Abstract
Drought stress limits the oilseed crops productivity in semi-arid areas. To alleviate drought stress effects during seed-filling stage, the effect of foliar application of different Zn concentration (0, 0.6 and 1.2 kg ha−1) on five safflower genotypes was investigated in a 2-year (2015–2016 and 2016–2017) field experiments. The results showed that supplemental Zn (1.2 kg ha−1) significantly increased drought resistance by enhancement in proline (20%) and carbohydrate accumulation (4.3%), relative water content (2.4%) and chlorophyll content (3.8%) in all studied safflower genotypes. The induced improve in safflower’s physiological traits achieved in the Zn supplemented treatment resulted in a significant increase in genotypes yield and its components. Moreover, Zn foliar application significantly reduced the drought adverse effect on oil yield and improved the unsaturated fatty acids content. Finally, Zn foliar application can represent an effective means to mitigate the adverse effects of drought stress on growth and the yield of safflower genotypes in water shortage condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought stress will increase in intensity in the future as a result of climate change, mainly due to decreases in regional precipitation but also because of increasing evaporation driven by global warming (Lobell et al. 2008; Sadeghi and Delaviz 2016). Water stress has severe adverse effect on plant physiology, growth and productivity. For this reason, need to crops with higher drought resistance strongly rising (Sadeghi and Robati 2015; Sadeghi and Rostami 2017). Safflower (Carthamus tinctorius L.) is a deep rooted drought tolerant crop that originated in desert environments of the Middle East and mainly grown as oilseed in the arid and semi-arid regions of the world (Hojati et al. 2011b; Singh et al. 2016). Nutritional edible oil contains the unsaturated fatty acids whereas, high oleic safflower cultivars contain over 85% oleic acid and the high linoleic safflower cultivars contain 87–89% linoleic acid in their storage oil (Li and Mündel 1996).
The importance of oil crops such as safflower has increased in recent years. Due to that safflower, can resistance in drought condition without reduction in yield, it can be a promising alternate crop in dryland agro-ecosystems (Kar et al. 2007). Plant have developed a wide variety of drought tolerance mechanisms in morphological and physiological levels (Ghanaatiyan and Sadeghi 2017). There are wide variations among the safflower genotypes with respect to seed and oil yields at drought stress condition (Yeilaghi et al. 2012). Genetic variations among genotypes with various drought tolerance have been reported in different crops (Kauser et al. 2006). Therefore, safflower cultivation constitutes a more profitable crop for the farmers in some countries especially arid and semi-arid areas compared to other crops (Yau 2004).
Zinc, as one of the essential microelements in crop plant, plays a crucial role in resistance to drought stress (Khan et al. 2003; Yavas and Unay 2016). In addition, this element is also very important in maintaining the bio membrane structure and for detoxifying ROS (Rehman et al. 2012). Zinc also plays an important role in the production of biomass (Cakmak 2008), as well as in the nitrogen and carbohydrate metabolism of plants (Pandey 2015). Zinc is required in seed development (Hänsch and Mendel 2009). Moreover, zinc is a ubiquitous micronutrient required as a structural and functional component of many enzymes and proteins (Coleman 1998). The mechanisms for Zn-mediated drought tolerance are still not fully understood, but have been suggested to be involved in the increases of water use efficiency (Karim et al. 2012; Khan et al. 2004), the leaf osmotic potential and transpiration rate changes (Khan et al. 2004; Sadoogh and Shariatmadari 2014), and involvement in modulating biochemical damages by antioxidant enzymes (Upadhyaya et al. 2013). Numerous studies have demonstrated marked increases in seed Zn concentrations due to foliar Zn spray, whereas soil Zn applications and seed priming are less effective (Cakmak et al. 2010; Hussain et al. 2012; Yang et al. 2011). Given that the root absorption capacity of Zn and macronutrients (e.g., N, P, and K) is easily compromised by drought or salinity, particularly during the seed-filling stage (Fernández and Eichert 2009), the simultaneous and effective delivery of these nutrients through foliar applications is of great importance from an economic, agronomic and environmental point of view (Wang et al. 2017).
Drought is very unpredictable among abiotic stresses in terms to occurrence, severity, timing and duration (Anosheh et al. 2011; Ashraf et al. 2005). Hence, the main objective of this study was to evaluate of Zn foliar application as an applied method to alleviate drought stress effects occurring unexpectedly almost in seed-filling stage on yield of different genotypes safflower.
Materials and Methods
Site Description
This study consisted of two field experiments was conducted during 2016–2017 and 2017–2018 at experimental field of seed and plant improvement institute (SPII) in Alborz province, Iran (35°49′12″N, 51° 06′33″E, 1321 m). The study location is characterized by a semi-arid climate with an annual average precipitation of 243 mm, and the annual mean maximum and minimum temperatures of 30 and 1 °C, respectively. Local climatic data of growing seasons are presented in Fig. 1. Soil type of the study site was clay-loam, and soil pH, electrical conductivity (EC) was 7.2 and 2.2, respectively.
Field Preparation and Planting
The experiment field was disked and ploughed before planting to incorporate residue and to form a seedbed each year. Two weeks before planting, soil and water samples were taken in order to determine the physical and chemical properties. A composite soil sample was collected at a depth of 0–30 cm. After air drying, the soil was passed through a 2 mm sieve to allow the measurement of a set of standard soil characteristics (soil texture, pH, electrical conductivity, organic carbon content, total nitrogen, available phosphorus and available potassium) (Guo 2009) (Table 1).
The experimental design was a factorial split plot in a randomized complete block with three replications. The irrigation was conducted at two levels routine irrigation (blank) and the elimination of watering after late of flowering stage (drought stress) in main plots. Subplots were 30 treatments in number and consisted of a factorial combination of five safflower genotypes (Soffe, Goldasht, Golmehr, Padideh and Parnian), and three foliar Zn applications including 0, 0.6 and 1.2 kg ha−1 Zn (0.5% surfactant-containing solution). The foliar application was applied with a pressurized backpack sprayer (12 l capacity) calibrated to deliver 1000 l ha−1 of spray solution. Prior to seeding according to results of soil analysis, half of urea and total of super phosphate were broadcasted and incorporated into the soil. The other Half of urea was applied when stem elongation began. Safflower seeds were disinfected with fungicide prior to planting and sown on 30 September 2016 and 1 October 2017 in six rows at 5 cm intervals (row length: 4 m, row distance: 30 cm). Between all main plots, a 2 m alley was kept to eliminate all influence of lateral water movement. In sampling, 50 cm from the side of plots was removed as marginal effect. Physiological parameters was measured by selecting specified leaves from five plants per plot under fair weather condition. To evaluate of yield components also at maturity stage, whole plants harvested in July.
Agronomic Measurements
At maturity in July of 2017 and 2018 plants were cut at ground level then oven dried at 70 °C until a constant weight. Seeds were separated from straw by crushing. Seed and straw (stem plus leaf, biological yield) were weighted by a balance and harvest index was computed as the ratio of seed yield to the total plant biomass.
Yield components were number of heads per plant, number of seeds per primary and secondary heads, number of branches per plant, number of seeds per plant which measured by counting and 1000-seeds weight measured with an analytical balance (0.001).
Physiological Measurements
Relative Water Content
Three young and developed leaves of each plot were sampled at noon and the RWC of the leaf samples was calculated using the equation:
where FW represented the fresh weight of the sample leaf, TW their overnight turgid weight and DW their weight after oven drying (Cornic 1994).
Chlorophyll Measurements
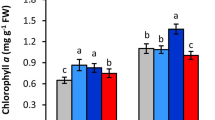
Chlorophyll readings were taken with a hand-held dual-wavelength meter (SPAD-502, Minolta, Japan). For each plot the 30 youngest fully expanded leaves per plot were used when the plants were at pod development stage.
Free Proline Content
The free proline content was extracted from 0.5 g leaf samples in 3% (w/v) aqueous sulphosalycylic acid and estimated using ninhydrin reagent according to the method described by Bates et al. (1973). The absorbance of fraction with toluene aspired from the liquid phase was read at 520 nm. Proline concentration was determined using a calibration curve and expressed as μmol proline g−1 FW.
Oil Content and Oil Yield
Oil content was determined by NMR spectrometer at 25 °C, fitted with a permanent magnet of 0.23 T (9 MHz for 1H) and a 13 mm × 30 mm catheter of useful area, using the Condor IDE software with CPMG pulse sequence with Qdamper (Colnago et al. 2011), expressed on a dry basis (DB%). Oil yield was determined by multiplying seed yield by oil content, hereby obtaining yielding oil in kg ha−1.
Linoleic, Oleic and Palmitic Acids Content
The fatty acid composition of the oil samples was determined by gas chromatography. The oil sample of each experimental unit (plot) was converted to its fatty acid methyl esters (FAME). Oil samples (0.2 ml) were dissolved in hexane and trans esterified with sodium methylate (0.1 M). Analyses of FAMEs were carried out using a Varian CP-3800 model gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and a fused silica capillary column (CP-Sil88, 50 m, 0.25 mm, 0.2 µm). The samples were injected in split mode (split ratio 1:40). The initial oven temperature was set at 150 °C for 1 min, elevated at a rate of 5 °C min−1 to 190 °C for 3 min, and then ramped at 5 °C min−1 to the final 240 °C for 8 min. The injector temperature was set at 250 °C and the detector temperature was set at 280 °C. Nitrogen with at a flow rate of 1.5 ml min−1 was used as the carrier gas. Peak identification was performed by comparing the relative retention times with those of a commercial standard mixture of FAME. The fatty acid content of palmitic (C16:0), oleic (C18:1) and linoleic (C18:2) were determined using a computing integrator and showed as the percentage of the oil.
Statistical Analysis
Routines implemented in the SAS statistical analysis software v9.1 package (SAS Institute, Cary, NC, USA) were used to derive analyses of variance. The least significant difference (LSD) test was used to compare treatment means, applying a p threshold of 0.05 to declare significance. Moreover, Microsoft Office Excel and R software were used to draw the figures.
Results
Effect of Foliar Application of Zn on Physiological Parameters in Drought Condition
The results showed that agronomic and physiological parameters of safflower genotypes significantly affected by climatic conditions in two growing season. Improved agronomic traits of genotypes were obtained in second year (Table 2), which can be the result of more precipitation in May and April (normally safflower flowering stage occurs in this period) and a higher average temperature of the second year.
The results of physiological assessment of different safflower genotypes treated by Zn in drought stress condition for 2-year experiment have been shown in Tables 3 and 4. The results a significantly reduction in RWC in stress condition for all genotypes in both experiment. Result of first year experiment showed that the foliar applied Zn significantly increased relative water content only in Soffe, Padideh and Parnian genotypes in drought stress condition and highest increase was related to treatment by Zn in amount of 1.2 kg ha−1 (Table 3). There was no significant effect of Zn on this trait in control condition in both experiments. Relative water content affected significantly by foliar application of Zn in drought stress condition just in Parnian genotype in second year (Table 4). Drought- enhanced proline accumulation observed in all genotypes and in both experiment. Interaction of drought and Zn foliar application led to highest proline accumulation in all genotypes so that most increase was related to Parnian genotype from 9.53 into 12.7 µmol g−1 FW (Table 3). In the second year experiment proline accumulation due to Zn treatment observed in all genotypes expect of Soffe (Table 4). Carbohydrate content in the plants grown in drought condition was considerably higher than in control plants in the both experiment. Foliar application of Zn noticeably raised carbohydrate content in all genotypes except of Goldasht in first experiment (Table 3) and Parnian in second experiment (Table 4). The highest carbohydrate accumulation in all genotypes and both experiment was achieved in with zinc treatment at 1.2 kg ha−1. Highest amount of carbohydrate content in response to 1.2 kg ha−1 Zn treatment observed in Parnian genotype in amount 106.6 and 142.1 µmol g−1 FW in first and second experiment, respectively. The SPAD value decreased significantly by drought stress in all genotypes. An increase trend observed in SPAD value measured in treated plants by Zn in both experiments. In the first year experiment there was no significant difference between Zn foliar concentrations treatments in increase SPAD value while in the second experiment higher SPAD value observed in Parnian genotype treated by 1.2 kg ha−1 compare to 0.6 kg ha−1.
Effect of Foliar Application of Zn on Yield Components in Drought Condition
Results of yield components of different safflower genotypes treated by 0.6 and 1.2 kg ha−1 Zn in drought condition represented in Figs. 2 and 3 for 2 years of field experiment as fold changes compare to control condition (calculated using formula: log2 (drought/control). Results showed that foliar application of Zn significantly alleviated effects of drought on total seed number (TSN) in both years so that it led to lower reduction of this trait in drought condition in all studied genotypes. Also highest increasing effect of Zn on TSN was 0.12-fold increase in this trait observed in Parnian genotypes harvested in drought condition compare to control plants (untreated with Zn) in the first year experiment. Drought stress significantly decreased thousand seed weight (TSW) in all genotypes expect of Golmehr in first year experiment (Fig. 2c) and Goldasht in second year experiment (Fig. 3b). Drought increased significantly total seed weight of Padideh genotype in first year experiment (Fig. 2d). Zn treatment in amount of 1.2 kg ha−1 reduced drought stress effects on TSW in Goldasht, Golmehr and Padideh genotypes harvested in second experiment (Fig. 3b–d). Seed yield reduced significantly in drought condition in Soffe, Golmehr and Parnian genotypes in first year experiment and there was no significant change in this trait in second year experiment. Seed yield was not affected by Zn foliar application in all genotypes expect of Golmehr which treatment by 1.2 kg ha−1 led to significant increase in this trait compare to control (Fig. 2c). Biological yield was found to be significantly decrease in drought stress condition in all genotypes in first year experiments, except Padideh genotype which positively correlated with drought (Fig. 2d). Drought had no significant effect on biological yield of Golmehr, Padideh and Parnian genotypes in second year experiment (Fig. 3c–e). Zn treatments were effective to reduce harmful effects of drought stress on biological yield of Soffe in the first year experiment (Fig. 2a) and Soffe, Goldasht, Golmehr and Padide genotypes in the second year experiment (Fig. 2a–d). Drought-caused increased biological yield of Padideh genotype affected negatively by foliar application of Zn in the first year experiment (Fig. 2d). Changes in harvest index (HI) as the ratio of seed yield to the total plant biomass in drought condition was different among various genotypes so that in Goldasht and Parnian genotypes increased in stress condition of the first year experiment (Fig. 2b, c). Zn treatment led to increase HI in Goldasht, Golmehr and Padideh genotype in drought condition compare to non-application of Zn in the first year experiment (Fig. 2b–d). Oil percentage significantly reduced in drought condition in Golmehr and Parnian genotypes in first year experiment (Fig. 2c, e) and also in Soffe genotype in second year experiment (Fig. 3a). In contrast Goldasht genotype showed significant enhancement in oil percentage in drought condition in the first year experiment (Fig. 2b). Interaction of drought stress and Zn foliar application in the first year experiment showed that oil percentage of Golmehr and Padideh genotypes positively affected by Zn 0.6 and 1.2 kg ha−1 respectively (Fig. 2c, d), while it reduced in Goldasht and Parnian compare to non-application of Zn (Fig. 2b, e). In the second year experiment Zn treatment led to significant increase in oil percentage in Golmehr too (Fig. 3c). Oil yield affected significantly by drought and Zn foliar application in some genotypes just in the first year experiment. No significant change in this trait observed in second year experiment. Drought led to significant reduction in oil yield of Soffe, Golmehr and Parnian genotype (Fig. 2a, c, e), and Zn foliar application reduced significantly drought effect on oil yield of Golmehr genotype (Fig. 2c).
Logarithmic ratio of yield components (TSN total seed number, TSW thousand seed weight, SY seed yield, Bio Y biological yield, HI harvest index, OP oil percentage, OY oil yield) of different safflower genotypes (a Soffe, b Goldasht, c Golmehr, d Padideh and e Parnian) in drought condition to control in first year experiment. Asterisk indicates significantly change (P < 0.05) in compare to control. The different lowercase letters indicate significant difference (P < 0.05) between Zn treatments
Logarithmic ratio of yield components (TSN total seed number, TSW thousand seed weight, SY seed yield, Bio Y biological yield, Hi harvest index, OP oil percentage, OY oil yield) of different safflower genotypes (a Soffe, b Goldasht, c Golmehr, d Padideh and e Parnian) in drought condition to control in second year experiment. Asterisk indicates significantly change (P < 0.05) in compare to control. The different lowercase letters indicate significant difference (P < 0.05) between Zn treatments
Effect of Foliar Application of Zn on Fatty Acid Composition in Drought Condition
Change in palmitic acid (C16:0), linoleic acid (C18:2) and oleic acid (C18:1) as major fatty acid of seed oil extracted from seeds of five safflower genotypes treated by Zn foliar application in drought condition in the first year experiment summarized in Fig. 4. Results showed that drought led to significant reduction in linoleic acid content in all studied safflower genotypes. Also drought stress caused a significant increase in oleic acid content in Soffe, Goldasht and Parnian genotypes. In contrast to drought-caused enhancement in palmitic acid content in Golmehr, Padideh and Parnian genotypes, significant decrease observed in this fatty acid content in oil of Soffe and Goldasht genotypes. Zn foliar application had various significant effects on fatty acid composition of all genotypes. Significant increase and decrease in linoleic acid content observed in Soffe and Padideh genotypes treated by Zn in drought condition respectively. There was no significant effect caused by Zn on linoleic acid content of other genotypes. Foliar application of Zn in stress condition resulted in oleic acid significant enhancement in Goldasht, Golmehr and Padideh genotypes. In contrast treatment by 0.6 and 1.2 kg ha−1 Zn in stress condition led to significant reduction in oleic acid content to 14.2 and 15.8% in Soffe and Parnian genotypes respectively. Relative to the effect of water deficiency, a progressive decrease in palmitic acid content from 7.5 to 6.4% in Goldasht and from 6.8 to 5.6% in Padideh genotypes observed in treated plants by 1.2 kg ha−1 of Zn. Also Zn-caused reduction in palmitic acid from 6.7 to 6.3 and 6.8 to 6.6 respectively observed in Soffe and Golmehr genotypes treated by 0.6 kg ha−1 of Zn.
Effect of Zn foliar application (Zn1: control, Zn2: 0.6 and Zn3: 1.2 kg ha−1) on linoleic, oleic and palmitic acids content of different safflower genotypes (a Soffe, b Goldasht, c Golmehr, d Padideh and e Parnian) in control (C) and drought (S) conditions. The different lowercase letters indicate significant difference (P < 0.05) between Zn treatments
Discussion
Results of physiological assessment showed that drought stress increased significantly proline and carbohydrate content in all safflower genotypes in both years experiment. Moreover, a reduction in relative water content and SPAD value were recorded in drought condition. In biochemical responses, proline is an important osmolyte involved in the control of osmotic pressure in the cells (Errabii et al. 2007; Gandonou et al. 2006; Patade et al. 2008). Also increase in carbohydrate content in drought condition reported in previous works (Valliyodan and Nguyen 2006). Decrease in RWC and SPAD value in safflower and Gossypium respectively reported under drought condition (Hojati et al. 2011a; Wu et al. 2015). Results showed that foliar application of Zn alleviated harmful effects of drought stress on yield of safflower genotypes. Zn is an essential plant nutrient and plays an important role in plant growth (Cakmak 2008). Previous research reported that the biochemical responses related to photosynthesis are regulated by Zn, such as repairs PSII process and integrates the structure of Rubisco (Tsonev et al. 2012). Supplemental Zn significantly enhanced SPAD value in all genotypes in both years experiment. It has been reported that zinc may be required for chlorophyll production (Cakmak 2008; Kaya and Higgs 2002; Pandey 2015). It has been reported that Zn application under adverse conditions, such as salt stress and cadmium toxicity, could increase chlorophyll a and b content and photosynthesis rate, and improve plant growth (Hassan et al. 2005; Tavallali et al. 2010). Zn also plays main roles in regulation of protein synthesis and carbohydrates metabolism (Mousavi 2011). Our results also indicated the significant increase in carbohydrate content resulted from Zn application in all genotypes. Proline as an osmo-protectant also increased by treatment safflower genotypes by Zn. Greater accumulation of proline due to Zn application can help in maintaining water content, prevent membrane distortion, and acts as a hydroxyl radical scavenger (Xu et al. 2009). Higher RWC observed in some treated genotypes by Zn also indicated role of Zn in increase resistance to drought. Apposite of resistance to drought, yield reduction of safflower due to drought stress has been reported in previous works (Ashrafi and Razmjoo 2010).
Results of current study showed that drought caused reduction of seed yield components in different safflower genotypes in 2 years’ experiments. Other researchers have also pointed out the harmful effect of drought on total seed number, thousand seed weight and seed yield of safflower (Abd El-Lattief 2013; Ghamarnia et al. 2010; Istanbulluoglu et al. 2009; Movahhedy-Dehnavy et al. 2009). Reduce of safflower seed yield under water stress has also been reported too by Pourdad (2008). Yield reduction in low water availabilities due to low biomass production is associated with decreased photosynthesis in these conditions (Pinheiro and Chaves 2011). Translocation of assimilates to the seed is a crucial physiological process during the filling phase of safflower seeds, especially under drought. Similar to plant seed yield, biological yield was significantly lower in drought condition in most genotypes. It has been reported that during seed filling stage, the drought stress exerted destructive consequences in relative water content, osmotic adjustment and leaf weight in five safflower genotypes (Eslam 2011). Genotype variation for HI was due to their different growth habits. Genotype with higher HI were more efficient in partitioning their biomass into seed yield. Change in fatty acid composition of oilseed crops in stress condition reported previously (Nazari et al. 2017; Reiahisamani et al. 2018). Our results also showed change in linoleic, oleic and palmitic acids content in most genotypes, which was different between genotypes. Linoleic acid content reduced significantly under drought condition in all genotypes. Under water-stress conditions, earlier maturity of plants will result in a shorter period of seed filling and as a consequence a shorter time span for conversion of oleic to linoleic acid, which can be the main reason for relative decline of linoleic acid content under drought stress (Nazari et al. 2017). Moreover, the results showed an increase in TSN and TSW caused by Zn foliar application in most of genotypes in both years. Foliar Zn application has also been shown to improve safflower seed yield and seedling dry weight grown under water deficit condition (Movahhedy-Dehnavy et al. 2009). Changes in fatty acid content in response to Zn foliar application was different among various genotypes and there was not a constant trend in the observations.
Conclusion
Drought stress had a negative effect on the growth and yield of safflower. Supplemental Zn dramatically increased the safflower resistance to drought stress. Therefore, a conclusion cloud be presumed that the enhanced proline and carbohydrate content in order to osmotic adjustment and having higher relative water content, protecting photosynthetic pigments and continue photosynthetic activity, resulted in the increase of yield in the Zn treatment in safflower genotypes under drought stress. Taken together, the present research clearly demonstrated that supplemental Zn could enhance the drought tolerance in safflower.
References
Abd El-Lattief, E. A. (2013). Safflower yields and water use efficiency as affected by irrigation at different soil moisture depletion levels and plant population density under arid conditions. Archives of Agronomy and Soil Science,59, 1545–1557. https://doi.org/10.1080/03650340.2012.735769.
Anosheh, H. P., Sadeghi, H., & Emam, Y. (2011). Chemical priming with urea and KNO3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress. Journal of Crop Science and Biotechnology,14, 289–295. https://doi.org/10.1007/s12892-011-0039-x.
Ashraf, M., Harris, P., & Harris, P. (2005). Use of genetic engineering and molecular biology approaches for crop improvement for stress environments. Abiotic Stress. https://doi.org/10.1201/9781482293609-11.
Ashrafi, E., & Razmjoo, K. (2010). Effect of irrigation regimes on oil content and composition of safflower (Carthamus tinctorius L.) cultivars. Journal of the American Oil Chemists Society,87, 499–506. https://doi.org/10.1007/s11746-009-1527-8.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil,39, 205–207. https://doi.org/10.1007/BF00018060.
Cakmak, I. (2008). Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant and Soil,302, 1–17. https://doi.org/10.1007/s11104-007-9466-3.
Cakmak, I., Kalayci, M., Kaya, Y., Torun, A. A., Aydin, N., Wang, Y., et al. (2010). Biofortification and localization of zinc in wheat grain. Journal of Agriculture and Food Chemistry,58, 9092–9102. https://doi.org/10.1021/jf101197h.
Coleman, J. E. (1998). Zinc enzymes. Current Opinion in Chemical Biology,2, 222–234. https://doi.org/10.1016/S1367-5931(98)80064-1.
Colnago, L. A., Azeredo, R. B. V., Marchi Netto, A., Andrade, F. D., & Venâncio, T. (2011). Rapid analyses of oil and fat content in agri-food products using continuous wave free precession time domain NMR. Magnetic Resonance in Chemistry,49, S113–S120. https://doi.org/10.1002/mrc.2841.
Cornic, G. (1994). Drought stress and high light effects on leaf photosynthesis. Photoinhibition of Photosynthesis, 297–313. https://ci.nii.ac.jp/naid/10006109918/. Accessed 23 Nov 2018.
Errabii, T., Gandonou, C. B., Essalmani, H., Abrini, J., Idaomar, M., & Skali Senhaji, N. (2007). Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiologiae Plantarum,29, 95–102. https://doi.org/10.1007/s11738-006-0006-1.
Eslam, P. (2011). Evaluation of physiological indices for improving water deficit tolerance in spring safflower. Journal of Agricultural Science and Technology, 13, 327–338. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Evaluation+of+physiological+indices+for+improving+water+deficit+tolerance+in+spring+safflower.+J+Agric+Sci+Technol+&btnG. Accessed 23 Nov 2018.
Fernández, V., & Eichert, T. (2009). Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. CRC Critical Reviews in Plant Sciences,28, 36–68. https://doi.org/10.1080/07352680902743069.
Gandonou, C. B., Errabii, T., Abrini, J., Idaomar, M., & Senhaji, N. S. (2006). Selection of callus cultures of sugarcane (Saccharum sp.) tolerant to NaCl and their response to salt stress. Plant Cell Tissue and Organ Culture,87, 9–16. https://doi.org/10.1007/s11240-006-9113-3.
Ghamarnia, H., Environ, S., & Sepehri, J. (2010). Different irrigation regimes affect water use, yield and other yield components of safflower (Carthamus tinctorius L.) crop in a semi-arid region of Iran. Journal of Food, Agriculture and Environment, 8, 590–593. https://www.researchgate.net/profile/Houshang_Ghamarnia/publication/234841399_Different_irrigation_regimes_affect_water_use_yield_and_other_yield_components_of_safflower_Carthamus_tinctorius_L_crop_in_a_semi-arid_region_of_Iran/links/56852df008ae19758394e. Accessed 23 Nov 2018.
Ghanaatiyan, K., & Sadeghi, H. (2017). Differential responses of chicory ecotypes exposed to drought stress in relation to enzymatic and non-enzymatic antioxidants as well as ABA concentration. Journal of Horticultural Science and Biotechnology,92, 404–410. https://doi.org/10.1080/14620316.2017.1286235.
Guo, M. (2009). Soil sampling and methods of analysis. https://doi.org/10.2134/jeq2008.0018br.
Hänsch, R., & Mendel, R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Current Opinion in Plant Biology,12, 259–266. https://doi.org/10.1016/J.PBI.2009.05.006.
Hassan, M. J., Zhang, G., Wu, F., Wei, K., & Chen, Z. (2005). Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. Journal of Plant Nutrition and Soil Science,168, 255–261. https://doi.org/10.1002/jpln.200420403.
Hojati, M., Modarres-Sanavy, S. A., Karimi, M., & Ghanati, F. (2011a). Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiologiae Plantarum,33, 105–112. https://doi.org/10.1007/s11738-010-0521-y.
Hojati, M., Modarres-Sanavy, S. A. M., Karimi, M., & Ghanati, F. (2011b). Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiologiae Plantarum,33, 105–112. https://doi.org/10.1007/s11738-010-0521-y.
Hussain, S., Maqsood, M. A., Rengel, Z., & Aziz, T. (2012). Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant and Soil,361, 279–290. https://doi.org/10.1007/s11104-012-1217-4.
Istanbulluoglu, A., Gocmen, E., Gezer, E., Pasa, C., & Konukcu, F. (2009). Effects of water stress at different development stages on yield and water productivity of winter and summer safflower (Carthamus tinctorius L.). Agricultural Water Management,96, 1429–1434. https://doi.org/10.1016/J.AGWAT.2009.04.004.
Kar, G., Kumar, A., & Martha, M. (2007). Water use efficiency and crop coefficients of dry season oilseed crops. Agricultural Water Management,87, 73–82. https://doi.org/10.1016/J.AGWAT.2006.06.002.
Karim, M. R., Zhang, Y.-Q., Zhao, R.-R., Chen, X.-P., Zhang, F.-S., & Zou, C.-Q. (2012). Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. Journal of Plant Nutrition and Soil Science,175, 142–151. https://doi.org/10.1002/jpln.201100141.
Kauser, R., Athar, H. U. R., & Ashraf, M. (2006). Chlorophyll fluorescence: A potential indicator for rapid assessment of water stress tolerance in Canola (Brassica napus L.). Pakistan Journal of Botany, 38(5 Spec issue), 1501–1509. https://www.escholar.manchester.ac.uk/uk-ac-man-scw:158076. Accessed 10 Feb 2019.
Kaya, C., & Higgs, D. (2002). Response of tomato (Lycopersicon esculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Scientia Horticulturae (Amsterdam),93, 53–64. https://doi.org/10.1016/S0304-4238(01)00310-7.
Khan, H. R., McDonald, G. K., & Rengel, Z. (2003). Zn fertilization improves water use efficiency, grain yield and seed Zn content in chickpea. Plant and Soil,249, 389–400. https://doi.org/10.1023/A:1022808323744.
Khan, H. R., McDonald, G. K., & Rengel, Z. (2004). Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arietinum L.). Plant and Soil,267, 271–284. https://doi.org/10.1007/s11104-005-0120-7.
Li, D., & Mündel, H. H. (1996). Safflower. Carthamus tinctorius L. Promoting the conservation and use of underutilized and neglected crops. 7. Rome: Institute of Plant Genetics and Crop Plant Research (p. 83).
Lobell, D. B., Burke, M. B., Tebaldi, C., Mastrandrea, M. D., Falcon, W. P., & Naylor, R. L. (2008). Prioritizing climate change adaptation needs for food security in 2030. Science,319, 607–610. https://doi.org/10.1126/science.1152339.
Mousavi, S. (2011). Zinc in crop production and interaction with phosphorus. Australian Journal of Basic and Applied Sciences, 5, 1503–1509. https://www.researchgate.net/file.PostFileLoader.html?id=589043c8dc332d7c8f1db4e5&assetKey=AS%3A456500230529029%401485849544613. Accessed 23 Nov 2018.
Movahhedy-Dehnavy, M., Modarres-Sanavy, S. A. M., & Mokhtassi-Bidgoli, A. (2009). Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Industrial Crops and Products,30, 82–92. https://doi.org/10.1016/J.INDCROP.2009.02.004.
Nazari, M., Mirlohi, A., & Majidi, M. M. (2017). Effects of drought stress on oil characteristics of Carthamus species. Journal of the American Oil Chemists Society,94, 247–256. https://doi.org/10.1007/s11746-016-2938-y.
Pandey, R. (2015). Mineral nutrition of plants. In Plant biology and biotechnology (New Delhi: Springer India), pp. 499–538. https://doi.org/10.1007/978-81-322-2286-6_20.
Patade, V. Y., Suprasanna, P., & Bapat, V. A. (2008). Effects of salt stress in relation to osmotic adjustment on sugarcane (Saccharum officinarum L.) callus cultures. Plant Growth Regulation,55, 169–173. https://doi.org/10.1007/s10725-008-9270-y.
Pinheiro, C., & Chaves, M. M. (2011). Photosynthesis and drought: Can we make metabolic connections from available data? Journal of Experimental Botany,62, 869–882. https://doi.org/10.1093/jxb/erq340.
Pourdad, S. (2008). Study on drought resistance indices in spring safflower. Acta Agron. Hungarica,56, 203–212. https://doi.org/10.1556/AAgr.56.2008.2.9.
Rehman, H., Aziz, T., Farooq, M., Wakeel, A., & Rengel, Z. (2012). Zinc nutrition in rice production systems: A review. Plant and Soil,361, 203–226. https://doi.org/10.1007/s11104-012-1346-9.
Reiahisamani, N., Esmaeili, M., Khoshkholgh Sima, N. A., Zaefarian, F., & Zeinalabedini, M. (2018). Assessment of the oil content of the seed produced by Salicornia L., along with its ability to produce forage in saline soils. Genetic Resources and Crop Evolution,65, 1879–1891. https://doi.org/10.1007/s10722-018-0661-2.
Sadeghi, H., & Delaviz, M. (2016). Response of three new Atriplex species (Atriplex spp.) to drought and its recovery. Acta Ecologica Sinica,36, 212–217. https://doi.org/10.1016/j.chnaes.2016.04.010.
Sadeghi, H., & Robati, Z. (2015). Response of Cichorium intybus L. to eight seed priming methods under osmotic stress conditions. Biocatalysis and Agricultural Biotechnology,4, 443–448. https://doi.org/10.1016/j.bcab.2015.08.003.
Sadeghi, H., & Rostami, L. (2017). Changes in biochemical characteristics and Na and K content of caper (Capparis spinosa L.) seedlings under water and salt stress. J. Agric. Rural Dev. Trop. Subtrop.,118, 199–206.
Sadoogh, F., and Shariatmadari, H. (2014). Adjusted nutrition of tomato with potassium and zinc in drought stress conditions induced by polyethylene glycol 6000 in hydroponic culture. Journal of Science and Technology of Greenhouse Culture, 5. https://www.cabdirect.org/cabdirect/abstract/20143245145. Accessed 29 Nov 2018.
Singh, S., Angadi, S. V., Grover, K., Begna, S., & Auld, D. (2016). Drought response and yield formation of spring safflower under different water regimes in the semiarid Southern High Plains. Agricultural Water Management,163, 354–362. https://doi.org/10.1016/J.AGWAT.2015.10.010.
Tavallali, V., Rahemi, M., Eshghi, S., Kholdebarin, B., and Ramezanian, A. (2010). Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L.’Badami’) seedlings. Turkish Journal of Agriculture and Forestry, 34, 349–359. http://journals.tubitak.gov.tr/agriculture/abstract.htm?id=11113. Accessed 30 Nov 2018.
Tsonev, T., Jose, F., & Lidon, C. (2012). Zinc in plants—An overview. http://ejfa.info/322. Accessed 23 Nov 2018.
Upadhyaya, H., Dutta, B. K., & Panda, S. K. (2013). Zinc modulates drought-induced biochemical damages in Tea [Camellia sinensis (L) O Kuntze]. Journal of Agriculture and Food Chemistry,61, 6660–6670. https://doi.org/10.1021/jf304254z.
Valliyodan, B., & Nguyen, H. T. (2006). Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Current Opinion in Plant Biology,9, 189–195. https://doi.org/10.1016/J.PBI.2006.01.019.
Wang, S., Li, M., Liu, K., Tian, X., Li, S., Chen, Y., et al. (2017). Effects of Zn, macronutrients, and their interactions through foliar applications on winter wheat grain nutritional quality. PLoS One,12, e0181276. https://doi.org/10.1371/journal.pone.0181276.
Wu, S., Hu, C., Tan, Q., Li, L., Shi, K., Zheng, Y., et al. (2015). Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium hirsutum). Acta Physiologiae Plantarum,37, 167. https://doi.org/10.1007/s11738-015-1919-3.
Xu, J., Yin, H., & Li, X. (2009). Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Reports,28, 325–333. https://doi.org/10.1007/s00299-008-0643-5.
Yang, X., Tian, X., Gale, W., Cao, Y., Lu, X., & Zhao, A. (2011). Effect of soil and foliar zinc application on zinc concentration and bioavailability in wheat grain grown on potentially zinc-deficient soil. Cereal Research Communications,39, 535–543. https://doi.org/10.1556/CRC.39.2011.4.8.
Yau, S. K. (2004). Yield, agronomic performance, and economics of safflower in comparison with other rainfed crops in a semi-arid, high-elevation Mediterranean environment. Experimental Agriculture, 40, 453–462. https://www.escholar.manchester.ac.uk/uk-ac-man-scw:158076. Accessed 10 Feb 2019.
Yavas, I., and Unay, A. (2016). Effects of zinc and salicylic acid on wheat under drought stress. Journal of Animal and Plant Sciences, 26, 1012–1018. http://www.thejaps.org.pk/docs/v-26-04/16.pdf. Accessed 29 Nov 2018.
Yeilaghi, H., Arzani, A., Ghaderian, M., Fotovat, R., Feizi, M., & Pourdad, S. S. (2012). Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chemistry,130, 618–625. https://doi.org/10.1016/J.FOODCHEM.2011.07.085.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahmani, F., Sayfzadeh, S., Jabbari, H. et al. Alleviation of Drought Stress Effects on Safflower Yield by Foliar Application of Zinc. Int. J. Plant Prod. 13, 297–308 (2019). https://doi.org/10.1007/s42106-019-00055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42106-019-00055-7