Abstract
Black pepper (Piper nigrum L.) is an important spice crop with high economic value. However, its production is severely hampered by the oomycete pathogen, Phytophthora capsici. Integrative disease management strategies have been developed to control the pathogen, but the pathogen is in the phase of evolving its virulence. Absolute resistance against Phytophthora rot was not reported in black pepper germplasm. However, Piper colubrinum, a wild species is reported as resistant. Resistance proteins are involved in continuous surveillance of pathogen entry and activation of plant defense signalling pathways for an effective hypersensitive response to prevent pathogen invasion. In this study, a sequence-based homology approach using the conserved nucleotide-binding site (NBS) of known plant resistance genes was used to isolate Resistance Gene Analogs (RGA) and assess their transcript level during Phytophthora infection. The RGA transcript level was evaluated in resistant wild species (P. colubrinum), two moderately resistant black pepper genotypes (IISR Sakthi and 04-P24-1), and one susceptible genotype (Subhakara). The identified RGAs of black pepper were found to be of non-TIR R gene class with NBS motifs. The expressions of six PnRGAs were assessed employing qRT-PCR at different time points after challenging with highly aggressive Phytophthora isolate. The kinetics of differential expression post-infection with P. capsici indicates the differential timing and magnitude of pathogen recognition in resistant P. colubrinum and moderately resistant black pepper genotypes compared to susceptible genotype. In silico analysis revealed that differentially expressed P. nigrum RGAs function through ADP phosphorylation, which is a key process in pathogen recognition. The identification of P. nigrum RGAs induced by P. capsici should provide valuable information for cloning and characterization of resistance genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black pepper (Piper nigrum L.) is an important spice crop grown for its berries, commercially traded both in fresh and dried form and used as a spice as well as medicine. Being native to the humid, tropical evergreen forests of the Western Ghats of the Indian Peninsula (Ravindran et al. 2000), black pepper are also cultivated in tropical and subtropical regions around the globe. Vietnam, Indonesia, Bulgaria, India, Brazil are the top five black pepper producing nations (FAOSTAT 2017). Among the diseases that threaten black pepper cultivation, the Phytophthora foot rot caused by Phytophthora capsici is the most devastating. The application of chemicals mainly based on contact and systemic fungicide practiced by pepper growers could protect the crop only to some extent but may lead to the emergence of resistant pathogen strains. Moreover in India, Phytophthora disease is monsoon bound, and the mixed cropping system practiced by pepper growers along with coconut or arecanut gives a conducive microclimatic condition for the pathogen spread and possibility of cross- infection. P. capsici can infect all parts of the black pepper through an aerial phase in which foliar infection in leaves, stem, and spike occurs or as soil phase in which root and collar will be infected (Anandaraj and Sarma 1995; Anandaraj 2000).
Complete resistance against P. capsici was not reported among the cultivated black pepper genotypes. However, resistance screening to Phytophthora foot rot disease has resulted in the identification of resistant P. colubrinum (Purseglove et al. 1981), and a few black pepper genotypes viz., IISR Sakthi (Sarma et al. 1994) and O4-P24-1 (Bhai et al. 2010). Defense mechanism in IISR Sakthi was correlated to membrane integrity of the host, accumulation of Phenylalanine ammonia lyase (PAL), and induction of pathogenesis-related proteins (Shamina 1997; Jebakumar et al. 2001). There are also reports on specific biochemical responses that lead to moderate resistance in 04-P24-1, an open-pollinated progeny of black pepper (Vandana et al. 2014). The compatible and incompatible interaction of black pepper and P. colubrinum with P. capsici involves transcriptional activation or repression of a large number of genes. A fraction of these pathogenesis-related genes has been characterized at the molecular level, including expression changes upon infection with P. capsici (Vandana and Bhai 2018; Malik and George 2018). However, recognition factors for pathogen recognition were not elucidated yet.
Resistance genes are involved in pathogen recognition either by direct interaction with the pathogen effectors or with pathogen modified host protein during the invasion and subsequent activation of signaling pathways leading to defense against the pathogen. R proteins were categorized into five classes based on the combination of conserved protein signatures possessed such as serine/threonine protein kinases (PKs), leucine-rich repeats (LRRs), nucleotide-binding sites (NBS), and transmembrane domains (TMs) (Gururani et al. 2012).
The majority of the R gene analogs (RGA) belong to NBS-LRR superfamily and the presence of such structural motifs makes them potential regions for providing disease resistance (Li et al. 2016). Earlier, the technique for identification of resistance gene analogs capitalizes on conserved regions for designing degenerate primers and isolation using the polymerase chain reaction (PCR) (Leister et al. 1996; Kanazin et al. 1996). Genome-wide investigations revealed that RGAs accounts for approximately 0.2–1.3% of genes predicted in plant genomes where they occur in clusters, and however RGA content varies with different plant genotypes (Ameline-Torregrosa et al. 2008; Yang et al. 2008; Bayer et al. 2019). Thus NBS-LRR genes are often polymorphic between individuals of a host population, and the complete set of these genes defines the repertoire for the detection of polymorphic pathogen effectors (Bakker et al. 2006; Zhang et al. 2009; Maekawa et al. 2011). More than 4500 NBS-LRR-type RGAs have been amplified via PCR from a wide range of plant species, and they have been arranged in clusters similar to R genes in plant genomes (Marone et al. 2013).
The main objective of the study was to identify RGAs in black pepper using degenerate primers designed to amplify conserved NBS domain between kinase-1a and GLPL motif and assess the RGA transcript level in response to P. capsici infection. The differentially expressed RGAs in resistant and susceptible genotypes would help develop markers linked to disease resistance in black pepper.
Materials and methods
Identification of RGAs from black pepper
Plant material and genomic DNA isolation
Black pepper genotypes, i) IISR Sakthi, moderately resistant to P. capsici, ii) O4-P24-1, the open-pollinated progeny of IISR Sakthi, moderately resistant to P. capsici, iii) Subhakara, susceptible to P. capsici were used for the isolation of R gene analogs. Rooted plants of 3-4 leaf stage raised in sterile potting mixture and maintained under greenhouse conditions at ICAR- Indian Institute of Spices Research, Kozhikode, Kerala, India were used. Leaves of black pepper were harvested, flash-frozen in liquid nitrogen, and stored at −80 °C until use. Genomic DNA was isolated using the protocol described by Doyle and Doyle (1987) with modifications.
Degenerate primers and PCR amplification
Four pairs of primers varying in degeneracy were used to amplify the conserved region between the P-loop and GLPL domain (Table 1) of plant R genes. PCR amplifications were conducted in a total volume of 25 μl containing 100 ng of template DNA, 1x PCR buffer with 2 mM MgCl2, 250 μM dNTPs, 2.5 U of Taq polymerase (Takara, Japan), 2.5 μM each of forward and reverse primer (Sigma, USA). The thermocycler profile was as follows: initial denaturation of 94 °C for 5 min, followed by denaturation at 94 °C for 30 s, annealing (45-60 °C) for 1 min, and extension of 72 °C for 1 min followed by a final extension at 72 °C for 10 min. The PCR products were separated by 1.5% agarose gel, and the amplicons were gel-purified by using Gen elute gel extraction kit (Sigma, USA). The purified DNA was ligated into pTZ57R/T vector (Insta clone T/A cloning kit, Thermo Scientific, USA) and transformed into Escherichia coli DH5α cells according to the manufacturer’s instructions.
Cloning and sequencing of PCR products
The purified plasmids DNA of twenty recombinant clones from each degenerate primer set were sequenced using ABI prism (Xcelris Labs Ltd., Ahmedabad, India) with universal M13 forward and reverse primers. Contigs were assembled using DNA baser v.3.5.4 (Heracle Biosoft, Romania), and vector sequences were removed using Vecscreen (http://www.ncbi.nlm.nih.gov/tools/vecscreen/), and primers were trimmed using BioEdit software (Hall 1999). Sequence homology was compared by searching NCBI using BLASTX. Open reading frames were identified using the ORF finder module of NCBI (http://www.ncbi.nlm.nih.gov/project/gorf). Out of the 60 random clones sequenced, sequences that do not have sequence similarity with R genes or RGAs and those possess internal stop codons were excluded from further analysis. The selected clones were translated using Emboss transseq (http://www.ebi.ac.uk/Tools/st/emboss_transeq) followed by BLASTP searches and InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan5) to analyze the identity of the sequences. Conserved motifs were predicted using the MEME Suite software package version 5.1.0 (Bailey et al. 2009). Sequence alignment of PnRGA sequences was carried out using Clustal omega (Sievers et al. 2011). Diversity analysis was performed with the deduced amino acid sequences of PnRGAs identified in this study and NB-ARC domain of known TIR and non-TIR R genes of Arabidopsis thaliana, Capsicum annuum, Solanum lycopersicum as well as NB-ARC domain of R gene transcripts of P. nigrum fruit and leaf transcriptome (Hu et al. 2015; Johnson et al. 2012) by using MEGA 6.0 (Tamura et al. 2011) using maximum likelihood method of amino acid substitution model, Jones-Taylor-Thornton (JTT) with 1000 bootstrap replications.
Gene expression studies of selected Piper nigrum RGA transcripts
Plant material and pathogen isolate
Three black pepper genotypes and a wild species known for its differential response to P. capsici infection were used: IISR Sakthi, 04-P24-1, Subhakara, and P. colubrinum. Rooted plants of 3-4 leaf stage raised in sterile potting mixture maintained under greenhouse conditions were used for challenge inoculation. A highly aggressive isolate of P. capsici (05-06) isolated from an infected spike from Peruvannamuzhi, Kozhikode, Kerala, India was used for infection. The genotype ‘Subhakara’ is highly susceptible to P. capsici isolate 05-06 and ‘IISR Sakthi’ and ‘04-P24-1’ are moderately resistant and P. colubrinum exhibits hypersensitive responses.
P. capsici inoculum plugs of 3 mm were excised from a 72-h old culture on carrot agar medium and used to inoculate leaves of P. colubrinum and P. nigrum genotypes. For artificial inoculation, the mycelial plugs were placed on the abaxial surface of the third or fourth leaf from the apex. A moist cotton strip was placed over the inoculum plug and was secured using cellophane tape. Another set of plants mock inoculated only with carrot agar plugs without P. capsici inoculums was used as a control. Leaf samples at different time intervals (0.5, 1, 2, 4, 8, 12, 16, 24, 48 and 72 h post-inoculation (hpi) were used for RGA expression studies. The remaining plants were also observed for the lesion development to confirm the occurrence of a viable infection. The experimental design comprised of three biological replicates (n = 3), each of which contained three technical replicates.
RT- qPCR analysis for differential expression of P. nigrum RGA
Total RNA was isolated from leaves of inoculated and uninoculated P. colubrinum and P. nigrum genotypes using TRIZOL (Invitrogen, USA) according to the manufacturer’s instructions. Total RNA was quantified and was treated with DNase I to remove genomic DNA. For cDNA synthesis, 1 μg of RNA was primed with 18mer oligodT and 200 units of RevertAid reverse transcriptase (Thermo Scientific, USA) for 1 h at 42 °C and 10 min at 72 °C.
Six sets of primers (Table 2) were designed from P. nigrum RGAs using the Primer quest tool (http://eu.idtdna.com/primerquest/home/index) to study the expression pattern of PnRGAs following Phytophthora challenge in black pepper. The endogenous control gene used for expression analysis in black pepper and P. colubrinum were glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Umadevi et al. 2019) and actin (Malik and George 2018) respectively. The qRT - PCR was set in a volume of 20 μl, containing 1x Fast start Essential SYBR green master mix (Roche, Germany), 0.5 μM each primer (Sigma, USA), 10 ng of RNA equivalent cDNA. Real-time PCR was carried out in Rotor Gene Q real-time PCR (Qiagen, Germany). The reaction conditions were set as follows: 94 °C for 10 min, 35 cycles of 94 °C for 10 min, and 60 °C for 30 s and 72 °C for 30 s. A dissociation curve analysis was performed with a stepwise increase of 1 °C per cycle from 62 to 99 °C at the end of the reaction to confirm a single PCR product. A non-template control (NTC) was also tested for all primer pairs to detect any genomic DNA contamination.
The expression level of PnRGAs in each genotype at different time points after inoculation was compared with the expression in mock-inoculated samples. Relative quantification of RGA differential expression among genotypes and time points post-inoculation was performed by a comparative (2-∆∆Ct) method (Livak and Schmittgen 2001). A heat map was generated based on the relative fold change in RGA expression compared to uninoculated control using Clustvis (Metsalu and Vilo 2015).
Results
Identification of RGAs from black pepper
The nucleotide-binding site-encoding DNA regions between Kinase-1a and GLPL were amplified using degenerate primers generated 450- 500 bp amplicon. Out of the 60 recombinant clones sequenced, after removing the repetitive sequences, 23 sequences with uninterrupted open reading frames (ORFs) having domains with high homology to resistance R-genes of other plant species were named as PnRGAs and were deposited in the GenBank (Supplementary table).
Sequence analysis, classification and motif characterization of PnRGAs
Multiple sequence alignment of 23 PnRGAs isolated from the study with NBS regions of characterized TIR and non-TIR-R genes of A. thaliana, C. annuum, S. lycopersicum revealed the presence of conserved domains such as P loop / Kinase 1a, RNBS A non-TIR, Kinase-2a, RNBS-B, RNBS-C and GLPL within the PnRGAs. All of the 23 Piper RGAs possessed a conserved tryptophan (W) residue at the end of the kinase-2a domain, confirming their similarity to a non-TIR class of NBS-LRR R genes. Percentage of amino acid identity was derived through pairwise comparison among isolated PnRGAs, NB-ARC domain of R gene transcripts of P. nigrum fruit and leaf transcriptome, and the NBS motifs of both TIR and non-TIR R genes (Table 3).
Multiple Expectation Maximization for Motif Elicitation analysis (MEME) confirmed that six major conserved motifs that determine the structural characteristics of NBS domain in identified PnRGAs. The P-loop (Kinase-1a) motif showed highest conservation (E value: 1.5e-218) followed by RNBS-B (E value: 2.6e-219), RNBS C (E value: 3.0e-208), Kinase-2a motif (E value: 8.7e-195), GLPL motif (E value: 5.5e-183) and RNBS-A non-TIR (2.1e-174) (Fig. 1).
A phylogenetic tree based on the Maximum likelihood algorithm constructed with PnRGAs identified in the present study and known R-genes (TIR and non-TIR). The resulting tree was divided into non-TIR and TIR-NBS LRR clades and 23 PnRGAs clustered with non-TIR R gene clade which was subdivided into four major groups (Fig. 2).
Interpro scan analysis of the predicted amino acid sequences of 23 PnRGA concluded that all the PnRGA possessed NB-ARC (Nucleotide-binding and similarity to human Apaf-1, R genes and Ced4) domain (IPR002182) and were classified into P-loop containing nucleoside triphosphatase hydrolase superfamily (IPR027417).
Expression profiling of the PnRGAs
The present study examined the temporal expression analysis of six PnRGAs that are involved in the compatible and incompatible interaction during the P. capsici invasion at different time periods. Differences in the transcription levels of the selected PnRGAs over time were observed after inoculating resistant wild species, moderately tolerant and susceptible genotypes of black pepper with highly aggressive isolate of P. capsici, 05-06 (Fig. 3). Basal level expressions of CC-NBS-PnRGAs were present in leaves of P. colubrinum, IISR Sakthi, O4-P24-1, and Subhakara.
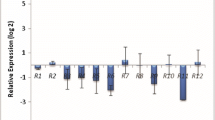
Relative transcript level of Piper nigrum RGAs namely PnRGA1, PnRGA3, PnRGA5, PnRGA8 PnRGA11 and PnRGA24 in IISR Sakthi, Subhakara, 04-P24-1 and P. colubrinum at different time points post inoculation with P. capsici. All real time experiments were performed in triplicates (n = 3). Data are represented as means±SD. Compared with P. colubrinum * means P < 0.05
The PnRGAs which are involved in pathogen recognition showed significant and noticeable higher expression in resistant P. colubrinum, when compared with the expression in moderately resistant and susceptible black pepper genotypes. PnRGAs (PnRGA1, PnRGA 3, PnRGA 8 and PnRGA 24) displayed a high level of expression in P. colubrinum at the very early time point. The highest relative expression of PnRGA in P. colubrinum was 22 fold for PnRGA24.
The PnRGA expression kinetics in moderately resistant IISR Sakthi, revealed upregulation of PnRGA1 and PnRGA24 at early time points. Interestingly, in the case of PnRGA24, the expression level remained high as long as 16 hpi. The highest mRNA transcript level of PnRGA in IISR Sakthi was six-fold at 0.5 hpi for PnRGA11.
A transient expression of most of the PnRGAs was observed in the case of susceptible genotype, Subhakara at the early time point (0.5 hpi). The mRNA transcript level of PnRGA8 persisted upto 2 hpi and afterwards downregulated compared to mock-inoculated control.
The expression of PnRGA3 was higher in 04-P24-1 up to 12 hpi and afterward transcript level was lower compared to the uninoculated control. Although in 04-P24-1, a higher level of transcription of PnRGA24 was noticed only at 0.5 hpi (5 fold higher), the transcript level remained lower till 72 hpi. The relationships among the differential mean fold-changes of expressions of the six PnRGAs during the time-course were observed in the heatmap (Fig. 4).
A heatmap representing the fold-change differences in expression among selected six PnRGA at 0.5, 1, 2, 4, 8, 12, 16, 24, 48 and 72 hpi in IISR Sakthi, Subhakara, 04-P24-1 and P. colubrinum. Red color represents up-regulation of PnRGAs and blue color represents down-regulation and color intensity indicates fold-change. The mean fold change expression values for each treatment and genotypes were normalized with mentioned reference genes and uninoculated control
Discussion
Integrated disease management strategy comprising of host resistance, biological control, and phytosanitary measures are necessary for safeguarding black pepper from its pathogens. The most effective and economical strategy for the management of Phytophthora quick wilt in black pepper relies on identifying or developing host resistant resources. The significant bottleneck towards the development of disease-resistant varieties in black pepper is the perennial nature of the crop, the complexity of the genome, and the existence of P. capsici in multiple hosts in the intercropping system. Considering the economic and medicinal properties of black pepper, the characterization of promising tolerant lines in germplasm is of top priority. Identification of RGAs in tolerant black pepper lines is an ideal approach towards the genetic improvement of non-model crops. In this study, 23 RGAs were identified using homology-based PCR cloning of NBS class disease resistance gene analogs in black pepper.
The identified R gene analogs of black pepper were found to be of non-TIR R gene type based on the criteria; the presence of tryptophan in the kinase 2a domain of the selected NBS region between P-loop and GLPL (Meyers et al. 1999). In dicots, the occurrence of both TIR and non-TIR RGA were reported by Meyers et al. (1999), however, later studies by Tarr and Alexander (2009) revealed both TIR and non-TIR R genes are restricted to basal angiosperms whereas Magnolidae family, to which black pepper belongs was reported to be devoid of TIR R genes. This may be because magnolids resemble monocots in which TIR R gene types are rare or absent. Persea americana and P. colubrinum, which belong to Magnolidae family were found to be devoid of the TIR NBS-LRR class of R genes (Tarr and Alexander 2009; Malik and George 2018). The presence of non-TIR RGA alone in black pepper is also in agreement with earlier reports in Magnolidae.
The alignment of deduced amino acid sequences of PnRGAs exhibited the typical features of the NBS domain [P loop (Kinase-1), RNBS-A-non-TIR, Kinase 2, RNBS-B, RNBS-C, and GLPL domains], indicating their possible function in disease resistance (McHale et al. 2006). In silico analysis predicted that amino acid sequences of all 23 PnRGAs belong to P-loop containing nucleoside triphosphate hydrolase (P-loop NTPase) superfamily, which functions in ADP binding by hydrolyzing of the beta-gamma phosphate bond of bound nucleoside triphosphate (NTP) (Zhou et al. 2019). So it is possible that identified PnRGAs may participate in ADP phosphorylation, which is a crucial process in pathogen recognition.
The distinction between resistant genes identified at the genetic level and those expressed during resistance response in plants that vary in degree of resistance is a pre-requisite for the functional validation of plant RGAs. Most plant R genes are transcriptionally induced in response to pathogen attack, and qPCR could be employed to detect even small fluctuations in gene activation compared to Northern blot analysis (Bustin et al. 2009).
Resistance responses of a plant during attempted infection can be studied by comparing the responses in tolerant/resistant genotype and susceptible genotype with and without pathogen stress. P. nigrum genotypes are susceptible to P. capsici and lesion appears in leaf within 12 hpi, with progressing margins and after 72 hpi the leaf falls. P. colubrinum exhibited hypersensitive response after 12 hpi and lesion does not progress even after 72 hpi when inoculated with highly aggressive isolate of P. capsici. This suggests that incompatible interactions occur between P. colubrinum and P. capsici. Hence, a time frame of 0-72 hpi was chosen for RGA expression studies in Piper spp. after pathogen challenge.
Differential expressions of PnRGAs were observed in resistant (P. colubrinum), moderately resistant (IISR Shakthi and 04-P24-1) and susceptible (Subhakara) black pepper genotypes and their relative transcript level varied at different time points. This infers that RGAs are present in all genotypes irrespective of its resistance or susceptible nature; however, its expression will have a significant role in disease resistance. PnRGA transcripts were also expressed in uninoculated leaf samples suggesting that constitutive expression of RGA transcripts occurs for continuously monitoring of pathogen invasion. Low-level transcript accumulation of RGAs under unchallenged conditions was also reported in banana, ginger, and turmeric (Peraza-Echeverria et al. 2008; Nair and Thomas 2007; Kar et al. 2013).
RGA expression in plants should be rapid enough for pathogen recognition and subsequent initiation of defense signaling pathways (Zhou et al. 2019). The expression of PnRGAs was found to be upregulated during the early hours of infection and down-regulated towards the later phases of infection. In C. annuum- P. capsici pathosystem, CaRGA2 encoding a blight resistance protein was differentially expressed in the resistant and susceptible genotypes following P. capsici infection and peak expression was recorded within 12 hpi followed by a decrease in expression (Zhang et al. 2013).
Since P. colubrinum is a potential donor for Phytophthora resistance and widely used as rootstock for black pepper propagation, it was essential to determine which of the responsive PnRGA expresses in this genetic background. The mRNA transcript level of PnRGAs was higher compared to P. nigrum genotypes after P. capsici challenge and this might be due to the hypersensitive response of P. colubrinum after 12 hpi.
In P. capsici- S. lycopersicum pathosystem, after 24hpi, P. capsici shifts from biotrophic to necrotrophic lifestyle and distinct transcriptome changes occurred in the host during initial infection and the transition from biotrophy to necrotrophy (24 to 48 hpi). The genes involved in pathogen invasion viz., those involved in germ tube formation and appressoria development were expressed in P. capsici during the time frame of 0-24 hpi (Jupe et al. 2013). In our study, we found that most of the PnRGAs were upregulated until 12 h post-inoculation with P. capsici. This suggests that RGAs were expressed more during the biotrophic stage of the pathogen. Mahadevan et al. (2016) also observed widespread colonization of P. capsici within 24 hpi and proliferation of mycelia towards other healthy tissues in black pepper variety Panniyur I. We presume that being a hemibiotroph, P. capsici changes to the necrotrophic phase of its life cycle with the active involvement of pathogen effectors and it might be responsible for the lowered expression of PnRGA from 24 hpi.
Phytophthora infection in black pepper occurs mainly through soil and aerial infections. Aerial infections occurs on the foliages, shoots, spikes and branches causing blight, spike shedding, defoliation which leads to plant death (Anandaraj 2000). The aerial infection of the plant happens from runner shoots or through the roots close to soil level, leading to black pepper wilt. The root infections culminating in collar rot takes long incubation time and takes nearly 2-3 rainy seasons, whereas the disease spread due to aerial infection in the fields are rapid due to the inoculums transferred by rain splashes and wind blown water droplets (Nambiar and Sarma 1982; Ristaino and Gumpertz 2000).
Our results envisaged that PnRGAs are constitutively expressed irrespective of the pathogen encounter and the transcriptional patterns of PnRGAs after P. capsici infection showed that the response was quicker during early infection stages. The future directions of this study should be focused on unraveling the protein functions of the selected RGAs that were differentially expressed in moderately resistant black pepper genotypes after P. capsici infection. This study will provide new insights and open new avenues in Piper breeding program against Phytophthora resistance. However, more studies are required to envisage the mechanism of various other gene families which are involved in the resistance response of black pepper during Phytophthora infection.
References
Ameline-Torregrosa C, Wang BB, O’Bleness MS, Deshpande S, Zhu H, Roe B, Young ND, Cannon SB (2008) Identification and characterization of nucleotide-binding site-leucine-rich repeat genes in the model plant Medicago truncatula. Plant Physiol 146:5–21
Anandaraj M (2000) Diseases of black pepper (Piper nigrum L.). In: Ravindran PN (ed) Black pepper- Piper nigrum L., medicinal and aromatic plants-industrial profiles. CRC Press Florida, Boca Raton, p 239
Anandaraj M, Sarma YR (1995) Diseases of black pepper (Piper nigrum L.) and their management. J Spices Aromat Crops 4:17–23
Bailey TL, Bodén M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208
Bakker EG, Toomajian C, Kreitman M, Bergelson J (2006) A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18:1803–1818
Bayer PE, Golicz AA, Tirnaz S, Chan CK, Edwards D, Batley J (2019) Variation in abundance of predicted resistance genes in the Brassica oleracea pangenome. Plant Biotechnol J 17:789–800
Bhai RS, Eapen SJ, Anandaraj M, Saji KV (2010) Identification of Phytophthora nematode resistant source-from opens pollinated progenies of black pepper (Piper nigrum) using a modified protocol. Indian J Agric Sci 80(10):893–897
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15
Food and Agriculture Organization of the United Nations (2017) FAOSTAT Database. FAO, Rome Retrieved 1 Dec 2018 from http://faostat3.fao.org/home/E
Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW (2012) Plant disease resistance genes: current status and future directions. Physiol Mol Plant P 78:51–65
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hu L, Hao C, Fan R, Wu B, Tan L, Wu H (2015) De novo assembly and characterization of fruit transcriptome in black pepper (Piper nigrum). PLoS One 10(6):e0129822
Jebakumar SR, Anandaraj M, Sarma YR (2001) Induction of PR proteins and defense related enzymes in black pepper due to inoculation with Phytophthora capsici. Indian Phytopathol 54:23e28
Johnson KG, VijeshKumar IP, Anandaraj M, (2012) Transcriptomics approaches for gene discovery in plants - a case study in Piper. International Conference on Agricultural and Horticultural Sciences. Hyderabad International Convention Centre, India, Agrotechnology 1(2)
Joshi RK, Kar B, Mohanty S, Subudhi E, Nayak S (2012) Molecular cloning, characterisation and expression analysis of resistance gene candidates in Kampferia galanga L. Mol Biotechnol 50(3):200–210
Jupe J, Stam R, Howden AJM, Morris JA, Zhang R, Hedley PE, Huitema E (2013) Phytophthora capsici-tomato interaction features dramatic shifts in gene expression associated with a hemi-biotrophic lifestyle. Genome Biol 14:R63
Kanazin V, Marek LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc Natl Acad Sci U S A 93:11746–11750
Kar B, Nanda S, Kumar PN, Nayak S, Joshi RK (2013) Molecular characterization and functional analysis of CzR1, a coiled-coil-nucleotide-binding site-leucine–rich repeat R-gene from Curcuma Zedoaria Loeb. That confers resistance to Pythium aphanidermatum. Physiol Mol Plant P 83:59–68
Leister D, Ballvora A, Salamini S, Gebhardt C (1996) A PCR based approach for isolating pathogen resistance genes from potato with potential for wide application in plants. Nat Genet 14:421–429
Li P, Quan X, Jia G, Xiao J, Cloutier S, You FM (2016) RGAugury: a pipeline for genome-wide prediction of resistance gene analogs (RGAs) in plants. BMC Genomics 17:852
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2 (-deltadeltaC(t)) method. Methods 25(4):402–408
Maekawa T, Kufer TA, Schulze-Lefert P (2011) NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 12:817–826
Mahadevan C, Krishnan A, Saraswathy GG, Surendran A, Jaleel A, Sakuntala M (2016) Transcriptome- assisted label-free quantitative proteomics analysis reveals novel insights into Piper nigrum—Phytophthora capsici Phytopathosystem. Front Plant Sci 7:785
Malik N, George JK (2018) Resistance genes in Piper colubrinum: In silico survey from leaf transcriptome and expression studies upon challenge inoculation with Phytophthora capsici. Appl Biochem Biotechnol 184:987–1008
Marone D, Russo MA, Laido G, Leonardis AMD, Mastrangelo AM (2013) Plant nucleotide binding site- leucine rich repeat (NBS-LRR) genes: active guardians in host defense responses. Int J Mol Sci 14:7302–7326
McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biol 7:212
Metsalu T, Vilo J (2015) Clustvis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res 43(W1):W566–W570
Meyers BC, Dickerman AW, Michelmore RW, Sivaramakrishnan S, Sobral BW, Young ND (1999) Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding super family. Plant J 20:317–332
Nair RA, Thomas G (2007) Isolation, characterization and expression studies of resistance gene candidates (RGCs) from Zingiber spp. Theor Appl Genet 116:123–134
Nambiar KKN, Sarma YR (1982) Some aspects of epidemiology of foot rot of black pepper. In: Nambiar KKN (ed) Phytophthora diseases of tropical cultivated plants. Central Plantation Crops Research Institute, Kasaragod, pp 225–232
Ohmori T, Murata M, Motoyoshi F (1998) Characterization of disease resistance gene-like sequences in near-isogenic lines of tomato. Theor Appl Genet 96:331–338
Peraza-Echeverria S, Dale JL, Harding RM, Smith MK, Collet C (2008) Characterization of disease resistance gene candidates of the nucleotide binding site (NBS) type from banana and correlation of a transcriptional polymorphism with resistance to Fusarium oxysporum f.sp. cubense race 4. Mol Breed 22:565e79
Purseglove JW, Brown EG, Green CL, Robbins SRJ (1981) Spices, vol I. Longman, London
Ravindran PN, Babu KN, Sasikumar B, Krishnamurthy KS (2000) Botany and crop improvement of black pepper. In: Ravindran PN (ed) Black pepper- Piper nigrum L., medicinal and aromatic plants-industrial profiles. CRC Press, Boca Raton, p 23
Ristaino JB, Gumpertz ML (2000) New frontiers in the study of dispersal and spatial analysis of epidemics caused by species in the genus Phytophthora. Annu Rev Phytopathol 38(1):541–576
Sarma YR, Anandaraj M, Venugopal, M (1994) Diseases of spice crops. In: Chadha KL, Rethinam P (eds.), Advances in horticulture, Vol 10 (2). Malhotra Publishing House New Delhi, pp. 1015-1058
Shamina A (1997) Biochemical studies on host-pathogen interactions in black pepper (Piper nigrum L.) infected with Phytophthora capsici. Ph.D. Dissertation, University of Calicut
Sievers F, Wilm A, Dineen DG, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal omega. Mol Syst Biol 7:539
Tamura K, Peterson D, Peterson N, Steecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tarr DEK, Alexander HM (2009) TIR-NBS-LRR genes are rare in monocots: evidence from diverse monocot orders. BMC Res Notes 2(1):197
Umadevi P, Suraby EJ, Ananaraj M, Nepolean T (2019) Identification of stable reference gene for transcript normalization in black pepper-Phytophthora capsici pathosystem. Physiol Mol Biol Pla 25(4):945–952
Vandana VV, Bhai RS (2018) Differential expression of PR genes in response to Phytophthora capsici inoculation in resistant and susceptible black pepper (Piper nigrum L.) lines. Eur J Plant Pathol 150(3):713–724
Vandana VV, Bhai RS, Shamina A (2014) Biochemical defense responses of black pepper (Piper nigrum L.) lines to Phytophthora capsici. Physiol Mol Plant Path 88:18e27
Yang S, Zhang X, Yue JX, Tian D, Chen JQ (2008) Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol Gen Genomics 280:187–198
Zhang Y, Wang J, Zhang X, Chen JQ, Tian D, Yang S (2009) Genetic signature of rice domestication shown by a variety of genes. J Mol Evol 68:393–406
Zhang YL, Li DW, Gong ZH, Wang JE, Yin YX, Ji JJ (2013) Genetic determinants of the defense response of resistant and susceptible pepper (Capsicum annuum) cultivars infected with Phytophthora capsici (Oomycetes; Pythiaceae). Genet Mol Res 12:3605–3621
Zhou Z, Bar I, Sambasivam PT, Ford R (2019) Determination of the key resistance gene analogs involved in Ascochyta rabiei recognition in chickpea. Front Plant Sci 10:644
Acknowledgments
The authors extend their gratitude to the Indian Council of Agriculture Research (ICAR) for financial support through ORP on Phytophthora, Fusarium and Ralstonia diseases of horticultural and field crops (PhytoFuRa). The authors acknowledge Dr. Santhosh J Eapen and Mrs. Rosana OB, DISC, ICAR- IISR, Kozhikode for bioinformatics support. The authors are thankful to Dr. Johnson K George, ICAR- IISR, Kozhikode for sharing data of leaf transcriptome of P. nigrum L. The authors are also grateful to Huasong Wu, Key Laboratory of Genetic Resources Utilization of Spice and Beverage Crops, Ministry of Agriculture, Wanning, Hainan 571533, China, for sharing data of fruit transcriptome of P. nigrum L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Suraby, E.J., Prasath, D., Babu, K.N. et al. Identification of resistance gene analogs involved in Phytophthora capsici recognition in black pepper (Piper nigrum L.). J Plant Pathol 102, 1121–1131 (2020). https://doi.org/10.1007/s42161-020-00586-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-020-00586-3