was conducted using R Language Software version 4.1.
The antagonistic potential of biosynthesized AgNPs on conidial germination
The antagonistic potential of biosynthesized AgNPs was examined against Cercospora canescens. We performed the cavity slide method as described by Mishra et al. (2014). The conidial suspension (5 × 105 concentration per ml) was mixed with AgNPs concentrations that showed statistically significant percent diseases reduction than fungicide in the greenhouse experiment. We filled the conidial suspension-AgNPs mixtures in a cavity slide. In the fungicide treated conidial suspension set, fungicide was mixed with conidial suspension and filled in cavity slide. We kept the control set separately by filling the cavity with conidial suspension without AgNPs and fungicide. The experiment was repeated in triplicate. The slides were maintained inside the Petri dishes, having sterilized moist blotting paper, and incubated at 25 ± 2 °C for 24 and 48 h. After incubation, slides were examined under the light microscope (HD1600T, camera fitted of Olympus, DP25 Meji Techno, Japan) to observe the antagonistic effect of AgNPs on conidial germination.
Effect of AgNPs on Cercospora leaf spot-induced biochemical changes in mungbean
For investigating the AgNPs mediated suppression of Cercospora leaf spot-induced biochemical changes in mungbean plant, we had taken the leaves of mungbean plants, (i) AgNPs treated plant-16 days post-inoculation (AgNPs application-I at 8 days post-inoculation), (ii) Control plant (16 days post-inoculation), (iii) and non-inoculated plant. Total phenols, polyphenol oxidase (PPO), and peroxidase (POD) of each sample were determined by following the protocols described by Hameed et al. (2017). Data were analyzed using a two-way analysis of variance, and means were compared using Tukey's HSD test.
Results
Biosynthesis and characterization of AgNPs
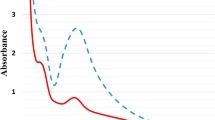
The extracellular synthesis of silver nanoparticles (AgNPs) was confirmed by the clear transition in yellow to dark brown sample color. The color transition of every concentration was different due to the variation in the concentration of AgNO3 solution. Plain LB and B. subtilis culture supernatant without AgNO3 were used as controls that retained their original color; however, the treated samples exhibited slight or more color transitions (Fig. 1). A clear light brown to dark brown color was observed for all four culture samples after 24 h of incubation which confirmed the successful biosynthesis of silver nanoparticles (AgNPs). The absorption spectra of all AgNO3 treated bacterial supernatant were recorded after 24, 36, 48, and 72 h incubation. The UV–vis spectral analysis of samples showing bioreduction of AgNO3 after 24 h of incubation revealed the robust and broad absorption peaks at 410 nm wavelengths (Fig. 2).
Biosynthesis of silver nanoparticles (AgNPs): Color transition in AgNO3 treated Bacillus subtilis culture supernatant in comparison to Control 1 (LB medium) and Control 2 (supernatant without AgNO3). The color transition, yellow to dark brown, in samples treated with different concentration of AgNO3 solution showing reduction of silver ion (Ag +) into Ag°
The zetasizing of biosynthesized AgNPs through Dynamic Light Scattering (DLS) gave the average zeta value of 100.4 d.nm (Supplementary File 1). The polydispersity index (PdI) of biosynthesized NPs was 0.432, showing the sample with the best operating distribution algorithm (Supplementary Table 1). The zeta potential of biosynthesized AgNPs showed a prominent and sharp peak at -35.5 mV with an average zeta potential value, -32.3 (Supplementary File 2), indicating their long-term stability in solution. Atomic Force Microscopy (AFM) was made for the morphological characterization of biosynthesized silver nanoparticles. The analysis revealed that biosynthesized silver nanoparticles were 8 nm in size and spherical (Fig. 3).
Fourier Transform Infrared (FTIR) analysis was performed to determine the interaction between silver and various bioactive molecules involved in the bioreduction and stability of biosynthesized AgNPs. FTIR spectrum showed the presence of a capping agent (Fig. 4). Furthermore, the image analysis revealed no traces of agglomeration and flocculation.
Fourier Transform Infrared (FTIR) spectrum of biosynthesized silver nanoparticles: FTIR spectrum showed intense absorption peaks at 3224.28 cm−1, 3060.87 cm−1, 2957.36 cm−1, 1554.63 cm−1, 1452.61 cm−1,1397.79 cm−1, 1080.88 cm−1, 922.25 cm−1 and 665.9 cm−1, indicating the presence of capping agent. A strong and broad absorption peak was observed at 3224.28 cm−1, showing stretching of OH, which indicated the presence of alcohol and phenol compounds. The band observed at 3060.87 cm−1 showed the stretching of = C-H attributed to the aliphatic alkene functional group. The absorption peak at 2957.36 cm−1 and 922.25 cm−1 attributed to the strong stretching of the C-H group, which shows the presence of the alkane group; however, the band observed at the 1554.63 cm−1 peak demonstrating N–H bending that depicted the presence of the amide group in the culture supernatant. The absorption peak at 1452.61 cm−1 could be attributed to the presence of the methyl group as it indicates CH3 bending. The band at 1397.79 cm−1 showed a strong NO2 stretching. A strong C–O–C stretching observed at 1080.88 cm−1, indicating the presence of an ether group. The strong C–Cl stretch at 665.9 cm.−1 credited the alkyl halides group
Characterization of Cercospora canescens
Isolated Cercospora canescens characterized on a morphogenomics basis was identified as C. canescens. Pure colonies were creamy on PDA. The conidiophores were simple, light to olivaceous brown, straight or geniculate (89.50 µm × 9.70 µm), scars of conidia bearing prominent and conidia were short, hyaline, straight to sub-straight, obclavate-cylindric, borne solitary measuring 129.34 µm × 5.69 µm. The isolate(s) bear a single conidium at the tip of conidiophores with a prominent scar, as Chand et al. (2012) described. The sequence homology search using BLASTn showed 100% similarity with Cercospora canescens isolate CBS 111,133. The sequences were then lodged to the NCBI database, and their assigned GenBank accession numbers are MT816500 (ITS), MT822289 (CAL), MT831970 (TEF), MT831969 (ACT). The characterized culture was labelled as FMB-Cerco-VR and deposited to Fungal Molecular Biology Laboratory-Culture Collection number FMB 0211.
In vitro assay for assessing the inhibitory dose of AgNPs
Due to the slow growth rate of Cercospora canescens, there was no visible mycelium growth after 24 h of incubation. However, an increase in fungal mycelium was prominently visible after 72 and 96 h of incubation. The control plate (plates without any AgNPs concentration) showed prominent growth of fungal mycelium, while, AgNPs containing plates showed less mycelium growth than the control (Fig. 5). The AgNPs concentrations with > 40% inhibitory effect against the fungal pathogen after 96 h were selected for in planta assay (Supplementary Table 2).
Silver nanoparticles mediated conidial germination inhibition
We examined the possible antagonistic impact of biosynthesized AgNPs on conidial germination of leaf spot causing phytopathogen Cercospora canescens, as this is the chief disease-causing step. Our investigation accentuated the substantial impact of biosynthesized AgNPs on Cercospora canescens. After 24 h of incubation, conidial germination was completely inhibited in AgNPs treated sets and in a fungicide treated set. While 70% conidial germination was observed in the control set. After 48 h of incubation, 100% inhibition in conidial germination was observed in AgNPs treated sets, whereas 30% inhibition in conidial germination was observed fungicide treated set. It demonstrated the efficacy and advantage of biosynthesized AgNPs over fungicide in controlling and managing the disease. However, 100% conidial germination was observed in the control set.
Tripartite interaction assay among Vigna radiata, Cercospora canescens, and biosynthesized silver nanoparticles (AgNPs)
The AgNPs concentrations with more than 40% fungal inhibition in AgNPs-fungus cultivation assay were used for in planta antifungal assay to evaluate their antifungal proficiency. The efficacy of AgNPs was evaluated by applying AgNPs suspensions on plants leaves, compared with fungicidal application and plants without any application were kept as a negative control. The 800 ppm AgNPs suspension showed maximum disease inhibition. The 800 ppm AgNPs concentration was statistically significant and higher than other treatments with 46.87 ± 3.74 percent diseases reduction. The disease inhibition with fungicide and AgNPs suspension of 200 ppm concentration was similar and statistically non-significant by scoring the mean ± SE values 27.00 ± 2.9 and 26.40 ± 1.57 respectively (Fig. 6 and Supplementary Table 3). The disease management scheme by nanoparticles application as nanofungicides experimented in a greenhouse with the best silver nanoparticles concentration (800 ppm) attained in the abovementioned experiment. The nanoparticles application plan was determined by three experiments (i) AgNPs treatment post pathogen challenge, (ii) AgNPs treatment pre pathogen challenge, and (iii) simultaneous treatment of AgNPs with pathogen challenge. In the first experimental trial, post-inoculation AgNPs application was made. AgNPs were applied on plants after the visible appearance of characteristic symptoms (after seven days of fungal inoculation), and at the time of first application of AgNPs, more than 40% disease severity was observed in all plants. After eight days of AgNPs application-I (16 days post-inoculation), disease severity in AgNPs treated plants was reduced to 35%; however, 65% disease severity was observed in control plants (pathogen challenged plants without AgNPs treatment). The second application of AgNPs was performed after 18 days post-inoculation (10 days after AgNPs application-I). After eight days of AgNPs application-II (26 days post-inoculation), the disease was reduced to 10%; however, control plants were 80–90% damaged due to disease (Supplementary Fig. 1).
In the second experimental trial, pre-inoculation AgNPs application was carried out. Silver nanoparticles suspension was sprayed 24 h and 48 h before inoculation. The pre-inoculation AgNPs application was performed on two sets of plants. One set was subjected to AgNPs application 24 h before inoculation, and AgNPs application on the second set was 48 h before inoculation. After eight days post-inoculation, ≤ 5% disease severity was recorded in both sets (Supplementary Fig. 1). While, in control plants (pathogen-challenged plants without pre-inoculation AgNPs application), approximately 40% disease severity was observed. It indicated that silver nanoparticle application on plants before disease appearance might reduce disease occurrence and suppress disease onset. The result directed that AgNPs protect mungbean plants against Cercospora canescens by hindering conidial germination, which alleviated fungal potential and disease occurrence.
In the third experimental trial, foliar spray of conidial suspension of C. canescens (pathogen) and AgNPs suspension was done simultaneously, though control plants were the only pathogen challenged. After seven days of inoculation, AgNPs treated plants remained healthy, showed no traces of disease, while pathogen challenged plants (control; without AgNPs application) showed approximately 40–45% disease severity (Supplementary Fig. 1). These observations indicated that AgNPs protect the plants against fungal pathogens and can overcome the inoculum potential of the pathogen responsible for disease onset.
AgNPs mediated impact on Cercospora leaf spot-induced biochemical alterations in mungbean
Total phenols, polyphenol oxidase (PPO), and peroxidase (POD) were estimated in inoculated mungbean plants after AgNPs applications, control (inoculated mungbean plants without AgNPs application), and non-inoculated plant. Total phenols were recorded to be significantly higher in the non-inoculated plant than control (16 days post-inoculated plant without AgNPs application), and statistically non-significant as compared to AgNPs treated challenged plant (16 days post-inoculated plant with AgNPs application at 8 days post-inoculation). We found POD activity significantly less in the control plant. However, significantly increased activity was recorded in non-inoculated plants and AgNPs treated challenged plants compared to control plants. Non-significant change in POD activity was observed in AgNPs treated challenged plant as compared to the non-inoculated plant. We recorded similar results in the total and polyphenol oxidase (PPO) assay (Supplementary Fig. 2).
Discussion
Silver nanoparticles possess an antimicrobial potential that increased their production at the commercial level. Due to its antimicrobial potential, silver nanoparticles are used in plant disease prevention and management. These nanoparticles display various modes of action for disease inhibition, making them suitable for controlling different phytopathogens in a much safer mode than other chemical fungicides (Park et al. 2006). The mechanism of AgNPs biosynthesis involves nitrate reductase in a mixture (supernatant + AgNO3), which helps in transferring electrons from silver ions resulting AgNPs synthesis. The change in colour during the biosynthesis process is due to the reduction of Ag + into Ag° by using the active biomolecules of supernatant (Sadowski et al. 2008). In the present study, the biosynthesized AgNPs showed an absorbance peak at 410 nm, indicating their smaller size and spherical shape. The peak of the UV–vis spectrum located at 420 nm is reported for metallic NPs with a size range of 2 nm to 100 nm (Sastry et al. 1997). In this study, the biosynthesized AgNPs revealed -35.5 zeta potential, which showed its stability in solution as the high negative zeta potential value depicts long-term stability of AgNPs due to negative-negative electrostatic repulsive force (Mukherjee et al. 2014). The particle size obtained through DLS in our study was100.4 d.nm (zeta average), i.e., the average size of our biosynthesized AgNPs along with the salvation layer. The DLS measurement gives the hydrodynamic diameter of nanoparticles; it does not give the "core" particle size. Hence, the particle size in zeta average (d.nm) is based on core particle size, ions concentration, and types of ions in the medium and surface structures (Singh et al. 2017). Atomic Force Microscopy (AFM) allows 3D imaging of NPs, so it is possible to measure the height of biosynthesized NPs qualitatively. However, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) determine 2D imaging. Therefore, AFM analysis was carried out that demonstrated nanoparticles of 8 nm size.
The present study focused on investigating the antifungal efficacy of biosynthesized silver nanoparticles (AgNPs) on Cercospora leaf spot (CLS) disease that causes 60–100% yield losses during the most favourable climatic conditions; warm temperature along with high humidity. Spore germination is an indispensable episode in protonema establishment, affects adversely under stressed environmental cues (Judelson and Blanco 2005). Hence, we attempted to evaluate the fungicidal potential of biosynthesized AgNPs, and their antagonistic impact on conidia (asexual spores). The result showed that in the presence of biosynthesized AgNPs, conidial germination was inhibited. The result of this study showed congruity with the investigation of Chen et al. (2020). Metal nanoparticles possess antimicrobial effects, which make them suitable to be used against various fungus and bacteria. The silver nanoparticles' in-vitro and in planta assessments revealed that AgNPs effectively inhibit fungal mycelial growth and diseases.
Nanoparticles inactivate the crucial microbial enzymes that lead to ROS production (reactive oxygen species), ultimately fatal for microbial cells (Allahverdiyev et al. 2011). The phenolic compounds of plants play a significant role in enhancing the mechanical strength to restrict the pathogen spread. These are involved in providing defense by synthesizing lignin and suberin that ultimately increase the strength of the plant cell wall. The level of phenolic compounds is higher in healthy plants than in diseased plants (Singh et al. 2014). Peroxidases (POD) provide rapid defense through suberification, lignification, healing of injuries, and regulating cell wall elongation (Sulman et al. 2001; Maksimov et al. 2014). Polyphenol oxidase (PPO) has a significant role in the primary stages of plant defense at which phenols, like chlorogenic acid are released due to damage to the plant membrane. Moreover, PPO helps catalyze phenolic oxidation to free radicals, which ultimately react with biological molecules and create unfavorable conditions for the development and survival of pathogens (Mohamed et al. 2012). Disease reduction in plants is associated with activation of defense responses responsible for thwarting disease infection upon host and pathogen interaction (Jones and Dangl 2006). In addition to physical and chemicals defense-related barriers, plants have evolved multiple defense responses which activate upon pathogen attack and prevent the spread of pathogen infection (Vanitha et al. 2009). We investigated the alteration in the biochemical dynamics of mungbean plants infected by Cercospora leaf spots before and after biosynthesized AgNPs application. In the present study, quantitative estimations of total phenolic compounds, peroxidase (POD), and polyphenol oxidase (PPO) were determined in inoculated mungbean plants after AgNPs application in non-inoculated plants and control plants (under pathogen challenge without AgNPs application). Variation in the levels of these defense-related compounds was observed. We found the level of total phenols, peroxidases, and polyphenol oxidase was significantly low in control plants (challenged plants) compared to non-inoculated plants and challenged plants with AgNPs application. However, the level of these compounds in non-inoculated plants and AgNPs treated challenged plants were statistically non-significant. In our study, Cercospora leaf spot-induced biochemical alterations were recorded to be reversed towards normal levels after biosynthesized AgNPs application on challenged mungbean plants.
Availability of data and material
I declare that the submitted manuscript is our work, which has not been published before.
Code availability
Not applicable.
References
Adabi M, Naghibzadeh M, Mohsen A, Zarrinfard MA, Esnaashari SS, Seifalian AM, Faridi-Majidi R, Aiyelabegan HT, Ghanbari H (2017) Biocompatibility and nanostructured materials: applications in nanomedicine. Artif Cells Nanomed Biotechnol 45:833–842. https://doi.org/10.1080/21691401.2016.1178134
Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE (2009) Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 61:428–437. https://doi.org/10.1016/j.addr.2009.03.009
Agrios GN (2005) Plant pathology, 5th edn. Elsevier Academic Press, Amsterdam
Agrios GN (2009) Plant pathogens and disease: general introduction. Elsevier Inc, University of Florida, Gainesville, FL USA
Allahverdiyev AM, Emrah SA, Malahat B, Miriam R (2011) Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 6:933–940. https://doi.org/10.2217/fmb.11.78
Altas Z, Ozguven MM, Yanar Y (2018) Determination of Sugar Beet Leaf Spot disease level (Cercospora Beticola Sacc.) with image processing technique by using drone. CIACR 5:621–631. https://doi.org/10.32474/CIACR.2018.05.000214
Bekker TF, Kaiser C, Merwe R, Labuschagne N (2006) In-vitro inhibition of mycelial growth of several phytopathogenic fungi by soluble potassium silicate. S Afr J Plant Soil 23:169–172. https://doi.org/10.1080/02571862.2006.10634750
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556. https://doi.org/10.1080/00275514.1999.12061051
Chand R, Singh V, Pal C, Kumar P, Kumar M (2012) First report of a new pathogenic variant of Cercospora canescens on mungbean (Vigna radiata) from India. New Dis Rep 26:2044–588. https://doi.org/10.5197/j.2044-0588.2012.026.006
Chen J, Wu L, Lu M, Lu S, Li Z, Ding W (2020) Comparative study on the fungicidal activity of metallic MgO nanoparticles and macroscale MgO against soilborne fungal phytopathogens. Front Microbiol 11:365. https://doi.org/10.3389/fmicb.2020.00365
Du H, Lo TM, Sitompul J, Chang MW (2012) Systems-level Analysis of Escherichia coli Response to Silver Nanoparticles: the Roles of Anaerobic Respiration in Microbial Resistance. Biochem Biophys Res Commun 424:657–662. https://doi.org/10.1016/j.bbrc.2012.06.134
El Hussein AA, Alhasan RE, Abdelwahab SA, Marmar A (2014) Isolation and identification of Streptomyces rochei strain active against Phytopathogenic Fungi. Microbiol Res J 4:1057–1068. https://doi.org/10.9734/BMRJ/2014/11074
Ellis SD, Boehm MJ, Mitchell TK (2008) Fungal and fungal-like diseases of plants. Fact Sheet Int J Agric Nat Resour, the Ohio State University
Hameed S, Akhtar KP, Hameed A, Gulzar T, Kiran S, Yousaf S, Abbas G, Asghar MJ, Sarwar N (2017) Biochemical changes in the leaves of mungbean (Vigna radiata) plants infected by phytoplasma. Turkish J Biochem 42(6):591–599. https://doi.org/10.1515/tjb-2016-0304
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043. https://doi.org/10.1094/PDIS-93-10-1037
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Judelson HS, Blanco FA (2005) The spores of phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3:47–58. https://doi.org/10.1038/nrmicro1064
Kim SW, Jung WJH, Lamsal K, Kim YS, Min JS, Lee YS (2012) Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40:53–58. https://doi.org/10.5941/MYCO.2012.40.1.053
Maksimov I, Troshina N, Surina O, Cherepanova E (2014) Salicylic acid increases the defense reaction against bunt and smut pathogens in wheat calli. J Plant Interact 9:306–314. https://doi.org/10.1080/17429145.2013.832424
Mishra S, Singh BR, Singh A, Keswani C, Naqvi AH, Singh HB (2014) Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 9:97881. https://doi.org/10.1371/journal.pone.0097881
Mohamed H, EL-Hady AA, Mansour M, El-Rheem, El-Samawaty A, (2012) Association of oxidative stress components with resistance to flax powdery mildew. Trop Plant Pathol 37:386–392. https://doi.org/10.1590/S1982-56762012000600002
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri Juan B, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346. https://doi.org/10.1088/0957-4484/16/10/059
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4:316. https://doi.org/10.7150/thno.7819
Oluwatoyin F, Gabriel O, Olubunmi A, Olanike O (2020) Preparation of bio-nematicidal nanoparticles of Eucalyptus officinalis for the control of CYST nematode (Heterodera sacchari). JAPS: J Anim Plant Sci 30:1172–1177. https://doi.org/10.36899/JAPS.2020.5.0134.
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720. https://doi.org/10.1128/AEM.02218-06
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol 22:295–302. https://doi.org/10.5423/PPJ.2006.22.3.295
Plattner A, Kim JJ, Reid J, Hausner G, Lim YW, Yamaoka Y, Breuil C (2009) Resolving taxonomic and phylogenetic incongruence within species Ceratocystiopsis minuta. Mycologia. 101:878-87. https://doi.org/10.3852/08-132
Pourali P, Yahyaei B (2016) Biological production of silver nanoparticles by soil isolated bacteria and preliminary study of their cytotoxicity and cutaneous wound healing efficiency in rat. J Trace Elem Med Biol 34:22–31. https://doi.org/10.1016/j.jtemb.2015.11.004
Sadowski Z, Maliszewska IH, Grochowalska B, Polowczyk I, Kozlecki T (2008) Synthesis of silver nanoparticles using microorganisms. Mater Sci-Poland 26:419–424
Sastry M, Mayya KS, Bandyopadhyay K (1997) pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf A Physicochem Eng Asp 127:221–228. https://doi.org/10.1016/S0927-7757(97)00087-3
Savithramma N, Rao ML, Rukmini K, Devi PS (2011) Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int J Chemtech Res 3:1394–1402
Singh D, Jackon G, Hunter D, Fullerton R, Lebot V, Taylor M, Iosefa T, Okpul T, Tyson J (2012) Taro leaf blight – a threat to food security. Agriculture 2:182–203. https://doi.org/10.3390/agriculture2030182
Singh HP, Kaur S, Batish DR, Kohli RK (2014) Ferulic acid impairs rhizogenesis and root growth, and alters associated biochemical changes in mung bean (Vigna radiata) hypocotyls. J Plant Interact 9:267–274. https://doi.org/10.1080/17429145.2013.820360
Singh T, Jyoti K, Patnaik A, Singh A, Chauhan R, Chandel SS (2017) Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J Genet Eng Biotechnol 15:31–39. https://doi.org/10.1016/j.jgeb.2017.04.005
Sulman M, Fox G, Osman A, Inkerman A, Williams P, Michalowitz M (2001) Relationship between total peroxidase activity and susceptibility to black point in mature grain of some barley cultivars. Proceeding of the 10th Australian Barley Technical Symposium. Canberra, ACT, Australia
Vanitha SC, Niranjana SR, Umesha S (2009) Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J Phytopathol 157:552–557. https://doi.org/10.1111/j.1439-0434.2008.01526.x
White TJ, Bruns T, Lee SJ, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. 18:315-22.
Zvereva AS, Pooggin MM (2012) Silencing and innate immunity in plant defense against viral and non-viral pathogens. Virus 4:2578–2597. https://doi.org/10.3390/v4112578
Acknowledgements
We are grateful to Higher Education Commission (HEC) for funding to conduct this study under the project "Transcriptomics based understanding of Cercospora leaf spot resistance in Mungbean and disease management through nanotechnology," Project No.7425; Department of Soil Science, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan for providing Bacillus subtilis strain; National Institute for Biotechnology and Genetic Engineering (NIBGE, FSD) for providing the facility of Atomic Force Microscopy (AFM) and National Textile University (NTU) for their ZetaSizer and FTIR analysis services. And Special thanks to Center for Advances Studies (CAS), University of Agriculture Faisalabad, Pakistan, for providing lyophilizer, and UV-vis BioSpectrometer (Eppendorf) facility, and Khalid Pervaiz Akhtar, Principal Scientist, Nuclear Institute for Agriculture & Biology (NIAB) in facilitating us to conduct biochemical assays.
Funding
The Higher Education Commission (HEC) funded this work under "Transcriptomics based understanding of Cercospora leaf spot resistance in Mungbean and disease management through nanotechnology," Project No.7425.
Author information
Authors and Affiliations
Contributions
Siddra Ijaz and Imran Ul Haq conceived, designed, and developed the experiment and supervised the experimentation. Maria Babar experimented. Maria Babar, Siddra Ijaz, and Imran Ul Haq drafted the manuscript. Siddra Ijaz, Imran Ul Haq, and Iqrar Ahmad Khan analyzed and interpreted the data and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors are participating in this research study.
Consent for publication
All authors are giving consent to publish this research article in the Journal of Plant Pathology.
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babar, M., Ijaz, S., Haq, I.U. et al. Morpho-functional characterization, in vitro study, and tripartite interaction assay evaluate the potential of biosynthesized silver nanoparticles to manage Cercospora leaf spot disease in Vigna radiata. J Plant Pathol 104, 1371–1381 (2022). https://doi.org/10.1007/s42161-022-01168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-022-01168-1