Abstract
In order to improve the mechanical properties of PPC, a hyperbranched polymer with a large number of active epoxy groups at the end (epoxy-terminated hyperbranched polyester, EHBP) was synthesized and characterized by FTIR, NMR and GPC. Then, PPC/EHBP blends were prepared through melt-mixing. The effect of EHBP on the mechanical properties, thermal properties, viscosity and gas permeability of the PPC/EHBP blends is studied in detail based on the expected micro-crosslinking theory between EHBP and PPC. The results indicate that when the content of EHBP is 0.5 wt%, the tensile strength increased by 73.8%; elongation at break increased by 131.7%; the impact strength increased by 88.4%. The glass transition temperature increased from 40.71 to 44.44 °C upon addition of EHBP. Also, the addition of EHBP is beneficial to the improvement of the gas barrier property of PPC. The modification mechanism is discussed. It indicates that upon addition of EHBP, both physical hydrogen bonding and chemical bonding occurred, based on gel content calculation, which is in line with expectations. When the content of EHBP is 0.5 wt%, the degree of chemical micro-crosslinking is about 9.2%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
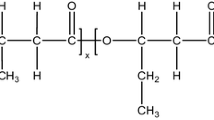
Poly(propylene carbonate) (PPC) is a type of commonly used biodegradable polymer, which can be produced through the copolymerization of carbon dioxide and propylene oxide [1,2,3,4]. It has attracted a vast of interests from both academic and industrial sources, mainly because it can be employed to reduce the emission of white pollutant and greenhouse gases. In addition, PPC has wide potential application in food preservation because of its excellent gas barrier performance. However, the presence of a large number of ether bonds has caused the material with low glass transition point, low thermal decomposition temperature and low tensile strength. These disadvantages have restricted the application of PPC. As a result, proper modifications of PPC are considered not trivial. Among all methods of modification, melting blend is found most effective. It has been reported that PPC can be modified by melting blend with many types of modifiers. Ge et al. [5] found that the stiffness, tensile strength and thermal stability of PPC composites increased upon addition of corn starch as the modifier. Qu et al. [6] prepared a type of easily dispersed PPC-based nanocomposites incorporated with SiC/GO nanohybrids, showing synergistic effects with superior thermal, mechanical, and barrier properties. Kuang et al. [7] reported an approach for the preparation of a PPC/PBS/PTFE ternary system for the aim of improving the strength of PPC without sacrificing its biodegradability. Other substances, such as polylactic acid (PLA) [8], poly(phthalic acid-butylene adipate) (PBAT) [9], straw fiber [10], wood flour [11], wool powder [12] and nano-montmorillonite [13], have also been employed to produce PPC-based blends with low cost and moderate mechanical properties by means of melting blend. On the other hand, PPC can be also chemically modified to alter its molecular structure via end-capping [14, 15], chain extension [16, 17] and crosslinking [14, 18]. Pang et al. [14] used 2,4-diphenylmethane diisocyanate (MDI) as end-capping reagent to modify PPC. It was found that the end-capping reaction of PPC chain only occurred when the content of MDI is 0.1 wt%. The thermal stability and mechanical properties of PPC were improved significantly by adding MDI. However, when the content of MDI exceeds 0.3 wt%, the end-capping, chain extension and crosslinking reactions are simultaneously happened. Wang et al. [16] reported that using ADR-4368 as the chain extender, the mechanical properties, thermal stability and gas barrier performance of PPC can all be effectively improved. Han et al. [18] investigated the direct chemical crosslinking of PPC by (PAPI). It was found that the glass transition point, decomposition temperature and the tensile strength all increased.
Hyperbranched polymer (HBP) is a new type of polymer [19,20,21], which has been developed for several decades. Compared with the linear polymer, HBP has many unique advantages, such as three-dimensional spherical structure, a host number of active terminal functional groups, the internal cavities and branch points, as well as the low probability of the entanglement of molecular chains. HBP can be widely used in the preparation of drug carriers, coatings, adhesion agent and ink printing [22, 23]. In recent years, the investigation of HBP as the blending modifier has attracted expanding attentions. Bhardwaj et al. [24] combined hydroxyl terminated HBP and polylactic acid (PLA) via melt blending to improve the toughness of PLA. Run et al. [25] prepared hyperbranched poly (amide-ester)/poly (butylene succinate) (HBP/PBS) blends. It was found that HBP not only facilitated the crystallization of PBS as the nucleating agent, but also improved the rheological property of PBS as the plasticizer. Chen et al. [26] modified bisphenol A (DGEBA) using a new synthesized heterocyclic nitrogen-containing hyperbranched epoxy resin (HTPE). The impact strength, fracture toughness, tensile strength and flexural strength of the blends were all found improved. Tang et al. [27] toughened epoxy with hyperbranched polyurethane (HBPU). It concluded that upon addition of 10 wt% of HBPU, the toughness of the blends was found to be improved significantly without reducing their processability.
In this study, EHBP is successfully synthesized and used as the modifier to toughen PPC. The effect of EHBP on the mechanical properties, thermal properties and viscosity of the PPC/EHBP blends is studied. The results indicate that by adding EHBP, the tensile strength, elongation at break, impact strength increased and the glass transition temperature all increased. The toughening mechanism is discussed in detail. It indicates that upon addition of EHBP, both physical hydrogen bonding and chemical bonding occurred.
Experimental
Materials
Poly(propylene carbonate) (PPC101, Technical Pure, \(\overline {{M_w}}\) = 7.5 × 104 g mol−1, DI = 2.17) was purchased from Nanyang Zhongjutianguan crown low-carbon Technology Co., Ltd. P-toluenesulfonic acid, boron trifluoride diethyl ether of analysis, sodium hydroxide of analysis and diaminodiphenylmethane were purchased from Sinopharm Chemical Reagent Co., Ltd. 2,2-Bis(hydroxymethyl)propionic acid was purchased from Tianjin Weiyi chemical technology Co., Ltd. Trimethylolpropane was purchased from Tianjin Bodi Chemical Co., Ltd. Trichloromethane was purchased from Beijing Chemical Works. Epichlorohydrin was purchased from Chengdu Huaxia Chemical Reagent Co., Ltd.
Synthesis of epoxy-terminated EHBP
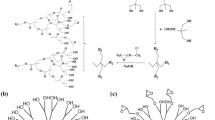
HBPE was prepared using established synthetic protocols [28, 29]. Into the system of HBPE, boron trifluoride diethyl ether (2.5 mL) was added at 75 °C. Epichlorohydrin (100 mL) was then added dropwise into the reaction mixture. After the reaction was carried out for 2 h, the excess of moisture, epichlorohydrin and small molecular by-products were pumped off under reduced pressure. Saturated sodium hydroxide solution (40 mL) was thereafter added and the reaction was continued at 75 °C for 2 h. After adding 300 mL of chloroform at room temperature, the product was dissolved in 30 min. The mixture was filtered and the filtrate was purified first using a rotary evaporator and then under vacuum to obtain the desired product as an orange viscous liquid [30]. The yield of the product is 92.1%. Figure 1 shows the detailed chemical structure schematic diagram of the prepared EHBP.
Sample characterization of EHBP
FTIR
Samples were mixed with KBr at a weight ratio of 1:100 and processed into pellets for the use in Fourier transform infrared (FTIR) measurements and to aid the possible correlation between the intensities of absorption bands and the quantity of corresponding functional groups. The scanning wavelength range of FTIR spectroscopy (Nicolet 8700, Thermo Electron, USA) was set to be 4000–500 cm−1. The scan number was 32.
NMR
The nuclear magnetic resonance (Agilent NMR Magnet, 600 MHz 54 mm ASC, Agilent) (1H-NMR and 13C-NMR) spectrum was recorded using a Bruker Advance 400 NMR spectrometer with a 5-mm tube at room temperature. The sample was dissolved in DMSO-d6 before analysis.
GPC
Gel permeation chromatography (GPC) (DAWN HELEOS-II, Wyatt, USA) was carried out using dimethylsulfoxide as the solvent. Concentration is 1 mg mL−1. Calibration was performed using polystyrene standards.
Epoxy value titration
Hydrochloric acid and acetone was mixed with the volume ratio of 1:40. A certain amount of EHBP was put into a 100-mL conical flask, where 25 mL of the above mixture hydrogen acid and acetone was added. The mixture was stirred at room temperature until EHBP was dissolved completely. After standing for 1 h, the mixture was titrated with 0.1 M NaOH ethanol solution using phenolphthalein as indicator. The blank test was also processed with the same method. For each sample, the analysis was performed three times. The epoxy value of EHBP is calculated according to Eq. 1.
where B and A are the volumes of NaOH solution used in titration and blank test; Nb is the concentration of the NaOH solution; W is the mass of the sample.
Sample preparation
-
(1)
PPC was dried at 40 °C for 12 h prior to use employing diaminodiphenylmethane (DDM) as the accelerator. The specific sample formula is shown in Table 1;
Table 1 Formula for the blending modification of PPC with EHBP -
(2)
PPC was evenly mixed with EHBP and DDM for 420 s in an Internal Mixer (SHR-10A, Zhangjiagang Hongji Machinery Co., Ltd.,) (temperature: 140 °C; rotor speed: 60 rpm). The blends were then cooled down to room temperature before crushing and granulation through a Crushing Machine (HP-150, Beijing Huanyatianyuan Mechanical and Technical Co., Ltd.).
-
(3)
Standard splines were made through an injection molding machine (TY-400, Hangzhou Dayu Machinery Co., Ltd.). The mixing temperature was set to 150 °C, and the injection pressure was 40 MPa.
Sample characterization of the blends
Mechanical properties
Tensile strength and elongation at break of the blends were tested by a computer-controlled electronic universal testing machine (CMT6104, MTS Industry System Co., Ltd., Shenzhen, Guangdong Province, PR China) according to GB/T 1040.2-2006 Standard. The tensile rate was chosen to be 5 mm/min. The size of the spline was determined according to the GB/T 1040.2-2006 standard: dumbbell shaped spline, L = 150 mm, d = 4 mm.
Impact strength was tested by an electronic Izod impact testing machine (XJUD-5.5, Chengde Jinjian Testing Instrument Co., Ltd., Chengde, Hebei Province, PR China) according to GB/T 1843-2008 standard (ISO180:2000). The size of the A-type notched spline was determined according the GB/T 1843-2008 standard (ISO180:2000): 80 mm × 10 mm × 4 mm. The impact energy of the pendulum was 4 J.
Shore hardness was tested by a D-Shore Durometer (XHS, Material Testing Machine Co., Ltd., Yingkou, Liaoning Province, PR China) according to GB/T 2411-2008 Standard.
DSC
The differential scanning calorimeter (DSC) (Q100, TA, USA) of the PHBV/PBAT blends with various HBP contents were measured under the protection of nitrogen gas. The sample (5.0–10.0 mg) was heated from room temperature to 200 °C, pre-annealed at 200 °C for 3 min, followed by cooling to − 70 °C at 20 °C/min and then reheated to 200 °C at 10 °C/min.
Rheological measurements
Rheological tests were carried out by Rotary Rheometer (MARS, Thermo Scientific Co., Ltd.) at 165 °C. The angular velocity was set in the range of 0.01–100 rad s−1.
Gel content
The sample was dissolved in chloroform at 55 °C for 8 h and dried at 80 °C for 4 h to calculate the gel content. The gel content was calculated by Eq. 2.
where m1 and m2 are the mass of the sample before and after dissolution, respectively.
Gas permeability test
The PPC resin and PPC/EHBP blends were compressed into tablet machine (LP-S-50, Lab Tech, Sweden) with 200-μm-thick sheets at 150 °C to get thin plates (Size: 10 cm × 10 cm × 200 μm size). Gas permeability tests of oxygen and carbon dioxide were then taken by permeation test instrument (VAC-V2, Ji’nan Languang Electromechanical Technology Co., Ltd., Shandong, China) through pressure differential gas method, respectively, according to GB 1038-2000. Each group of samples was tested three times.
Results and discussion
FTIR analysis of EHBP
Figure 2 demonstrates the FTIR spectra of EHBP and HBPE. Compared to that of HBPE, the spectrum of EHBP shows the C–O–C trans-epoxy ring vibration band at 845 cm−1 and the C–O–C cis-epoxy ring vibration band at 908 cm−1. It is proven that the epoxy terminal group had been successfully grafted onto hyperbranched polyesters.
NMR analysis of EHBP
Figure 3a shows the 13C NMR spectrum of EHBP. The spectrum of EHBP shows the absorption peaks of secondary and tertiary carbon atoms directly bonded to the oxygen atom at 44.2 ppm and 50.9 ppm, demonstrating that the terminal hydroxyl groups of the hyperbranched polyesters have been epoxidized.
Figure 3b shows the 1H NMR spectrum of EHBP. The chemical shift at 2.6 ppm, 2.8 ppm and 3.1 ppm represent the signal of protons bonded to the secondary (H-1, H-2) and tertiary (H-3) carbon atoms on the epoxy group. The area ratio of these three signals is ~ 1.0:1.0:1.1, which is of the order the same as expected from theoretical calculation (1:1:1).
The above results indicate that the target product EHBP has been synthesized.
GPC analysis of EHBP
The measured number-average molecular weight of EHBP is 4.86 × 103. It is calculated that the molecular weight of the molecular chain segment of EHBP is 351. The molecular weight of the nth generation EHBP is 131 + 351 × 2n−1. Therefore, it is calculated that n = 5, indicating that the generated EHBP contains 96 terminal groups.
Epoxy value of EHBP
The epoxy value of EHBP is determined by titration. It concludes that the epoxy value of the synthesized EHBP is 0.23 mol 100 g−1, while the one for the fifth generation of EHBP should be 0.88 mol 100 g−1, as expected from theory. As a result, it is calculated that the grafting degree of the epoxy group is 26.1%.
Mechanical properties of the PPC/EHBP blends
Figure 4 shows the tensile strength, elongation at break and the impact strength of the PPC/EHBP blends. As shown in Fig. 4a, b, the tensile strength and the elongation at break of the PPC/EHBP blends increased firstly and then decreased upon addition of EHBP. Compared to those of the neat PPC, the tensile strength and the elongation at break of the PPC/EHBP blends are increased by 73.8% (from 15.24 to 26.49 MPa) and 131.7% (from 344.3% to 797.8%), respectively, when the content of EHBP was 0.5 wt%.
Figure 4c shows the impact strength of the PPC and PPC/EHBP blends. It can be seen that the impact strength of the PPC/EHBP blends increased firstly and then decreased upon addition of EHBP. The impact strength of the PPC/EHBP blends with 0.5 wt% content of EHBP is increased by 88.4% (from 17.06 to 32.14 kJ/m−2) when compared to that of the neat blends.
The above results show that the addition of EHBP can enhance the PPC resin without destroying its toughness. Taking into account the tensile strength and impact strength, when the content of EHBP was 0.5%, the modification effect of PPC is the best, and the tensile strength of PPC/EHBP blends increased by 73.8%, the elongation at break increased by 131.7%, and the impact strength increased by 88.4%.
The reasons are as follows. Firstly, two types of interactions, the hydrogen bonding effect and/or the formation of the ether bond, might take place between oxygen atoms of EHBP and hydroxyl groups of PPC, which subsequently enhance the compatibility of the blends. Further, a large amount of such two types of interactions give rise to the formation of both physical and chemical micro-crosslinking. Below a certain micro-crosslinking concentration, i.e. 0.5 wt% of EHBP, it enhances the entanglement and interweaving of the molecular chain and thus the strength and the elasticity of the blends. Meanwhile, the molecular cohesive energy density and the plastic deformation resistance can also be improved, which lead to the increase in the shore hardness.
However, when the content of the EHBP is more than 0.5 wt%, the degree of micro-crosslinking is too high. Under this condition, the movement and rotation of the molecular chain segment are restricted because of the chemical bonding. It causes the reality that the tensile strength, elongation at break and the impact strength all decreased along with the increase in the EHBP content.
Figure 4d illustrates the shore hardness of the blends as a function of EHBP content. It concludes that upon addition of EHBP, the hardness of the blends is improved. With the increase in EHBP content, the hardness is also found increased. Due to the formation of micro-crosslinking structure (both physical and chemical), the molecular cohesive energy density and the plastic deformation resistance are both improved. Consequently, the shore hardness is found increased.
Gel content of the PPC/EHBP blends
The gel content of the PPC/EHBP blends was measured to prove the existence of the chemical micro-crosslinking effect. During the dissolving process, molecular chains with physical micro-crosslinking are completely soluble because of the scission of the hydrogen bonds; however, those molecular chains with chemical micro-crosslinking can be only swelled.
The pure PPC can be completely dissolved in chloroform. However, after adding EHBP, the blends can only be partially dissolved. It means that both the physical micro-crosslinking and the chemical micro-crosslinking occurred between PPC and EHBP. The detailed gel content results are shown in Table 2. It suggests that when then content of EHBP is 2.5 wt%, the degree of chemical micro-crosslinking is about 15.3%.
DMA analysis of the PPC/EHBP blends
The DMA curves of glass transition point for and the PPC/EHBP blends are shown in Fig. 5. Upon addition of EHBP (0.5 wt%), the glass transition point of the PPC/EHBP blends increased from 40.71 to 43.83 °C. Tg is increased along with the increase in EHBP content. When the content of EHBP is 2.5 wt%, Tg of the blends is 44.44 °C.
As has been explained in the above section, the addition of EHBP causes the formation of the micro-crosslinking structure, including both physical and chemical interactions. It enhances the inter-molecular entanglement and interweaving of PPC, which subsequently restricts the movement of the molecular chain and leads to the increase in the glass transition point. The increase in the glass transition point would be meaningful to improve the critical temperature (Tc) of PPC in the future.
Rheological performance analysis of PPC/EHBP
The storage modulus (G′)-angular frequency (ω) relation curves for the PPC/EHBP blends are shown in Fig. 6a. It indicates that upon addition of EHBP, G′ for the blends is increased, compared to that of the neat PPC. Furthermore, it is found that with the increase in EHBP content, G′ for the blends firstly get increased and is followed by a subsequent decrease. The peak value for G′ is achieved when the content of EHBP is 0.5 wt%. It is well known that G′ is a characteristic indicator of the melt viscosity. Upon addition of EHBP, the micro-crosslinking effect gives rise to the enhancement of the molecular interweaving, which causes the increase in the elasticity and thus the G′ of the blends. When then content of EHBP is 0.5 wt%, such an effect reaches the peak value. When adding excessive EHBP, the degree of micro-crosslinking becomes too high. Under this condition, the movement and rotation of the molecular chain segment are restricted by the chemical bonding, which leads to the decline of the elasticity of the blends. As a result, the G′ of the blends is found decreased.
Similar phenomena can also be observed in the case of loss modulus (G″)-angular frequency (ω) relation curves, as illustrated in Fig. 6b. With the increase in the EHBP content, the G″ of the blends is also found firstly increased and then decreased. It suggests that upon addition of EHBP, due to the formation of the micro-crosslinking structure, the movement of the molecular chain would consume more energy. It leads to the increase in the G″. However, EHBP is of spherical structure and low viscous nature. Consequently, it can lubricate the PPC molecular chain. With the increase in EHBP content, such a lubrication effect is also increased. Therefore, the overall observation of the G″ is the combination of the above two effect. When the content of EHBP is higher than 0.5 wt%, the lubrication effect might be stronger than the micro-crosslinking effect. As a result, it is observed that G″ is decreased. However, the overall liquidity of the blends is still better than the neat PPC.
The loss factor (tan δ)-angular frequency (ω) relation curves for the PPC/EHBP blends are shown in Fig. 7. It proposes that under low shear frequency, tan δ (the ratio of loss modulus to storage modulus) increases firstly and then decreases, along with the increase in EHBP content. The reason lies in: (1) upon addition of EHBP, the increase in the energy loss due to molecular movement gives rise to the enhancement of tanδ. The peak value of tan δ is found when the content of EHBP is 0.5 wt%. (2) When adding more EHBP into the blends, the internal lubrication becomes the main effect, which causes the decrease in tan δ. It is found that when then content of EHBP is more than 0.5 wt%, tan δ of the blends is even smaller than that of the neat PPC.
On the other hand, under high shear frequency, tanδ is found decreased firstly and then increased. It is well known that under high shear frequency, molecular chains with high molecular weight would be more sensitive to the change of shear frequency. Due to the formation of micro-crosslinking structure, the shape change of PPC molecular chain becomes bigger, with the increase in the shear frequency. It leads to the decrease in tan δ. However, when the content of EHBP exceeds 0.5 wt%, the formed chemical bonding would restrict the movement of the molecular chain. It causes the decrease in the elasticity and the increase in the viscidity of the blends, meaning that tan δ is increased.
The mechanism of modification
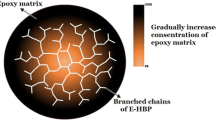
The toughening mechanism is mainly ascribed to the following factors, as shown in Fig. 8.
-
(1)
Both the physical hydrogen bonding and the chemical bonding can cause the formation of micro-crosslinking structure of the PPC/EHBP blends. As a result, the tensile strength, impact strength and glass transition temperature of PPC are all improved.
-
(2)
EHBP is a spherical structure with a large number of cavities inside. After adding EHBP, these cavities could absorb a part of the impact energy. At the same time, the crosslinking point between PPC and EHBP caused PPC molecular chain to be blocked.
-
(3)
EHBP could lubricate the molecular chain motions of PPC because of its low viscosity. The impact strength of the PPC/EHBP was reduced because of the lubrication of EHBP when the content of EHBP increased.
Analysis of gas permeability
As demonstrated in Table 3, upon addition of EHBP, the permeability coefficient of carbon dioxide and oxygen both decreased. When the content of EHBP is 0.5 wt% (mechanical property is best), the carbon dioxide and oxygen permeability coefficient of PPC/EHBP decreased by 49.6% and 60.5%, respectively. It indicates that the addition of EHBP is beneficial to the improvement of the gas barrier property of PPC, which is of great significance for food storage and preservation in packaging.
The gas permeability of amorphous polymers is mainly based on free volume model [31]. The decline of gas permeability coefficient of PPC after adding EHBP lies in the strong micro-crosslinking between EHBP and PPC. On the one hand, the micro-crosslinking network structure of PPC/EHBP restraints the movement of PPC molecular chain segment obviously, which reduces the free volume of the PPC, and also the permeation of gas molecules (oxygen, carbon dioxide). On the other hand, the micro-crosslinking network structure also delays the relaxation of the PPC molecular chain segments, prolongs the diffusion processing of gas molecules and thus improves the gas barrier properties of PPC.
However, if the degree of micro-crosslinking is too high, there would be voids present in the micro-crosslinking network. It would cause the decrease in the gas permeability. As shown in Table 3, when the content of EHBP is 2.5 wt%, the carbon dioxide and oxygen permeability coefficient of PPC/EHBP both increase, compared to that with 0.5 wt% of EHBP.
Conclusions
EHBP was successfully synthesized and used as a modifier for the reinforcing and toughening of PPC. Due to the formation of micro-crosslinking structure, the tensile strength, elongation at break and the impact strength all increased. It is found that when the EHBP content is 0.5 wt%, the tensile strength increased by 73.8%; the elongation at break increased by 131.7%; the impact strength increased 88.4%. Upon addition of EHBP, Tg is increased from 40.71 to 44.44 °C, and the oxygen permeability coefficient decreased by 60.5%. The modification mechanism is discussed in detail. It concludes that both hydrogen bonding effect and chemical reaction have occurred during melting blend.
References
Stewart R (2007) Biopolymers—greater consumer awareness drives growth of biobased-resins market. Plast Eng 63:24
Inoue S, Koinuma H, Tsuruta T (1969) Copolymerization of carbon dioxide and epoxide with organometallic compounds. Macromol Chem Phys 130:210–220
Nakano K, Hashimoto S, Nakamura M, Kamada T, Nozaki K (2011) Stereocomplex of poly(propylene carbonate): synthesis of stereogradient poly(propylene carbonate) by regio- and enantioselective copolymerization of propylene oxide with carbon dioxide. Angew Chem Int Edit 50:4868–4871
Aikawa S, Yoshida Y, Nishiyama S, Noguchi H, Shoji A (2006) Optical properties of poly(propylene carbonate) which contains C-60(OH)(n) structure in the end of polymer chain. Mol Cryst Liq Cryst 445:315–321
Ge X, Li X, Zhu Q, Li L, Meng Y (2004) Preparation and properties of biodegradable poly(propylene carbonate)/starch composites. Polym Eng Sci 44:2134–2140
Qu H, Wang Y, Ye Y, Zhou W, Bai S, Zhou X, Peng H, Xie X, Mai Y (2017) A promising nanohybrid of silicon carbide nanowires scrolled by graphene oxide sheets with a synergistic effect for poly(propylene carbonate) nanocomposites. J Mater Chem A 5:22361–22371
Kuang T, Li K, Chen B, Peng X (2017) Poly(propylene carbonate)-based in situ nanofibrillar biocomposites with enhanced miscibility, dynamic mechanical properties, rheological behavior and extrusion foaming ability. Compos Pt B Eng 123:112–123
Di Lorenzo ML, Ovyn R, Malinconico M, Rubino P, Grohens Y (2015) Peculiar crystallization kinetics of biodegradable poly(lactic acid)/poly(propylene carbonate) blends. Polym Eng Sci 55:2698–2705
Zhang N, Zeng C, Wang L, Ren J (2013) Preparation and properties of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with epoxy-functional styrene acrylic copolymer as reactive agent. J Polym Environ 21:286–292
Li X, Meng Y, Wang S, Rajulu AV, Tjong SC (2004) Completely biodegradable composites of poly(propylene carbonate) and short, lignocellulose fiber Hildegardia populifolia. J Polym Sci Pt B Polym Phys 42:666–675
Ge X, Zhu Q, Meng Y (2006) Fabrication and characterization of biodegradable poly(propylene carbonate)/wood flour composites. J Appl Polym Sci 99:782–787
Chang H, Li Q, Xu C, Li R, Wang H, Bu Z, Lin T (2017) Wool powder: an efficient additive to improve mechanical and thermal properties of poly(propylene carbonate). Compos Sci Technol 153:119–127
Kong J, Li Z, Cao Z, Han C, Dong L (2017) The excellent gas barrier properties and unique mechanical properties of poly(propylene carbonate)/organo-montmorillonite nanocomposites. Polym Bull 74:5065–5082
Pang S, Xu N, Xu G, Pan L, Lin Q, Wang X (2013) Preparation and properties of 4,4-diphenylmethane diisocyanate blocking modified poly(propylene carbonate). J Appl Polym Sci 128:2020–2029
Peng S, An Y, Chen C, Fei B, Zhuang Y, Dong L (2003) Thermal degradation kinetics of uncapped and end-capped poly(propylene carbonate). Polym Degrad Stabil 80:141–147
Wang X, Weng Y, Wang W, Huang Z, Wang Y (2016) Modification of poly(propylene carbonate) with chain extender ADR-4368 to improve its thermal, barrier, and mechanical properties. Polym Test 54:301–307
Wang X, Diao X, Yang N, Weng Y, Wang W (2015) Chain extension and modification of polypropylene carbonate using diphenylmethane diisocyanate. Polym Int 64:1491–1496
Hao Y, Ge H, Han L, Liang H, Zhang H, Dong L (2013) Thermal, mechanical, and rheological properties of poly(propylene carbonate) cross-linked with polyaryl polymethylene isocyanate. Polym Bull 70:1991–2003
Kim YH, Webster OW (1992) Hyperbranched polyphenylenes. Macromolecules 25:5561–5572
Mourey TH, Turner SR, Rubinstein M, Frechet JMJ, Hawker CJ, Wooley KL (1992) Unique behavior of dendritic macromolecules—intrinsic-viscosity of polyether dendrimers. Macromolecules 25:2401–2406
Yao Q, Li C, Huang H, Chen H, Liu B (2017) Waterborne carboxyl-terminated hyperbranched oligomer polyester ligand: synthesis, characterization and chelation with chromium(III). J Mol Struct 1143:371–377
Yu J, Chen Y (2010) Thermally cross-linkable hyperbranched polymers containing triphenylamine moieties: synthesis, curing and application in light-emitting diodes. Polymer 51:4484–4492
Wang T, Li M, Gao H, Wu Y (2011) Nanoparticle carriers based on copolymers of poly(epsilon-caprolactone) and hyperbranched polymers for drug delivery. J Colloid Interface Sci 353:107–115
Bhardwaj R, Mohanty AK (2007) Modification of brittle polylactide by novel hyperbranched polymer-based nanostructures. Biomacromolecules 8:2476–2484
Run M, Wang J, Yao M, Guo L, Wang H, Ba X (2013) Influences of hyperbranched poly(amide-ester) on the properties of poly(butylene succinate). Mater Chem Phys 139:988–997
Chen S, Zhang D, Jiang S, Jia D (2012) Preparation of hyperbranched epoxy resin containing nitrogen heterocycle and its toughened and reinforced composites. J Appl Polym Sci 123:3261–3269
Tang B, Liu X, Zhao X, Zhang J (2014) Highly efficient in situ toughening of epoxy thermosets with reactive hyperbranched polyurethane. J Appl Polym Sci 131:40614
Yokohara T, Yamaguchi M (2008) Structure and properties for biomass-based polyester blends of PLA and PBS. Eur Polym J 44:677–685
Li S, Wu Q, Lv T, Zhu H, Hou H, Lin Q, Li Y, Cui C, Guo Y (2017) Synthesis and characterization of hyperbranched polymer with epoxide-terminated group and application as modifier for epoxy/polyamide system. Polym Sci Ser B 59:328–336
Chen S, Xu Z, Zhang D (2018) Synthesis and application of epoxy-ended hyperbranched polymers. Chem Eng J 343:283–302
Liu D, Bian Q, Li Y, Wang Y, Xiang A, Tian H (2016) Effect of oxidation degrees of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Compos Sci Technol 129:146–152
Acknowledgements
The authors thank the National Nature Science Foundation (51503007), Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD201804030) and Beijing Municipal Natural Science Fund-Key project of science and technology plan of Beijing Education Committee (KZ201810011017) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, Y., Sima, Y., Weng, Y. et al. Simultaneously reinforcing and toughening of poly(propylene carbonate) by epoxy-terminated hyperbranched polymer(EHBP) through micro-crosslinking. Polym. Bull. 76, 5733–5749 (2019). https://doi.org/10.1007/s00289-018-02676-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-02676-w