Abstract
The potential of antagonistic bacteria isolated from tomato rhizosphere soils of Oman in the control of damping-off disease of tomato was investigated. A total of 27 bacterial isolates were isolated from 18 soil samples collected from the rhizosphere of tomato from Al-Batinah South, Al-Sharqia North and Muscat Governorate. These bacterial isolates were tested in vitro for their antagonistic activity against Pythium aphanidermatum using a dual culture technique. Of the 27 bacterial isolates tested, four isolates designated D1/3, D1/8, D1/17 and D1/18 were effective in inhibiting the mycelial growth of P. aphanidermatum, by inducing an inhibition zone of 32.3, 10.3, 6.3 and 9.9 mm, respectively. Compatibility tests using a cross-streak assay on nutrient agar medium indicated that these four bacterial isolates were compatible with one another. The bacterial isolates were identified as Klebsiella oxytoca (D1/3), Exiguobacterium indicum (D1/8) and Bacillus cereus (D1/17 and D1/18), on the basis of the rRNA gene sequences. Among the isolates tested for in vitro plant growth promoting activity, D1/8 induced the maximum shoot length and seedling vigor. The potential of bacterial antagonists either individually or in combination in the control of damping-off disease of tomato was tested under greenhouse conditions. Among the biocontrol treatments, the combined application of D1/8 and D1/17 was the most effective, where damping-off incidence was reduced by 27% relative to the infected control. These bacterial antagonists appear to be potential candidates to be developed as bio-inoculants for the ecofriendly management of damping-off of tomato under desert farming ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Mill.) is the most important vegetable crop cultivated in Oman, where drought, salinity and diseases are the major constraints to its production.. Damping-off caused by P. aphanidermatum is one of the most important diseases of tomato causing high mortality of seedlings in the nursery and field. Pythium spp. infects seeds or seedlings before emergence from the soil, resulting in “pre-emergence damping-off” (Hendrix and Campbell 1973). This pathogen also infects roots and the hypocotyl of seedlings after germination and causes their death, resulting in “post-emergence damping-off”. Pythium sp. oospores survive in the soil for a long time and act as the primary source of inoculum (Stanghellini and Nigh 1972).
Since P. aphanidermatum is a soil-borne pathogen, its control is very difficult. Development of resistant cultivars could be one of the best approaches. However, there are no known sources of resistance identified in the existing tomato cultivars. At present this disease in tomato and other vegetable crops in Oman is being managed by adopting few prophylactic methods including adequate drainage, application of an optimum dose of nitrogenous fertilizers, implementing crop rotation, soil solarization, biofumigation and removal of top 30 to 60 cm of greenhouse soil and replacement with fresh uncultivated soil (Deadman et al. 2006; Al-Sadi et al. 2008). Though management of damping-off can be partly achieved through the use of fungicides under greenhouse condition, treatment of soil with fungicides is expensive and impracticable under large-scale field cultivation. Although phenylamide fungicide metalaxyl and its isomer metalaxyl-M have shown high potential in controlling oomycetes, there are several reports of development of metalaxyl resistance in Pythium spp. (Sanders 1984; Mazzola et al. 2002). Furthermore, application of fungicides in the soil may cause undesirable effects to the environment. To address these concerns, new strategies for the control of damping-off in susceptible crops are continually explored.
The use of plant growth-promoting rhizobacteria (PGPR) with antagonistic properties on soil-borne plant pathogens has been suggested as a promising alternative to synthetic chemical fungicides (Weller 1988; Bloemberg and Lugtenberg 2001; Compant et al. 2005). The PGPR are a heterogeneous group of bacteria that can be found naturally in the rhizosphere or rhizoplane or in association with roots of plants (Ahmad et al. 2008). Many bacterial genera, including Agrobacterium, Azotobacter, Azospirillum, Bacillus, Burkholderia, Erwinia, Flavobacterium, Klebsiella, Micrococcous, Rhizobium, Pseudomonas and Serratia have been reported as PGPR, as they have shown potential as biocontrol agents against different fungal pathogens (Ahemad and Kibret 2014). Registered biocontrol products viz., Mycostop (Streptomyces griseoviridis) and Supresivit (Trichoderma harzianum) have been used for controlling plant diseases caused by Pythium sp. in greenhouses (Hansen et al. 2010). However, the release of “exotic” antagonistic microorganisms in soil may occasionally disrupt local ecosystem and produce undesirable ecological impacts on the rhizosphere microbiota (Jackman et al. 1992). Furthermore, introduced biocontrol agents may affect non-target microorganisms (Pereira et al. 2009). In Oman, the climate is extremely hot and dry most of the year and the summer temperature may exceed 50 °C. Sub-surface soil temperature variation may influence the growth and diversity of soil microbial communities. Soil salinity is another major constraint, as farm soils are exposed to secondary salinization through the use of highly saline irrigation water (Al-Rashdi and Sulaiman 2015). Under these environmental conditions, native antagonists that can maintain threshold populations in the soil may be ideal to be used as biocontrol agents.
The objectives of the present study were: (i) to isolate native antagonistic bacteria from rhizosphere soil of tomato collected from different tomato-growing regions of Oman; (ii) to study their antagonistic properties against P. aphanidermatum in vitro; (iii) to test the compatibility between effective antagonistic bacteria; (iv) to study the plant-growth promoting ability of potential bacterial antagonists and (v) to evaluate the efficacy of selected bacterial antagonists in the control of damping-off disease of tomato under greenhouse conditions.
Materials and methods
Fungal culture
A virulent isolate of Pythium aphanidermatum was recovered from infected tomato plants and the culture was maintained on potato dextrose agar (PDA) (Oxoid UK) medium. Pathogenicity of the isolate was confirmed by inoculating the susceptible tomato cv. ‘Pusa Ruby’. The fungus was identified based on the morphological characters and confirmed by internal transcribed spacer (ITS) sequence analysis (White et al. 1990; Al-Sadi et al. 2012).
Isolation of bacterial antagonists
A total of 18 soil samples were collected from different tomato fields in Al-Batinah South, Al-Sharqia North and Muscat Governorate of the Sultanate of Oman. For collection of rhizosphere soil, tomato plants were gently uprooted and soil adhering to the roots was collected. Bacteria from these soil samples were isolated using a serial dilution technique on nutrient agar (NA) medium and King’s medium B (KMB) (King et al. 1954). One gram of soil was suspended in 100 ml of sterile distilled water, then serial dilutions of 10−3, 10−4 and 10−5 were prepared in sterile distilled water. One hundred μl of the diluted soil suspension were applied on to the surface of the NA/ KMB plates using a sterile pipette, and spread over the medium under aseptic conditions, then the plates were incubated at 27 °C for 2–3 days. Bacterial colonies with different morphological characters such as shape, size, texture, colour, elevation and margin were selected from the plates and pure cultures of the bacteria were obtained.
In vitro testing for antagonistic activity
A total of 27 bacterial isolates were tested in vitro for their antagonistic activity against P. aphanidermatum using a dual culture technique. A bacterial isolate from an overnight culture was streaked at 1.0 cm from the outer edge of a 9-cm Petri dish containing 20 ml of NA/ KMB medium and incubated for 24 h at 27 °C. A mycelial disc of 7 mm cut with a sterile cork borer from the outer edge of a three-day-old culture of P. aphanidermatum was placed on the opposite side. The dual culture was then incubated for 3–5 days at 25 ± 2 °C. The inhibition zone was recorded at the end of the incubation period by measuring the distance between the edges of the fungal mycelium and the antagonistic bacterium (Vijayasamundeeswari et al. 2010).
Testing compatibility between antagonistic bacteria
Four bacterial isolates viz., D1/3, D1/8, D1/17 and D1/18, which showed antagonistic activity in vitro against P. aphanidermatum were tested for compatibility between them. An antagonistic bacterial isolate was streaked on one side of the Petri dish containing NA medium, 1 cm away from the periphery, then other three bacterial isolates were streaked perpendicularly to the first bacterium. In another method, a bacterial isolate was streaked twice in parallel lines and other three bacterial isolates were streaked perpendicularly to the first isolate. The plates were incubated at 27 °C for 2–3 days and the growth of all test bacteria was observed, especially at the intersection (Shifa et al. 2015).
Testing antifungal metabolites production by antagonists
The production of antifungal metabolites by the four bacterial antagonists viz., D1/3, D1/8, D1/17 and D1/18 was tested as described by Manhas and Kaur (2016). The antagonistic bacteria were grown on a liquid medium containing 20 g of dextrose, 5 g of glutamic acid, 1.02 g of MgSO4 • 7H2O, 1.0 g of K2HPO4, 0.5 g of KCl and 1 ml of trace element solution (0.5 g of MnSO4 • H2O, 0.16 g of CuSO4 • 5H2O and 0.015 g of FeSO4 • 7H2O in 100 ml of water) per liter (McKeen et al. 1986) at 27 °C on a rotary shaker at 150 rpm for 48 h. The cultures were then centrifuged at 12000 g for 10 min and the cell-free supernatants were collected. P. aphanidermatum was grown in 50 ml of potato dextrose broth (PDB) in a 250 ml conical flask for 3 days at 27 °C. At the end of incubation, the mycelium was collected by filtration through sterile filter paper and washed with sterile distilled water. Three mg of the mycelium were added to 20 ml of cell-free culture filtrate of bacterial antagonists. The electrical conductivity of the suspension was measured at 0, 6, 12, 24 h after incubation using a conductivity meter. The suspension was centrifuged each time at 10,000 rpm for 10 min before measuring conductivity.
Testing plant-growth promoting ability of bacterial antagonists
The efficacy of four bacterial antagonists viz., D1/3, D1/8, D1/17 and D1/18 in promoting plant growth was tested. The test bacteria were grown on 50 ml of nutrient broth for 48 h at 27 °C. At the end of incubation period, the culture was centrifuged at 3500 rpm for 15 min, the pellet was collected and suspended in 10 ml of sterile distilled water. Tomato seeds (cv. ‘Pusa Ruby’) were soaked in the bacterial suspension for 2 h at room temperature (25 ± 2 °C) then dried in the shade. The treated seeds were placed in a Petri dish lined with two layers of wet blotter papers and incubated at room temperature. After 14 days of incubation the percent germination was recorded and root and shoot length were measured using a ruler. Vigour index was calculated by multiplying percent plant stand with the sum of shoot and root length (Baki and Anderson 1973).
Identification of bacteria
The four bacterial isolates viz., D1/3, D1/8, D1/17 and D1/18 were identified by 16S rRNA gene sequence analysis. Each bacterial isolate was grown in 100 ml of nutrient broth at 27 °C on a shaker for 48 h and DNA was extracted using a foodproof StarPrep Two kit (BIOTECON Diagnostics, Germany). The concentration of DNA in the preparation was determined using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, USA). Extracted DNA was amplified by PCR using the universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′), 1429R (5′-TACGGYTACCTTACGACTT-3′) and 534R (5′-ATTACCGCGGCTGCTGG-3′) (Frank et al. 2008; Albertsen et al. 2015). PCR amplification was performed in 25 μl reaction volume using PuReTaq Ready-To-Go PCR beads (GE Healthcare, UK). The PCR bead was first placed in 21 μl of sterile distilled water, to which 2 μl of DNA (50 ng μl−1) and 1 μl (20 pmol μl−1) of each primer were added. Amplifications were performed in a Veriti 96-well Thermal cycler (Applied Biosystems, Singapore) with the following thermal cycle profile: initial DNA denaturation at 95 °C for 2 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s and extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR products (5 μl) were electrophoresed in 1% agarose (Thermo Scientific, USA) gel in Tris-borate-EDTA buffer, pH 8.0. Gels were stained with ethidium bromide (10 mg ml−1) and were visualized and documented using the Gene Flash (Syngene Bio-imaging) system. PCR products were sequenced in both directions at the Central Analytical and Applied Research Unit, Sultan Qaboos University, using the same forward and reverse primers used for PCR amplification. The 16S rDNA sequences were compared with those available in GenBank using the BLASTN programme Phylogenetic analysis of the sequences was performed using the RAxML GUI v.1.3 programme (Silvestro and Michalak 2012).
Greenhouse experiments
Plastic pots (15 cm diameter) were filled with sterile potting mixture (Bulrush Horticulture, Ireland, UK) and placed on greenhouse benches. A cell suspension of each bacterial isolate (D1/8, D1/17 and D1/18) was prepared from two-day-old nutrient broth cultures, the soil was drenched with 10 ml of each bacterial suspension (1 × 108 cfu ml−1) and incubated for three days. In the case of bacterial mixtures, an equal quantity (5 ml + 5 ml) of bacterial suspension was added. The fungicide Metalaxyl was included as chemical control. The fungicide solution (0.1%) was prepared in distilled water and 10 ml of the solution was added to each pot as a soil drench. To produce inoculum, Pythium sp. was cultured on sterile barley grains (15 g barley grain+10 ml distilled water) for 5–7 days at room temperature (27 ± 2 °C). The barley grain inoculum was incorporated into each pot at the rate of 10 grains per pot and mixed well. The pots containing non-infested soil were used as control. On the following day, tomato seeds (cv. ‘Pusa Ruby’) were sown in the infected soil at the rate of 10 seeds per pot. The percentage of damping-off incidence was recorded 21 days after sowing by counting the number of infected plants. The population density of antagonistic bacteria in the soil was determined at 0 and 21 days after sowing (das) by dilution-plating on NA medium and colonies were counted after two or three days of incubation at 27 °C (Vidhyasekaran and Muthamilan 1995). Each treatment was replicated 10 times with 10 plants per replicate. The relative humidity in the greenhouse was maintained at around 80%, and the temperature at 26 °C (day) and 20 °C (night). The experiment was repeated twice.
Statistical analysis
The experimental design used was completely randomized design (CRD). For each response, the validity of model assumptions (normal distribution and constant variance of the error terms) was verified by examining the residuals as described by Montgomery (2013). Independence of the error terms assumption was validated through randomization of the treatments within each block. A least significant difference (LSD) was first applied to the transformed values and then transferred to the original means for greenhouse experiment. When treatment effect was significant (p < 0.05) LSD was used. The data were analyzed using SAS statistical software version 9.2 GLM procedure (SAS Institute Inc. 2009).
Results
Isolation and in vitro screening of antagonists
A total of 27 bacterial isolates showing different morphological characters were isolated using NA and KMB media from the 18 soil samples collected from the rhizosphere of tomato. These bacterial isolates were tested for their ability to inhibit the growth of P. aphanidermatum in vitro by using dual culture technique. Among the various bacterial isolates tested, only four viz., D1/3, D1/8, D1/17 and D1/18 were found effective in restricting the mycelial growth of P. aphanidermatum in co-culture inducing inhibition zones of 32.3, 10.3, 6.3 and 9.9 mm, respectively (Table 1). The remaining 23 isolates failed to produce or produced only small inhibition zones (<5 mm) and they were not included in subsequent studies.
Production of antifungal metabolites by antagonists
The production of antifungal metabolites by the putative bacterial antagonists was tested by using the electrolyte leakage assay. The culture filtrate of all the four antagonistic bacteria caused leakage of cellular electrolytes from the mycelium of P. aphanidermatum as evidenced by increased levels of electrical conductivity (Table 2). Among the antagonists, D1/3 recorded the maximum release of electrolytes from the fungal mycelium followed by D1/8.
Plant growth promoting activity of antagonists
Seedling vigour tests revealed that the bacterial antagonists, except for D1/3, promoted plant growth as evidenced by increased seedling vigor compared to control (Table 3). Among the bacterial isolates tested, D1/8 recorded the maximum shoot length and seedling vigor. Seeds had 100% germination in all treatments. Treatment of tomato seeds with the bacterial antagonist D1/3 resulted in reduction of root length, shoot length and seedling vigor compared to control.
Characterization of the antagonistic bacteria
Based on the phylogenetic analysis of 16S rRNA gene sequences using the RAxMl GUI v.1.3 multiple sequence alignment programme, the antagonistic bacteria were identified as Klebsiella oxytoca (D1/3), Exiguobacterium indicum (D1/8) and Bacillus cereus (D1/17 and D1/18) (Table 4). The nucleotide sequences were deposited in GenBank under accession numbers MF687735, MF687736, MF687737 and MF687738.
Compatibility between antagonists
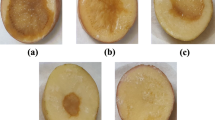
Compatibility tests using a cross-streak assay on nutrient agar medium indicated that the bacterial isolates were compatible with each other as they merged at the intersection (Fig. 1).
Greenhouse studies
The potential of bacterial antagonists in the control of tomato damping-off was evaluated under greenhouse conditions. Since a few strains of Klebsiella oxytoca have been implicated in human and animal diseases, D1/3 was not included in the greenhouse studies. When tomato seeds were sown in the soil artificially infested with P. aphanidermatum, 91% damping-off incidence was observed in the untreated control, whereas all other treatments provided various levels of disease control (Table 5). When comparing single inoculants, treatment with D1/8 was the most effective, where disease incidence was reduced to 79% with a control efficacy of about 13%. However, application of bacterial mixtures containing D1/8 and D1/17 resulted in a significantly better disease suppression compared to single antagonists, where the damping-off incidence was reduced to 66%, which is 27% less than in the infected control (Fig. 2). The fungicide Metalaxyl reduced the incidence of damping-off by 89% compared with the infected control. The rhizosphere population of the antagonists was assessed at the beginning (0 das) and end of the experiment (21 das). Since the antagonists were not used as marker strains, the total population of bacteria in the NA medium was counted. The results indicated that the antagonists multiplied well in the rhizosphere after application and their population increased with the increase of the crop age (Table 6). In general, the populations of antagonists in the soil treated with bacterial mixtures were higher than single strain treatments at 21 das.
Discussion
Several biocontrol agents including Trichoderma virens (Howell 2002), Aspergillus terreus (Halo et al. 2018), Burkholderia cepacia (Heungens and Parke 2001), Lysobacter enzymogenes (Postma et al. 2009), Actinoplanes campanulatus, Micromonospora chalcea and Streptomyces spiralis (El-Tarabily et al. 2009), Pseudomonas fluorescens (Khabbaz and Abbasi 2014), Pseudomonas aeruginosa (Al-Hinai et al. 2010) and Bacillus subtilis (Jayaraj et al. 2005) have been reported to be effective in the control of damping-off of various crops. In the present study four rhizobacterial isolates viz., K. oxytoca (D1/3), E. indicum (D1/8) and B. cereus (D1/17 and D1/18) isolated from Omani soil were found effective in inhibiting the mycelial growth of P. aphanidermatum in vitro. Among them, D1/3 was the most effective followed by D1/8, D1/18 and D1/17. The formation of inhibition zone might be due to the production of antifungal metabolites by these bacterial strains (Leifert et al. 1995; Shafi et al. 2017).
Electrolyte leakage from mycelial walls of fungi is an indicator of cell membrane damage (Manhas and Kaur 2016; Halo et al. 2018). In this study, the conductivity of mycelial suspension increased upon exposure to the cell-free culture filtrates of all the four antagonists and suggests production of antifungal metabolites as one of the possible mechanisms of control of P. aphanidermatum. Although K. oxytoca (D1/3) was highly effective in suppressing the growth of P. aphanidermatum, few strains of K. oxytoca have been implicated in various clinical diseases in humans and animals (Darby et al. 2014). The above characteristics of K. oxytoca may be a barrier for its use as a biocontrol agent for control of plant diseases due to biosafety issues. Further, treatment of tomato seeds with K. oxytoca resulted in reduction of root length, shoot length and seedling vigor compared to the control. This might be due to production of phytotoxic metabolites by this bacterium. Under these circumstances, E. indicum (D1/8) appeared to be a potential candidate to be developed as a bio-inoculant to control damping-off of tomato since it inhibited the mycelial growth of P. aphanidermatum in dual culture assay to the tune of 10.3 mm. Further E. indicum (D1/8) exhibited plant growth promoting potential and recorded the maximum shoot length and seedling vigor. Compatibility tests provided also evidence that this strain is compatible with other bacterial antagonists tested. Pot culture experiments under artificial inoculation condition indicated that soil application of E. indicum (D1/8) resulted in a significantly lower percentage of damping-off affected plants as compared with the infected control. Our results also indicated that the combined application of D1/8 and D1/17 suppressed the development of damping-off disease significantly compared with the untreated control. The antagonists multiplied well in the rhizosphere after application and the rhizosphere population increased with increase in the age of the crop, indicating the greater survival capacity of the antagonists in the tomato rhizosphere. Several species of Exiguobacterium have been widely used for industrial applications including production of enzymes, bioremediation and degradation of toxic substances in the environment (Kasana and Pandey 2017). Few strains of Exiguobacterium sp. were shown to have plant growth promoting potential (Kasana and Pandey 2017). E. indicum was shown to reduce chromium from saline environments (Mohapatra et al. 2017). To our knowledge, this is the first report showing effectiveness of E. indicum in controlling damping-off of tomato.
The results presented here indicate that mixtures of bacterial antagonists containing D1/8 and D1/17 were superior to individual antagonists in suppressing damping-off of tomato. The enhanced disease suppression by the antagonist mixture might be due to their different disease-suppressive mechanisms. Several researchers have suggested that mixtures of biocontrol agents with different modes of action offer better protection against diseases than the application of single isolates (Raupach and Kloepper 1998; Guetsky et al. 2001; Jetiyanon et al. 2003). Roberts et al. (2005) reported that combined application of T. virens and Burkholderia ambifaria significantly improved the suppression of damping-off of cucumber caused by Pytium ultimum. Similarly, Muthukumar et al. (2011) demonstrated that combined application of Trichoderma viride and Pseudomonas fluorescens significantly reduced the incidence of pre- and post-emergence damping-off of chilli and increased the fruit yield.
It is known that biocontrol agents perform well when pathogen pressure is low to moderate. The present study was conducted under high inoculum conditions with a highly aggressive isolate of P. aphanidermatum. Under natural field conditions, application of these biocontrol agents would be expected to give better results because of generally low inoculum levels of the pathogen in soil. Biofumigation, by incorporation of cabbage residues into the top 20 cm of soil and soil solarization have been reported to be effective in the control of Pythium damping-off in vegetable crops in Oman (Deadman et al. 2006). The results of this study clearly indicate that the bacterial biocontrol agents viz., E. indicum (D1/8) and B. cereus (D1/17) can be used in concert with other cultural methods in the integrated disease management (IDM) for environmentally friendly management of damping-off of tomato under desert farming system. Further studies are needed to evaluate the performance of these antagonists under field conditions. Also, studies are needed to evaluate the potential of these biocontrol agents against other soil-borne diseases of tomato, determine their mode of action, test the population dynamics of the introduced biocontrol agents and to assess biosafety of the antagonists.
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ - Sci 26:1–20
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH (2015) Back to basics. The influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS One 10:e0132783
Al-Hinai AH, Al-Sadi AM, Al-Bahry SN, Mothershaw AS, Al-Said AA, Al-Harthi SA, Deadman ML (2010) Isolation and characterization of Pseudomonas aeruginosa with antagonistic activity against Pythium aphanidermatum. J Plant Pathol 92:653–660
Al-Rashdi TT, Sulaiman H (2015) Assessment of physiochemical properties of farm soils and irrigation water around a major industrial area in Oman. Procedia Environ Sci 28:265–270
Al-Sadi AM, Drenth A, Deadman ML, Al-Said FA, Khan I, Aitken EAB (2008) Association of a second phase of seedling mortality in cucumber seedlings with a rapid rate of metalaxyl biodegradation in greenhouse soils. Crop Prot 27:1110–1117
Al-Sadi AM, Al-Ghaithi AG, Al-Balushi ZM, Al-Jabri AH (2012) Analysis of diversity in Pythium aphanidermatum populations from a single greenhouse reveals phenotypic and genotypic changes over 2006 to 2011. Plant Dis 96:852–858
Baki AAA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria. Crop Sci 31:630–633
Bloemberg GV, Lugtenberg BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Darby A, Lertpiriyapong K, Sarkar U, Seneviratne U, Park DS, Gamazon ER, Batchelder C, Cheung C, Buckley EM, Taylor NS, Shen Z, Tannenbaum SR, Wishnok JS, Fox JG (2014) Cytotoxic and pathogenic properties of Klebsiella oxytoca isolated from laboratory animals. PLoS One 9:e100542
Deadman M, Al-Hasani H, Al-Sadi A (2006) Solarization and biofumigation reduce Pythium aphanidermatum induced damping-off and enhance vegetative growth of greenhouse cucumber in Oman. J Plant Pathol 88:335–337
El-Tarabily KA, Nassar AH, Hardy GE, Sivasithamparam K (2009) Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J Appl Microbiol 106:13–26
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74:2461–2470
Guetsky R, Shtienberg D, Elad Y, Dinoor A (2001) Combining biocontrol agents to reduce the variability of biological control. Phytopathology 91:621–627
Halo BA, Al-Yahyai RA, Al-Sadi AM (2018) Aspergillus terreus inhibits growth and induces morphological abnormalities in Pythium aphanidermatum and suppresses Pythium-induced damping-off of cucumber. Front Microbiol 9:95. https://doi.org/10.3389/fmicb.2018.00095
Hansen VM, Winding A, Madsen AM (2010) Exposure to bioaerosols during the growth season of tomatoes in an organic greenhouse using Supresivit (Trichoderma harzianum) and Mycostop (Streptomyces griseoviridis). Appl Environ Microbiol 76:5874–5881
Hendrix FF, Campbell WA (1973) Pythiums as plant pathogens. Annu Rev Phytopathol 11:77–98
Heungens K, Parke JL (2001) Postinfection biological control of oomycete pathogens of pea by Burkholderia cepacia AMMDR1. Phytopathology 91:383–391
Howell CR (2002) Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathology 92:177–180
Jackman SC, Lee H, Trevors JT (1992) Survival, detection and containment of bacteria. Microb Releases 1:125–154
Jayaraj J, Ramakrishnan NV, Kannan R, Sakthivel K, Suganya D, Venkatesan S, Velazhahan R (2005) Development of new formulations of Bacillus subtilis for management of tomato damping-off caused by Pythium aphanidermatum. Biocontrol Sci Tech 15:55–65
Jetiyanon K, Fowler WD, Kloepper JW (2003) Broad-spectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis 87:1390–1394
Kasana RC, Pandey CB (2017) Exiguobacterium: an overview of a versatile genus with potential in industry and agriculture. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2017.1312273
Khabbaz SE, Abbasi PA (2014) Isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber. Can J Microbiol 60:25–33
King EO, Ward MN, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HAS, Harbour A (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78:97–108
Manhas RK, Kaur T (2016) Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping off and black leaf spot of Raphanus sativus. Front Plant Sci 7:1869. https://doi.org/10.3389/fpls.2016.01869
Mazzola M, Andrews PK, Reganold JP, Levesque CA (2002) Frequency, virulence, and metalaxyl sensitivity of Pythium spp. isolated from apple roots under conventional and organic production systems. Plant Dis 86:669–675
McKeen CD, Reilly CC, Pusey PL (1986) Production and partial characterization of antifungal substances antagonistic to Monilinia fructicola from Bacillus subtilis. Phytopathology 76:136–139
Mohapatra RK, Parhi PK, Thatoi H, Panda CR (2017) Bioreduction of hexavalent chromium by Exiguobacterium indicum strain MW1 isolated from marine water of Paradip port, Odisha, India. Chem Ecol 33:114–130
Montgomery DC (2013) Design and analysis of experiments, 7th edn. Wiley, New York
Muthukumar A, Eswaran A, Sangeetha G (2011) Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol Plant 33:1933–1944
Pereira P, Nesci A, Etcheverry M (2009) Impact of two bacterial biocontrol agents on bacterial and fungal culturable groups associated with the roots of field-grown maize. Lett Appl Microbiol 48:493–499
Postma J, Stevens LH, Wiegers GL, Davelaar E, Nijhuis EH (2009) Biological control of Pythium aphanidermatum in cucumber with a combined application of Lysobacter enzymogenes strain 3.1T8 and chitosan. Biol Control 48:301–309
Raupach GS, Kloepper JW (1998) Mixtures of plant growth- promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88:1158–1164
Roberts DP, Lohrke SM, Meyer SLF, Buyer JS, Bowers JH, Baker CJ, Wei L, de Souza JT, Lewis JA, Chang S (2005) Biocontrol agents applied individually and in combination for suppression of soil-borne diseases of cucumber. Crop Prot 24:141–155
Sanders PL (1984) Failure of metalaxyl to control Pythium blight on turfgrass in Pennsylvania. Plant Dis 68:776–777
SAS Institute Inc (ed) (2009) SAS/STAT user’s guide: version 9.2, 2nd edn. SAS Institute Inc., Cary, NC, USA
Shafi J, Mingshan J, Zhiqiu Q, Xiuwei L, Zumin G, Xinghai L, Yang Z, Peiwen Q, Hongzhe T, Wunan C, Kai W (2017) Optimization of Bacillus aerius strain JS-786 cell dry mass and its antifungal activity against Botrytis cinerea using response surface methodology. Arch Biol Sci 69:469–480
Shifa H, Gopalakrishnan C, Velazhahan R (2015) Efficacy of Bacillus subtilis G1 in suppression of stem rot caused by Sclerotium rolfsii and growth promotion of groundnut. Int J Agric Environ Biotechnol 8:91–98
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front end for RAxML. Org Divers Evol 12:335–337
Stanghellini ME, Nigh EL (1972) Occurrence and survival of Pythium aphanidermatum under arid soil conditions in Arizona. Plant Dis Rep 56:507–510
Vidhyasekaran P, Muthamilan M (1995) Development of formulations of Pseudomonas fluorescens for control of chickpea wilt. Plant Dis 79:782–786
Vijayasamundeeswari A, Vijayanandraj S, Paranidharan V, Samiyappan R, Velazhahan R (2010) Integrated management of aflatoxin B1 contamination of groundnut (Arachis hypogaea L.) with Burkholderia sp. and zimmu (Allium sativum L. x Allium cepa L.) intercropping. J Plant Interact 5:59–68
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgements
This study was supported in part by a grant (IG/AGR/DEAN/17/01) from Sultan Qaboos University, Muscat, Oman. We thank the Central Analytical and Applied Research Unit, Sultan Qaboos University for nucleic acid sequencing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Hussini, H.S., Al-Rawahi, A.Y., Al-Marhoon, A.A. et al. Biological control of damping-off of tomato caused by Pythium aphanidermatum by using native antagonistic rhizobacteria isolated from Omani soil. J Plant Pathol 101, 315–322 (2019). https://doi.org/10.1007/s42161-018-0184-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0184-x