Abstract
Aim
The aim of this study is the systematic review and meta-analysis of controlled trial studies to assess the antioxidant effects of selenium (Se) supplementation.
Methods
The systematic review and meta-analysis were performed according to the previously published protocol. The PubMed, Web of Sciences, and Scopus databases were meticulously searched for relevant data, without time or language restriction, up to June 1, 2017. All clinical trials which assessed the effect of Se supplementation on antioxidant markers, including oxidative stress index (OSI), antioxidant potency composite (APC) index, plasma malonaldehyde (MDA), total antioxidant capacity (TAC), antioxidant enzymes (superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT)), and total antioxidant plasma (TAP), were included. The effect of Se supplementation on antioxidant markers was assessed using standardized mean difference (SMD) and 95% confidence interval (CI). The random-effect meta-analysis method was used to estimate the pooled SMD.
Results
In total, 13 studies which assessed the effect of Se supplementation on antioxidant markers were included. The random-effect meta-analysis method showed that Se supplementation significantly increased GPX (SMD = 0.54; 95% CI = 0.21–0.87) and TAC (SMD = 0.39, 95% CI = 0.13, 0.66) levels and decreased MDA levels (SMD = − 0.54, 95% CI = − 0.78, − 0.30). The effect of Se supplementation on other antioxidant markers was not statistically significant (P > 0.05).
Conclusion
The findings showed that Se supplementation might reduce oxidative stress by increasing TAC and GPX levels and decreasing serum MDA, both of which are crucial factors for reduction of oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential micronutrient for human health. It plays a key role in regulation of selenoprotein synthesis, selenoproteins being the means by which Se exerts its biological functions throughout the body [1, 2]. The selenoproteins, including glutathione peroxidase (GPX), iodothyronine deiodinases (IDD), and thioredoxin reductase (TrxR), participate in antioxidant defense and prevent oxidative stress damage [3, 4]. Se is hence a critical factor for optimal functioning of the immune system, as well as of cellular processes and metabolic cycling [5,6,7].
Oxidative stress, which is a disturbance in the balance between antioxidant defense and reactive oxidant species can cause toxic effects and damage cellular function and biomolecules (e.g., lipids), lipoproteins, DNA, and proteins [5, 8, 9]. Oxidative stress has thus been implicated in initiation and development of such diseases as insulin resistance, diabetes mellitus, cardiometabolic diseases, cancer, and neurodegenerative diseases [8,9,10,11].
A number of studies have shown that low Se status, and hence selenoprotein insufficiency, is associated with suboptimal health while also causing several diseases [5, 12]. More specifically, Se deficiency impairs immune function and selenoprotein synthesis, which results in immune cells being unable to protect against oxidative stress [13, 14]. Furthermore, animal studies have demonstrated that low levels of selenoproteins are related to hyperglycemia and insulin resistance [15].
Previous studies have demonstrated that Se supplementation may have beneficial effects in reducing the risk of chronic metabolic disorders, hyperglycemia, hyperlipidemia, and cancer [16,17,18]. Meanwhile, there is scientific evidence that dietary Se intake is inversely associated with all-cause mortality [18]. There is also an inverse relationship between the onset of cardiovascular diseases and blood Se levels [19, 20]. An increase of Se intake in Beijing, China, led to a reduction in the cardiovascular disease mortality rate from 1984 to 1990 [1]. Given the critical role of selenoproteins in antioxidant defense and regulation of circulating lipoproteins, vascular endothelial cells, and cardiac function, it is hypothesized that Se supplementation may be protective against cardiometabolic disease [5, 21].
Due to the numerous controversies surrounding Se and its impact on both health and disease, it is clear that further studies are required to provide solid evidence which may be used by both clinicians and policymakers for the setting up of relevant health programs and nutritional interventions [22, 23].
Although several controlled trials have investigated the effects of Se supplementation on antioxidant markers, its pooled effect on antioxidant markers is as yet unclear and controversial. The aim of this systematic review and meta-analysis was therefore to assess the effects of Se supplementation on antioxidant markers.
Methods
This systematic review followed the PRISMA guidelines [24], while the meta-analysis was performed according to a previously published protocol [23].
Data sources and search strategy
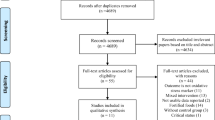
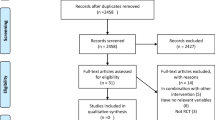
The PubMed, ISI/WOS, and Scopus databases were analyzed meticulously for relevant data, without time or language restriction, up to June 1, 2017 (Fig. 1). The reference lists of the retrieved reviews and meta-analyses were also screened to identify pertinent data. We excluded non-relevant articles and those with duplicate citation.
Study selection and data extraction
We included randomized controlled trials (RCTs) and quasi-RCT crossover studies, in which the control group received a placebo. Studies were considered regardless of dosage or duration of administration of antioxidants. Regarding the crossover studies, data from the first period were recorded and analyzed.
With regard to the RCTs, all studies among the different target groups of diabetic patients, polycystic ovarian syndrome (PCOS) patients, obese participants, and healthy subjects were included. Studies in which Se was applied as a single therapy or even combination therapy were considered as acceptable.
All clinical trials assessing the effect of Se supplementation on antioxidant markers, including oxidative stress index (OSI), antioxidant potency composite (APC) index, plasma malonaldehyde (MDA), total antioxidant capacity (TAC), antioxidant enzymes (superoxide dismutase (SOD), GPX, thiobarbituric acid-reactive substances (TBARS), catalase (CAT)), and total antioxidant plasma (TAP), were included.
Studies were excluded if they were (1) non-relevant; (2) a duplicate citation; (3) more than one paper from a specific study; (4) case reports, reviews, editorials, and studies performed on children (age < 18); and (5) concerned specific sub-group populations.
To establish inter-rater reliability, titles and abstracts were screened by two experts independently. Possible discrepancies were resolved by the main researcher. After assessment of relevancy, based on the inclusion/exclusion criteria, the full-text review determined the eligibility of papers.
The comprehensive recommended guidelines of the Consolidated Standards of Reporting Trials (CONSORT) 2010 25-item checklist [25], quality assessment, and data extraction and analysis were used, followed by two independent experts.
The main fields of relevant data were citation, type of study, study subjects, publication year, sample size, dose of supplementation, intervention group, control group, mean age of participants, outcome, intervention duration, follow-up duration, measurement interval, results, and effect size.
Data analysis
A meta-analysis was conducted for relevant antioxidant markers on which data were available from more than two individual studies. The mean change from baseline in antioxidant marker concentrations and standard deviation for both intervention and control groups was used to calculate the effect size. The effect size is presented as standardized mean difference (SMD). To pool the data, we used the random-effect model based on the result of heterogeneity among studies. A random-effect model was used if the Q-statistic for heterogeneity was significant at the level of 0.1 [26]. In other cases, the fixed-effect model was used [27]. The degree of heterogeneity was quantified, using I2 statistics, which is the estimation of the total variation across studies due to heterogeneity [28]. Possible sources of heterogeneity (such as quality assessment score, duration of intervention, study subjects, mean age of participants, dose of Se supplementation, type of study subjects, subjects with metabolic diseases, and healthy subjects) and sex ratio were explored by random-effect meta-regression analysis. Subgroup analyses were performed according to type of study population (type of disease) to identify between-study heterogeneity. Subgroup heterogeneity was evaluated using the fixed-effect model. A forest plot was used to present the results of the meta-analysis schematically. Publication bias was evaluated by visual inspection of the funnel plot and the Egger’s regression test. In addition, sensitivity analysis was conducted according to the study quality to test the robustness of the results.
The statistical analysis was carried out using Stata software, version 10 [25]. P value ≤ 0.05 was considered statistically significant.
Results
Search results and characteristics of included studies
From 3320 searched documents, following the refinement processing according to the inclusion/exclusion criteria, in total, 13 RCTs were included. These publications were dated from 2004 to 2016. Figure 1 shows the selection process of the articles included.
The meta-analysis included 13 studies (12 randomized controlled trials and 1 crossover). All studies had assigned a total of 2790 participants randomly to intervention and control groups. The age of the patients was from 10 to 85 years. Nine trials had recruited both men and women, while, in four other studies, only female subjects were enrolled [29,30,31,32]. Four trials used Se combined with other vitamins or minerals [10, 30, 33, 34] and nine trials used Se only as an oral supplement. The daily Se dose was 200 m/day in seven trials [29, 30, 32, 34,35,36,37]; one trial used 300 mg/day [33]; one RCT used 50 mg/day [10], and one study used 60 and 960 mg/day [31, 38]. In two studies, three different groups were given three different doses (100, 200, 300 mg/day); hence, they were considered as separate studies [15, 39]. Six trials were conducted in Iran, six in Europe, and one in the USA. All trials were placebo-controlled and all were double-blinded. The length of the intervention periods ranged from 42 days [25] to 180 days [15, 39] (Table 1).
Meta-analysis
Four trials involving 228 participants in the Se or placebo groups reported the effect of Se supplementation on TAC. Three studies reported GSH (glutathione), five studies MDA, 10 Se, three GPX, two TBARS, and four adiponectin as outcome at baseline and follow-up (Table 2).
The effect of Se supplementation on antioxidant levels is shown in Table 2. The pooled results indicated that Se supplementation significantly increased GPX levels (SMD = 0.54; 95% CI = 0.21–0.87). There was no significant heterogeneity between the three included trials (Q = 5.74, I2 = 65.2%, P = 0.057).
There was a significant improvement of antioxidant profile such as TAC [(SMD) = 0.39, 95% CI = (0.13, 0.66)] with obvious heterogeneity (Q = 10.27; P = 0,016; I2 = 70.8%), Se [(SMD) = 3.24, 95% CI = (3.1, 3.4)] with obvious heterogeneity (Q = 358.82; P = 0.0; I2 = 97.5%), and MDA [(SMD) = − 0.54, 95% CI = (− 0.78, − 0.30)] without significant heterogeneity (Q = 7.50; P = 0.11; I2 = 46.6%) through Se supplementation.
According to the meta-analysis, the intake of Se compared with placebo resulted in no significant improvement in GSH [(SMD) = 0.16, 95% CI = (− 0.12, 0.45)] and significant heterogeneity (Q = 10.94; P = 0.004; I2 = 81.7%), TBARS [(SMD) = − 0.12, 95% CI = (− 0.50, 0.25)], and adiponectin [(SMD) = 0.04, 95% CI = (− 0.09, 0.18)]. There was no significant evidence of heterogeneity for TBARS (Q = 0.14; P = 0.71; I2 = 0%) and adiponectin (Q = 0.40; P = 0.94; I2 = 0%), respectively. The pooled effect of Se supplementation on each antioxidant marker is depicted in Fig. 2.
Quality assessment
Table 3 shows the quality of the included studies. Six studies were classified as high quality, with the CONSORT score higher than 30 [15, 29, 30, 36, 37, 39]; four studies were classified as medium quality, with the CONSORT score in the range of 25–29 [10, 31, 32, 35]; and three studies were classified as low quality, with the CONSORT score lower than 25 [33, 34, 38]. Randomization as a prerequisite for inclusion in this meta-analysis was conducted in 13 studies.
All 12 RTCs were double-blind, but only four studies were described as blinding [15, 32, 37, 39].
Meta-regression and subgroup analysis
The effect of influencing factors was analyzed using a random-effect meta-regression. There was no effect of influencing factors, such as duration, mean age, study subjects, dose, antioxidant profile, and female ration on heterogeneity (P > 0.05). The results of meta-regression showed that the quality score was also non-significant (coefficient = 0.12, P = 0.2). To determine the source of heterogeneity, the subgroup analyses were divided according to study subjects (subjects with metabolic diseases and healthy subjects). Subgroup analysis showed that the effect of Se supplementation on antioxidant markers did not change according to study subjects.
Publication bias
Publication bias was estimated by Egger’s test. The results of Egger’s test did not support the existence of publication bias by Se supplementation on antioxidant profile (coefficient = 2.2, P = 0.5) (Fig. 3).
Sensitivity analysis
To test the robustness of the results, we conducted sensitivity analysis according to the study quality. After the exclusion of three low-quality studies, a total of 10 trials were analyzed. There was no significant difference in the results of the effect of Se supplementation on the level of plasma Se [(SMD) = 3.36, 95% CI = (3.2, 3.5)] and overall effect [(SMD) = 1.09, 95% CI = (0.5, 1.6)] by sensitivity analysis.
Discussion
The meta-analysis of this study was carried out on the basis of pooled data from 13 RCTs to assess the effect of Se supplementation on antioxidant factors. The results of analysis showed that Se supplementation increased GPX and Se levels and the total antioxidant capacity decreased MDA levels, whereas it had no significant effect on GSH, TBARS, and adiponectin levels.
According to the study analysis, Se intake increased Se and GPX levels increased considerably. Epidemiological studies have shown that Se intervention increases GSH-PX activity in plasma and erythrocytes [40, 41].
Various published reports support the fact that Se supplementation increases TAC and reduces MDA levels. A randomized, double-blind, placebo-controlled trial in 2016 reported that high Se-yeast supplement is effective in increasing TAC and decreasing MDA levels [42]. Several trials have shown that Se supplementation can reduce MDA levels [43, 44] and improve TAC and GSH levels [44]. Mahmoodpoor et al. [45] conducted a randomized, placebo-controlled trial in 2018 to investigate the effect of high-dose Se on antioxidant reserve of the lungs and ventilator-associated pneumonia (VAP) in critically ill patients. They demonstrated that serum Se and GPX-3 activity levels increased steadily in the treatment group within 10 days (P b 0.025), while they remained unchanged in the placebo group. They also stated that despite increasing antioxidant activity, Se supplementation did not affect the incidence of VAP in critically ill patients.
The results of one meta-analysis in 2017 on the effect of Se supplementation on coronary heart disease demonstrated that Se supplementation decreased serum CRP and increased GSH-PX levels, suggesting a positive effect on reducing oxidative stress and inflammation in CHD [46].
In an animal study carried out in 2017 by Mansour on the effect of Se yeast supplementation on growth, antioxidant status in meagre, it was shown that catalase and superoxide dismutase activity, as well as total antioxidant status, significantly increased, while thiobarbituric-reactive substances in liver homogenate significantly decreased with Se supplementation [47].
In another study, Se supplementation in 1-day-old male broilers resulted in amelioration of GPX activity, total antioxidant capacity, and malondialdehyde formation (P < 0.05) [48].
Significant heterogeneity was observed regarding the impact of Se supplementation on TAC, Se, and GPX levels. A random-effect model was subsequently applied. Meta-regression was performed to identify the source of heterogeneity. According to the meta-regression analysis, influencing factors, such as duration, mean age, dose, and female ratio had no effect on heterogeneity. However, there was a significant association between the type of antioxidant factors and heterogeneity (P = 0.008). We therefore conducted subgroup analyses based on antioxidant factors to determine the effect of Se supplementation on each factor.
The meta-analysis of the study has some limitations. In many investigations, Se has been used in combination with other vitamins or minerals, which makes it impossible to isolate the specific effects of Se or of different Se forms in these trials. In addition, the included studies were heterogeneous in type of patients, species of Se, and duration of treatment, as well as Se content of the soil in the various environments. These factors could well have affected the results of the studies. As other challenges assessed the complementary required data through the time-consuming process, we contacted the corresponding authors.
In the future, carefully designed double-blind clinical trials with large sample sizes with stratified participants based on the initial Se levels are recommended.
Conclusion
The study findings indicated that Se supplementation might be effective in reducing oxidative stress and inflammation through increasing TAC and GPX levels and decreasing serum MDA, both of which are crucial influencing factors for reduction of oxidative stress. However, the results also showed that Se supplementation is not sufficient to reduce TBARS or affect adiponectin and GSH levels. There was no evidence of adverse events. To the best of our knowledge, this study is the first published meta-analysis assessing the effect of Se supplementation on oxidative stress markers.
Change history
01 July 2020
The original version of this article, published on 10 December 2019 contained a mistake.
References
Brown KM, Arthur J (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr:593–599
Wrobel JK, Power R, Toborek M (2016) Biological activity of selenium: revisited. IUBMB Life 68:97–105
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13:102
Tapiero H, Townsend D, Tew K (2003) The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother 57:134–144
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Wang N, Tan H-Y, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med Cell Longev 2017:7478523
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Battin EE, Brumaghim JL (2009) Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 55:1–23
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Asp Med 26:256–267
Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW et al (2013) Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr 144:193–201
Stranges S, Galletti F, Farinaro E, D’Elia L, Russo O, Iacone R et al (2011) Associations of selenium status with cardiometabolic risk factors: an 8-year follow-up analysis of the Olivetti Heart study. Atherosclerosis. 217(1):274–278
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics. 6(1):25–54
Carlson BA, Yoo M-H, Shrimali RK, Irons R, Gladyshev VN, Hatfield DL et al (2010) Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc 69:300–310
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268
Rayman MP, Blundell-Pound G, Pastor-Barriuso R, Guallar E, Steinbrenner H, Stranges S (2012) A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS One 7:e45269
Wei J, Zeng C, Gong Q-Y, Li X-X, Lei G-H, Yang T-B (2015) Associations between dietary antioxidant intake and metabolic syndrome. PLoS One 10:e0130876
Milner JA, Romagnolo DF, Connor J, Lee S (2010) Bioactive compounds and cancer. Springer Science, LLC
Bleys J, Navas-Acien A, Guallar E (2008) Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med 168:404–410
Joseph J, Weber KT (2013) Selenium and cardiometabolic health: inconclusive yet intriguing evidence. Am J Med Sci 346:216–220
Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E (2006) Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 84:762–773
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422:11–22
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Djalalinia S, Khosravi M, Hasani M, Saeedi Moghaddam S, Kazemzadeh Atoofi M, Asayesh H et al (2017) Effects of selenium supplementation on cardiometabolic risk factors, inflammatory factors and antioxidant factors: a systematic review and meta-analysis protocol. IJPM(Accepted).
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8:18
StataCorp L (2007) Stata data analysis and statistical software. Spec Ed Release 10:733
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Whitehead A, Whitehead J (1991) A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med 10:1665–1677
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 327:557–560
Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A (2015) Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: randomized, double-blind, placebo-controlled trial. Nutrition 31:1235–1242
Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A et al (2012) Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab 60:157–168
Mao J, Bath SC, Vanderlelie JJ, Perkins AV, Redman CW, Rayman MP (2016) No effect of modest selenium supplementation on insulin resistance in UK pregnant women, as assessed by plasma adiponectin concentration. Br J Nutr 115:32–38
Razavi M, Jamilian M, Kashan ZF, Heidar Z, Mohseni M, Ghandi Y et al (2016) Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm Metab Res 48:185–190
Ravn-Haren G, Bügel S, Krath BN, Hoac T, Stagsted J, Jørgensen K et al (2008) Dragsted. A short-term intervention trial with selenate, selenium-enriched yeast and selenium-enriched milk: effects on oxidative defence regulation. Br J Nutr 99:883–892
Guertin KA, Grant RK, Arnold KB, Burwell L, Hartline J, Goodman PJ, Minasian LM et al (2016) Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic Biol Med 95:349–356
Faghihi T, Radfar M, Barmal M, Amini P, Qorbani M, Abdollahi M et al (2014) A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: effects on glucose homeostasis, oxidative stress, and lipid profile. Am J Ther 21:491–495
Farrokhian F, Bahmani M, Taghizadeh SM, Mirhashemi MH, Aarabi F, Raygan E et al (2016) Selenium supplementation affects insulin resistance and serum hs-CRP in patients with type 2 diabetes and coronary heart disease. Horm Metab Res 48:263–268
Bahmani F, Kia M, Soleimani A, Asemi Z, Esmaillzadeh A (2016) Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol Trace Elem Res 172:282–289
Faure P, Ramon O, Favier A, Halimi S (2004) Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Investig 34(7):475–481
Cold F, Winther KH, Pastor-Barriuso R, Rayman MP, Guallar E, Nybo M et al (2015) Randomised controlled trial of the effect of long-term selenium supplementation on plasma cholesterol in an elderly Danish population. Br J Nutr 114:1807–1818
Huang K, Liu H, Chen Z, Xu H (2002) Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis 162:137–144
Xia Y, Hill KE, Byrne DW, Xu J, Burk RF (2005) Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr 81:829–834
Bahmani F, Kia M, Soleimani A, Mohammadi AA, Asemi Z (2016) The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial. Br J Nutr 116:1222–1228
Chakrabarti SK, Ghosh S, Banerjee S, Mukherjee S, Chowdhury S (2016) Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocr Metab 20:674–678
Mesdaghinia E, Rahavi A, Bahmani F, Sharifi N, Asemi Z (2017) Clinical and metabolic response to selenium supplementation in pregnant women at risk for intrauterine growth restriction: randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 178:14–21
Mahmoodpoor A, Hamishehkar H, Sanaie S, Behruzizad N, Iranpour A, Koleini E et al (2018) Antioxidant reserve of the lungs and ventilator-associated pneumonia: a clinical trial of high dose selenium in critically ill patients. J Crit Care 44:357–362
Ju W, Li X, Li Z, Wu GR, Fu XF, Yang XM et al (2017) The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol 44:8–16
Mansour A, Goda A, Omar E, Khalil H, Esteban M (2017) Dietary supplementation of organic selenium improves growth, survival, antioxidant and immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol 68:516–524
Bakhshalinejad R, Akbari Moghaddam Kakhki R, Zoidis E (2018) Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br Poult Sci 59:81–91
Buijsse B, Lee D-H, Steffen L, Erickson RR, Luepker RV, Jacobs DR et al (eds) (2012) Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc’s. Zirlik A, ed. PLoS One 7:e38901
Acknowledgments
The authors would like to express their appreciation to all participants and scientific and executive partners who took part in this study.
Funding
The research reported in this publication was supported by the Elite Researcher Grant Committee under award number [963478] from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
MQ and RH participated in the study design and drafted the manuscript. SD and MQ participated in the study design and statistical analysis and drafted the manuscript. MH and HA contributed to the protocol development and drafted the manuscript. MK, AM, and MZ contributed to the data acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasani, M., Djalalinia, S., Khazdooz, M. et al. Effect of selenium supplementation on antioxidant markers: a systematic review and meta-analysis of randomized controlled trials. Hormones 18, 451–462 (2019). https://doi.org/10.1007/s42000-019-00143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-019-00143-3