Abstract
The deterioration in water quality of freshwater bodies and its implication on human health, ecological sustainability and economic development; has elicited a global concern and the need for water resource management and conservation. The water quality of Ikpoba reservoir, Benin City, Nigeria was evaluated using various approaches to ascertain its suitability for human consumption and pollution status. Water sampling was carried out from February 2018 to January 2019, and fifteen (15) water quality indicators were determined using standard analytical procedures. All physico-chemical parameters with the exception of water temperature, pH, dissolved oxygen, biochemical oxygen demand, turbidity, iron, copper, lead and cadmium were within their permissible limits recommended by the NSDWQ and FMEnv. (2007). Water Quality Index value of 207.37 (WQI > 50) indicate that the water is unsuitable for human consumption and use for other domestic purposes. The Comprehensive Pollution Index value of 4.16 (CPI > 2.01) for Ikpoba reservoir indicates that the freshwater body is severely polluted, with turbidity, lead, iron and cadmium identified as contributing significantly to the water pollution. Natural run-off and anthropogenic activities with the catchment area were identified sources of pollutants. The study has provided updated valuable information for water quality monitoring, pollution control strategies and water resource management of the Ikpoba reservoir.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Good water quality is essential for the well-being of human population as well as our environment, as the quality of water source significantly affects choice of land-use strategies such as agriculture, industrial and residential within a catchment area (Brion et al. 2011; Gulbaz & Kayeyzilmaz-Alhan 2012; Omran 2012). Also, the quality of any freshwater source at a specific location is a reflection of the combined effects of several natural processes and human activities along the water pathway. Presently, the water quality deterioration of freshwater bodies has become a global concern and a common challenge to many countries. This is attributed to increasing human population, industrial and agricultural growth, climate change and the high vulnerability of freshwater systems to pollution. These has resulted in threat to human and ecological health, drinking water availability and economic development (Houser and Richardson 2010; Gyamfi et al. 2013; IGES 2015; Ji et al. 2016). In Nigeria and in most countries, deterioration in surface water quality of rivers, reservoirs, lakes and streams due to uncontrolled anthropogenic activities, rapid urbanization and pesticide utilization in agriculture have been reported (Anyanwu 2012; Egun and Ogiesoba-Eguakun 2018; Oboh and Agbala 2017; Omoigberale and Ogbeibu 2007). This has necessitated the need for water pollution control and water quality monitoring.
The evaluation of water quality is of paramount importance in the optimum utilization of water resources as human and land-use activities have local effects on the water quality, and water quality significantly affects choice of land-use strategies (Omran 2012). In Nigeria, two types of water quality assessment methods are generally utilized. The first is the direct comparison of water physico-chemical data with various water quality standards such as the National standard for drinking water (SON 2007), Federal Ministry of Environment standard for surface water (FMEnv. 2007) and World Health Organization (WHO) standard for drinking water. The second is the Water Quality Index (WQI) which expresses the overall water quality of a location as a single number that is classified according to the suitability of the water for domestic use (Egun and Ogiesoba-Eguakun 2018; Oboh and Agbala 2017; Tyagi et al. 2013). The comprehensive assessment of water quality using the Comprehensive Pollution Index (CPI), indicates the degree of the overall pollution of a water body within a locality and identifies the main pollution factors (Zang 2017; Tao et al. 2011; Abbasi and Abbasi 2012). The CPI has garnered interest as a valuable tool for reliable management and conservation of water resources with rapid measurements of large bodies of water. And the supply of helpful information for management and control of the pollution in a watershed based on physiochemical parameters (Idris and Mirac 2018; Xu et al. 2015; Yan et al. 2015).
The Ikpoba reservoir was established in 1977 as a result of the impounding of Ikpoba River by a dam at Okhoro in Benin City, Edo State. The reservoir was established to provide public water supply to about 30% of the inhabitants of Benin City. Over the years, the reservoir has been a receptor to effluents and wastewater with mixed constituents of organic solids and inorganic materials containing a wide range of pollutants as a result of the channelling of the municipal storm water drainage into the reservoir. This has resulted in the alteration of the freshwater habitat thereby leading to decreased biodiversity (Tawari-Fufeyin and Ekaye 2007; Wangboje and Ekundayo 2013). This study, therefore, evaluates the water quality of the Ikpoba reservoir using water quality indices of WQI and CPI to determine the water suitability for human usage and the pollution status of the water body.
Materials and methods
Study area and sampling stations
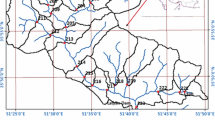
The Ikpoba reservoir (Latitudes 006° 22′ 50″ N and 006° 22′ 43″ N and Longitudes 05° 38′ 36″ E and 05° 38′ 46″ E) is situated in Okhoro, Benin City along the Ikpoba River which flows through Egor and Ikpoba in Okha Local Government Area in Edo state (Fig. 1). The reservoir is about 3.00 km long and 0.60 km wide; covers a total surface area of about 1.1 million m2 and impounds about 1.5 million m3 of water at full capacity. This capacity has reduced due to heavy silting and deposition of solid waste materials into the reservoir. The major land use types in the study area are agriculture, settlement and erosion control. Three (3) sampling stations were established along the stretch of the reservoir for water quality monitoring using the stratified random sampling approach along the downstream gradient (U.S. Geological Survey, 2018).
Analytical methods
Water samples were collected monthly for a duration of eighteen (18) months from February 2018 and January 2019. Water samples were collected between 07:00 and 09:00 on each sampling day. In situ measurements were carried out for water temperature using a Gilson mercury-in-glass thermometer; water pH and electrical conductivity was measured using a digital electronic pH meter (Hanna instrument: HI—1922 model) and conductivity meter (Hanna instrument: HI—96107), respectively. Turbidity was determined using the the HACH colorimeter (DR/920). Surface water samples for physico–chemical analysis were collected in clean and sterilised 1-L polyethylene bottles and tightly closed. The water samples were placed in a cooler box and then taken to the laboratory for analysis.
Samples for dissolved oxygen (DO) determination were collected in plain glass bottles, fixed in the field using 0.1 ml each of Winkler solutions A and B and determined titrimetrically in the laboratory using the Azide modification technique of the Winkler method (APHA 2012). Samples for biochemical oxygen demand (BOD) were collected in amber 250-ml reagent bottles and taken to the laboratory for a 5-day incubating period before analysis using the Winkler’s method. Sulphate, nitrate and phosphate were determined using the HACH colorimeter (DR/890). The respective reagent powder was added to 10 ml of water sample in a beaker and thoroughly mixed, then allowed to stand for 5 min. Water sample is then poured into the cell bottle and placed in the colorimeter, and the concentration read from the display screen in mg/L. Chloride content was determined by MOHR’s titration method. Two drops of potassium heptaoxodichromate (vii) (K2CrO4) indicator was added to 50 ml of water sample in conical flask and titrated against a 0.01 M silver nitrate (AgNO3) solution to give a brick red end point, which is compared to the end point of the blank titration of only the reagents and distilled water (Ademoroti 1996). For copper, lead, iron, zinc and cadmium determination, 10 ml of water sample was digested using an Aluminum Block Digester (BD 110) having added 4 ml of perchloric acid, 20 ml of concentrated nitric acid and 2 ml of concentrated sulphuric acid. The mixture was heated until white fumes evolved and a clear solution obtained. Respective metal ion content in the clear solution obtained was then determined colorimetrically using the Atomic Absorption Spectrophotometer (AAS).
Water Quality Index (WQI)
The Water Quality Index (WQI) was calculated using the Weighted Arithmetic Water Quality Index Method (Akter et al. 2016; Egun and Ogiesoba-Eguakun 2018; Oboh and Agbala 2017; Tyagi et al. 2013). The reference standard utilized for the calculation was Nigerian Standard for Drinking Water Quality (SON 2007) (Table 1).
WQI was calculated using the following equation:
The quality rating scale (Qi) for each parameter was calculated using the following equation:
Vi is the estimated concentration of the ith parameter of interest in the analysed water; Vo is the The ideal value of the ith parameter in pure water; Vo is the 0 (except pH = 7.0; and DO = 14.6 mg l−1); Si is the recommended Standard value of the ith parameter (SON 2007).
The unit weight (Wi) for each water quality parameter
where K is the proportionality constant; K is the 1/Σ (1/Si).
The rating of water quality according to WQI is given in Table 2.
Comprehensive Pollution Index (CPI) method
The Single Factor Pollution Index (PI) method is used to create a Comprehensive Water Pollution Index (CPI). The PI (excluding DO) increases with the pollutant’s concentration and its equation is as follows:
where, Pi is the pollution index of pollutant i; Ci is the measured concentration of the pollutant (mg l−1); Si is the National water quality standard permissible limit for the pollutant in surface water (FMEnv.2007).
The water quality factor Pi is classified into five grades, as listed in Table 3 (Li et al. 2010).
Comprehensive Pollution Index (CPI) is the arithmetic mean of n water pollution indexes. The equation was as follows:
where, Ci is the the measured concentration of the pollutant (mg l−1), Si is the National water quality standard permissible limit for the pollutant in surface water, n is the the number of chosen pollutants.
CPI is classified into the water quality levels listed in Table 4.
Results and discussion
Physical and chemical characteristics
The descriptive statistics of the physico-chemical parameters of Ikpoba reservoir are shown in Table 5 and Figs. 2, 3, 4, 5, 6 and 7. The water temperature (26–28 °C) did not exceed the permissible limit of 30 °C and is attributed to the surrounding secondary forest vegetation around the reservoir. Similar water temperature range have been reported for Ado-Ekiti reservoir (23.50–30.04 °C) by Idowu et al. (2013). The mean pH of 5.8 was below the NSDWQ (2007) and FMEnv. (2007) permissible range (6.5–8.5) of values, and indicates the acidic nature of the water body. The acidic nature of Nigerian freshwater bodies had been reported in several studies (Egun and Ogiesoba-Eguakun 2018; Oboh and Agbala 2017). Low pH levels enhances the corrosive nature of the water and influences the water taste and appearance negatively. Electrical conductivity values reflects the level of ionisation of most chemical compounds in a water body. Electrical conductivity values in this study (70–265.33 μS/cm) were within permissible limits. Similar electrical conductivity value of 158.55 µS/cm was reported for Ajiwa reservoir by Usman et al. (2017). Suspended solids in water are undesirable as they reduce the utilitarian and productivity of the water body. The range of water turbidity values (13–46 NTU) exceeded the NSDWQ and FMEnv. (2007) permissible limit of 5 NTU, an indication of high levels suspended solids in the water body arising mainly from the channelling of municipal wastewater/storm water towards the reservoir particularly at Station 1 (inlet). Dissolved oxygen levels (2.90–5.80 mg/L) were below the minimum acceptable limits by NSDWQ and FMEnv. (2007) an indication of high organic content and oxygen demand. Lowest values of DO were recorded during the raining season (August–November 2018) during which the reservoir received high volume of municipal wastewater and storm water from erosion. Biochemical oxygen demand is an important factor affecting water quality and it is used as organic pollutant indicator in water bodies. Mean BOD value of 3.99 mg/L exceeded the NSDWQ (2007) limit for drinking water, but did not exceed the FMEnv. (2007) limit of 6 mg/L for surface water. According to Oram (2014), water sample with a BOD > 5 mg/L indicates a polluted water with nearby sources of pollution. This buttresses the impact of anthropogenic activities within the Ikpoba reservoir catchment area on the water quality. The range of values for sulphate (15.67–20.67 mg/L), chloride (15.62–23.98 mg/L), nitrate (12.42–21.62 mg/L), phosphate (3.20–4.57 mg/L) and zinc (0.14–0.83 mg/L) did not exceed their respective NSDWQ (2007) and FMEnv.(2007) permissible limits. The mean concentration values for iron (0.90 mg/L), copper (1.28 mg/L), lead (0.023 mg/L) and cadmium (0.006 mg/L) exceeded their respective NSDWQ (2007) and FMEnv. (2007) permissible limits in drinking water and surface water. This implies that the consumption of the water in its untreated form exposes the population to health risk from heavy metal toxicity and predisposes the aquatic fauna in the reservoir to heavy metal bioaccumulation and its attendant physiological effects.
Comparing results in this study with previous studies on Ikpoba reservoir, copper concentration (1.28 mg/L) was higher than the values of 0.14 mg/L (Tawari-Fufeyin and Ekaye 2007) and 0.24 mg/L (Wangboje and Ekundayo 2013) previously reported. This indicates and increase in the activities associated with the release of copper compounds as constituents of effluents within the reservoir catchment area. Recorded values for cadmium, iron, lead and zinc in this study were lower than reported values for cadmium (0.009 mg/L and 0.02 mg/L), iron (2.80 mg/L and 5.60 mg/L), lead (0.05 mg/L and 0.07 mg/L) and zinc (4.41 mg/L and 116.59 mg/L) by Tawari-Fufeyin and Ekaye (2007) and Wangboje and Ekundayo (2013), respectively. The decline in values for these heavy metals in the water was to be associated with the period (seasonal) and duration of the respective studies.
Water Quality Index (WQI)
Concentrations of the considered physico–chemical parameters with specified permissible limit values were used to determine the WQI of the reservoir and ascertain its suitability for consumption and domestic use. A water source is considered suitable for human consumption when its WQI value is < 50. The computed WQI value of 207.37 recorded for Ikpoba reservoir is an indication that the water in its present state is unsuitable for human consumption and use for other domestic purposes (grade E) (Table 6). Also, elevated WQI values recorded in this study is indicative of the increased levels of pollutants in the water body.
Comprehensive Pollution Index assessment (CPI)
In this study, the computed CPI value of 4.40 for Ikpoba reservoir indicates that the freshwater body is severely polluted (Table 7). The ratio (PI) of the physico-chemical parameters content in the water relative to their permissible limits numerically expresses how each physicochemical parameter contributes to the overall pollution of the water body. A high ratio identifies the physico-chemical parameter as a principal pollutant in the water body. The obtained PI values for the various water quality indicators showed that the concentration levels of electrical conductivity, sulphate and chloride did not contribute to the water pollution. Water temperature and phosphate content were slight pollutants; pH, biochemical oxygen demand, copper and zinc—medium pollutants; nitrate—heavy pollutant; while turbidity, iron, lead and cadmium seriously contributed to the water pollution (Fig. 8). Increase in lead, turbidity, iron and cadmium is linked to the mixed constituents of municipal waste water discharged into the reservoir. Also, turbidity, BOD and nitrate pollution index values is reflective of how human activities and land use within the reservoir catchment area—farming, cattle grazing on the river banks, decay of organic matter, etc. have impacted on the water quality. The elevated levels of iron, lead and cadmium is an indication of the potential human health risk associated with the domestic consumption of the water in its present state by inhabitants within the catchment area.
The main objectives of water resource management and contamination control are the classification of water quality and the identification of principal pollutants, which will impact on treatment steps required to ensure safe water, and preventive action to improve water quality. The water quality of Ikpoba reservoir have been classified as severely polluted, with concentration levels of pH, BOD, copper, zinc, nitrate, turbidity, lead, iron and cadmium identified as contributing significantly to the surface water pollution. Therefore, the establishment of vegetative screens to curtail the degree of water turbidity and erosion control; desilting of the reservoir to prolong its functionality, periodic monitoring of land use activities within the reservoir catchment area to ascertain the type and constituents of effluents which is discharged directly or indirectly into the reservoir is recommended to curtail the further deterioration of the water quality. Also, the utilization of surface water bodies for the drainage of flood water has impacted negatively on the aquatic ecosystem. There is need for the consideration of other options such as establishment of retention pools for flood water management. This will reduce the amount of pollutants entering the reservoir. At present, the provision of potable water supply to the inhabitants of Benin City is inoperative due to the comatose state of water processing and supply infrastructure at the reservoir. Achieving the sustainable development goal of provision of potable water for human consumption, necessitates the need for the State Government to review its water resources management policies and make the requisite financial investments to revamping the Ikpoba reservoir.
Conclusion
The water quality of Ikpoba reservoir have been evaluated using various approaches/methods which have provided useful information about the water suitability for human consumption and pollution status. The comparism of physico-chemical parameters’ content in water with their respective permissible limits, showed that turbidity, iron, copper, lead and cadmium levels exceeded their respective permissible limits; while water temperature, pH, dissolved oxygen and biochemical oxygen demand were below their minimum permissible value for surface water. The WQI value of 207.37 (WQI > 50) indicate that the water is unsuitable for human consumption and use for other domestic purposes. The CPI value of 4.40 for Ikpoba reservoir indicates that the freshwater body is severely polluted (CPI > 2.01), with turbidity, lead, iron and cadmium identified as contributing significantly to the water pollution. Natural and anthropogenic activities with the reservoir catchment area were identified as both point sources and non-point sources of the principal pollutants. This study has further demonstrated the effectiveness of the WQI and CPI as coherent tools for water quality monitoring and evaluating the influence of pollutants from human and land-use activities on the water quality. The need for the regulation of effluents discharge into the reservoir, human activities and land use within the catchment area is recommended to prevent further deterioration in water quality.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Abbasi, T., & Abbasi, S. A. (2012). Water Quality Indices. Elsevier.
Ademoroti, C. M. A. (1996). Standard Methods for Water and Effluents Analysis (pp. 186–200). Foludex Press Ltd.
Akter, T., Jhohura, F. T., Akter, F., Chowdhury, T. R., Mistry, S. K., Dey, D., Barua, M. K., Islam, M. D., & Rahman, M. (2016). Water Quality Index for measuring drinking water quality in rural Bangladesh: A cross-sectional study. Journal of Health, Population and Nutrition, 35, 4–8.
Anyanwu, E. D. (2012). Physico-chemical and some trace metal analysis of Ogba River, Benin City, Nigeria. Jordan Journal of Biological Sciences, 5, 47–53.
APHA/AWWA/WEF. (2012). Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
Brion, G., Brye, K., Haggard, B., West, C., & Brahana, J. (2011). Land-use effects on water quality of a first-order stream in the Ozark Highlands, mid-southern United States. River Research and Applications., 27(6), 772–790.
Egun, N. K., & Ogiesoba-Eguakun, C. U. (2018). Physico-Chemical and Water Quality Index Analysis of the Okhuaihe River, Edo State Nigeria. African Journal of Aquatic Science, 43(4), 345–351.
Ezugwu, C. N. (2013). Sediment Deposition in Nigeria Reservoirs: Impacts and Control Measures. Innovative Systems Design and Engineering, 4(15), 54–60.
U.S. Geological Survey. (2018). Lakes and reservoirs—guidelines for study design and sampling: U.S. Geological Survey Techniques and Methods, book 9, chap. A10. https://doi.org/10.3133/tm9a10.
Gulbaz, S., & Kayeyzilmaz-Alhan, C. (2012). Impact of land use/cover changes on water quality and quantity in a calibrated hydrodynamic model. In Paper presented at the Conference 10th International Congress on Advances in Civil Engineering Middle East Technical University. Ankara.
Gyamfi, C., Boakye, R., Awuah, E., & Anyemedu, F. (2013). Application of the Ccme-Wqi Model in Assessing the Water Quality of the Aboabo River, Kumasi-Ghana. Journal of Sustainable Development, 6(10), 1–7.
Houser, J. N., & Richardson, W. B. (2010). Nitrogen and phosphorus in the Upper Mississippi River: Transport, processing, and effects on the river ecosystem. Hydrobiologia, 640(1), 71–88.
Idowu, E. O., Ugwumba, A. A. A., Edward, J. B., & Oso, J. A. (2013). Study of the Seasonal Variation in the PhysicoChemical Parameters of a Tropical Reservoir. Greener Journal of Physical Sciences, 3(4), 142–148.
Idris, I., & Mirac, A. (2018). Water quality assessment for Elmali Stream and Karacomak stream using the comprehensive pollution Index (CPI) in Karacomak Watershed, Kastamonu, Turkey. Fresenius Environmental Bulletin, 27(10), 7031–7038.
Institute for Global Environmental Strategies (IGES). (2015). WEPA Outlook on Water Environmental Management in Asia 2015. http://www.wepa-db.net/activities_201504outlook2015.htm.
Ji, X., Dahlgren, R. A., & Zhang, M. (2016). Comparison of seven water quality assessment methods for the characterization and management of highly impaired river systems. Environmental Monitoring and Assessment., 188(1), 1–16.
Li, Q., Zhou, Y., & Li, P. (2010). Evaluation of water quality status of coastal water in Sanya Bay in sunmmer. Transactions of Oceanology and Limnology, 3, 100–106.
Oboh, I. P., & Agbala, C. S. (2017). Assessment of the quality of River Siluko, Edo State, Southern Nigeria using water quality index (WQI). African Journal of Aquatic Science, 42(3), 279–286.
Omoigberale, M. O., & Ogbeibu, A. E. (2007). Assessing the environmental impacts of oil exploration and production on the water quality of Osse River, southern Nigeria. Global Journal of Environmental Sciences, 6, 1–13.
Omran, E.-S. (2012). A proposed model to assess and map irrigation water well suitability using geospatial analysis. Water, 4(3), 545–567.
Oram, B. (2014). Water quality terms glossary. https://www.water-resaerch. Accessed 4 Apr 2017.
Standard Organization of Nigeria (SON). (2007). Nigerian Standard for Drinking Water Quality (NSDWQ) (pp. 15–17). Nigerian Industrial Standard NIS 554, Standard Organization of Nigeria.
Tao, T., Yujia, Z., & Kai, H. (2011). Water Quality Analysis and Recommendations through Comprehensive Pollution Index Method-Take Qilu Lake as Example. Management Science and Engineering, 5(2), 95.
Tawari-Fufeyin, P., & Ekaye, S. A. (2007). Fish species diversity as indicator of pollution in Ikpoba River, Benin City, Nigeria. Reviews in Fish Biology and Fisheries, 17, 21–30.
Tyagi, S., Sharma, B., Singh, P., & Dobhal, R. (2013). Water quality assessment in terms of Water Quality Index. American Journal of Water Resources, 1, 34–38.
Usman, L. U., Namadi, S., & Nafiu, S. A. (2017). Effects of Physico-Chemical Parameters on the Composition and Abundance of Phytoplankton in Ajiwa Reservoir Katsina State, North Western Nigeria. Bayero Journal of Pure and Applied Science, 10(2), 16–24.
Wangboje, O. M., & Ekundayo, O. T. (2013). Assessment of Heavy Metals in Surface Water of the Ikpoba Reservoir, Benin City, Nigeria. Niger J Technol (NIJOTECH), 32(1), 61–66.
Xu, S., Wang, T., & Hu, S. (2015). Dynamic Assessment of Water Quality Based on a Variable Fuzzy Pattern Recognition Model. International Journal of Environmental Research and Public Health, 12(2), 2230–2248.
Yan, C. A., Zhang, W., Zhang, Z., Liu, Y., Deng, C., & Nie, N. (2015). Assessment of water quality and identification of polluted risky regions based on field observations & GIS in the Honghe river watershed. China. Plos One., 10(3), e0119130.
Zhang, L. (2017). Different methods for the evaluation of surface water quality: The Case of the Liao River, Liaoning Province, China. International Review for Spatial Planning and Sustainable Development, 5(4), 4–18.
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
EGUN, N.K., OBOH, .P. Surface water quality evaluation of Ikpoba reservoir, EDO State, Nigeria. Int J Energ Water Res 6, 509–519 (2022). https://doi.org/10.1007/s42108-021-00139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-021-00139-z