Abstract
Thalassemia Major (TM) is a clinical entity with a high prevalence of low bone mass. The aim of the present study was to perform a meta-analysis of all available data on the role of bisphosphonates (BPs) in the therapy of thalassemia major-induced osteoporosis. The PRISMA recommendations for reporting systematic reviews and meta-analyses were used to guide the present study. We searched PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) through March 31, 2017 for articles related to thalassemia and BPs. To meta-analytically synthesize the primary endpoint, we used the standardized mean difference (SMD) after Hedges’s g transformation under the scenario of a random effects model. Heterogeneity across studies was examined using the I2 statistic. Nine randomized controlled trials (RCTs) containing original data were included in this review. Three studies were performed in Italy, one in Australia, three in Greece, one in Cyprus, and one in China. The BPs investigated included zoledronate, alendronate, pamidronate, clodronate, and neridronate. Zoledronate and alendronate showed a tendency to perform best as compared to neridronate and the placebo effect with respect to femoral neck, lumbar spine, total hip, and total body in terms of bone mass density (g/cm2). BPs and in particular, zolendronate, were quite effective in the treatment of osteoporosis. These findings suggested that bisphosphonates are still a front-line treatment of osteoporosis in TM. However, to draw more meaningful and significant conclusions for the use and efficacy of BP in TM, larger and more complete RCTs should be conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a serious health problem not only because it affects quality of life but also, importantly, because it is associated with increased morbidity and mortality, while it additionally represents a significant economic burden for individuals and society alike.
Thalassemia is a hereditary disease caused by defective globin synthesis resulting not only in abnormal but also in a decreased quantity of globin chains [1]. Thalassemia intermedia (TI) is a term used to define a group of patients with β-thalassemia in whom the clinical severity of the disease is somewhere between the mild symptoms of the β-thalassemia trait and the severe manifestations of β-thalassemia major (TM). However, it has been reported that TI progresses over time to TM. The diagnosis is a clinical one that is based on the patient maintaining a satisfactory hemoglobin (Hb) level of at least 6–7 g/dL at the time of diagnosis without the need for regular blood transfusions [2]. β-thalassemia minor is characterized by reduced β-hemoglobin chain synthesis and sometimes mild anemia, although carriers of β-thalassemia minor are usually clinically asymptomatic [3].
A clinical entity with high incidence of low bone mineral density is thalassemia major (TM) is a clinical entity with high incidence of low bone mineral density. TM is a clinical hereditary hemolytic anemia caused by a defect in the ability of erythroblasts to synthesize the β-chain of adult hemoglobin. Several bone abnormalities beyond osteoporosis are present in patients with TM, including an enlargement of the cranial and facial bones, spinal deformities, scoliosis, spinal nerve compression, spontaneous fractures, and bone loss [4, 5]. The incidence of low bone mass in well-treated TM patients is approximately 40–50%. Therefore, osteoporosis represents a leading cause of morbidity in TM patients of both genders [6]. The introduction of transfusion therapy resulted in the reduction or prevention of bone deformities; however, the detection of low bone mass in many regularly transfused and well-chelated TM patients over the last decade has been quite unexpected. There is growing awareness that many transfusion-dependent adult patients with TM and TI suffer from long-standing bone pain, low bone mass, and fractures collectively referred to as bone disease [5].
The pathogenesis of bone disease in TM is quite complicated. Several genetic and acquired factors are implicated in bone turnover changes and diminished bone mineral density (BMD), such as delay in sexual maturation, endocrine dysfunction (diabetes mellitus, hypothyroidism, hypoparathyroidism, GH deficiency, etc.). In addition, iron chelation therapy has been associated with growth failure and bone abnormalities, while high desferrioxamine dosage has been also associated with cartilage alterations. With regular transfusion, most patients keep acceptable Hb levels, but even when within the normal range, as well as when adequate hormone replacement therapy is provided, these patients show increased bone turnover and decreased BMD [7,8,9].
During the last decade, there has been significant progress in our understanding of the pathogenesis of low BMD in TM patients. The RANK/RANKL/OPG pathway is of great importance for the activation and proliferation of osteoclast precursors and, hence, bone resorption. However, there is evidence that in these patients there is also reduced osteoblast activity, compounding the detrimental effect of TM on the bone [10].
It appears that prevention and treatment of early bone loss is the best strategy in TM. For the management of bone disease, many of the currently approved drugs employed primarily in post-menopausal osteoporosis have been used, such as sex hormone replacement therapy [11] and bisphosphonates (BP) [12]. Of course, adequate calcium and vitamin D intake as well as physical activity are encouraged in these patients during their skeletal development, while early diagnosis and treatment of comorbidities are a sine qua non [13].
Bisphosphonates, which are potent inhibitors of osteoclastic bone resorption, have been used to manage bone abnormalities in TM patients, with encouraging results. This class of drugs are the most commonly prescribed to treat osteoporosis. They act by inhibiting both osteoclastic recruitment and maturation, preventing the development of monocyte precursors into osteoclasts, inducing osteoclast apoptosis, and interrupting their attachment to the bone [14]. Even though the data regarding the effects of bisphosphonates on differentiation are limited, it is well established that their main effect is at the osteoclasts. The aim of the present study is to perform a meta-analysis of all available data concerning the role of BP in thalassemia major-induced osteoporosis. Specifically, we systematically examined the possibly beneficial time-dependent effect of BP on bone density and metabolism. The method of meta-analysis was therefore used to examine this effect.
Material and methods
Search strategy
The PRISMA recommendations for reporting systematic reviews and meta-analyses were used to guide the present study (Supplementary File: Tsartsalis et al. 2017_PRISMA 2009 Checklist.docx). We searched PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) through March 31, 2017 for articles related to the specified disease (thalassemia) and pharmacologic agents, i.e., bisphosphonates (BPs), using these precise keywords. The search was limited to studies carried out in humans, with no language restriction and included articles ahead of publication. The reference lists of the publications identified were hand-searched for additional relevant studies. Abstracts from International Meetings were not considered unless published as original peer-reviewed articles. A second reviewer (GIL) blinded to the primary reviewer’s (AT) decisions checked the paper selection, data extraction, and risk of bias assessment stages of the review. In each instance, the number of papers checked was the larger of either ten studies or 10% of the studies to be appraised. Any differences of opinion were discussed and a third reviewer (CS) was available to arbitrate any issues that remained unresolved.
Eligibility criteria
We focused on bisphosphonates (BP) randomized controlled trials (RCTs) on thalassemia designed to evaluate the effect of BP on all possible studied variables or surrogate anti-fracture endpoints (BMD and markers of bone turnover) in patients with thalassemia, with no age, race/ethnicity, gender, clinical, or other restrictions. Both non-amino-BPs and amino-BPs were considered. RCTs were excluded if they did not include BP studies or did not provide data that could be analyzed. Review of the selected RCTs was performed by a second reviewer (GIL), blinded to the primary reviewer’s (AT) decisions, checked the paper selection, data extraction, and risk of bias assessment stages of the review. In each instance, the number of papers checked was the larger of either ten studies or 10% of the studies to be appraised. In the case of discrepancies, a consensus was reached by discussing the issues with a third investigator (CS).
Data extraction and handling
For each study identified and included in this review, we aimed to collect the following information: (i) biochemical, bone markers studied, and their respective units, (ii) authors of the study, (iii) publication year, (iv) patient populations examined and type of clinical study design (e.g., placebo-controlled, double-blind control group, placebo, bisphosphonates), (v) BP dosage, (vi) duration of BP therapy (in years, including eventual extensions), (vii) method of BP administration (e.g., intravenously, per os), (viii) total study duration, (ix) frequency of BP administration, (x) total BP doses, (xi) number of participants per study population, (xii) country where the study was performed, (xiii) the presence of co-intervention (e.g., calcium, vitamin D), (xiv) type of co-intervention, (xv) dosage of co-intervention, (xvi) gender, (xvii) male/female ratio, (xviii) number of males and females, (xix) chelation therapy or not, (xx) type of chelation therapy, (xxi) dosage of chelation therapy, (xxii) patients maintained on a regular transfusion program, and (xxiii) proportion of patients presenting with hypogonadism. Continuous data were summarized as the mean or median as provided by the respective study. Data are provided as supplementary data (file Supplementary Data.xlsx).
Assessment of risk of bias
Quality assessment and risk of bias (RoB) evaluations of included RCTs were rated using the Cochrane Collaboration assessment tool: [15], adequate generation of allocation sequence, concealment of allocation to conditions, the prevention of knowledge of the allocated intervention (blinding of assessors), and dealing with incomplete outcome data. Then for each domain, the quality of each RCT was classified as high (that is, low risk of bias), moderate (that is, unclear risk of bias), and low (that is, high risk of bias). We also computed a “risk of bias” score for each RCT by giving one point to each domain for which a study could be rated as low RoB.
Statistical analyses
To meta-analytically synthesize the primary endpoint, we used the standardized mean difference (SMD) after Hedges’s g transformation under the scenario of a random effects model. The latter transformation was necessary because the BMD was reported using different units as T scores, Z scores, or g/cm2. Further, BP effect over time has been taken into account by calculating SMDs with respect to time zero for the respective drug [16, 17]. A SMD > 0 indicates positive effects of the bisphosphonates over time, while a SMD < 0 indicates negative effects (e.g., worsening) of the bisphosphonates over time. Heterogeneity across studies was examined using the I2 statistic and an I2 value above 50% was considered large. We then examined whether there existed any significant publication bias by graphically using a funnel plot and statistically using Egger’s test. Finally, we also summarized the reported evidence on the potential beneficial effects on markers of bone turnover and the reported adverse effects of bisphosphonate therapy in thalassemia-induced osteoporosis (TIO). All statistical analyses were conducted using Stata 10.0 (College Station, TX, USA) software package and the Matlab ® Simulation Environment (Mathworks, Inc., Natick, MA).
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Literature search
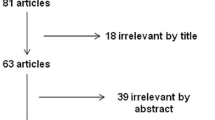
The flow diagram of our literature search is presented in Fig. 1. Our total search resulted in 50 entries, which after title and abstract assessment rendered a final total of 38 eligible for further inclusion in the present meta-analysis. From these 38 studies, 29 were further excluded because they were either not RCTs, or were not relevant to the topic, or were non-original reviews, or were case control studies. The remaining nine RCTs studies containing original data were included in this review [12, 18,19,20,21,22,23,24,25].
Study characteristics and methods
The characteristics of the nine studies under consideration are summarized in Table 1. Three studies were performed in Italy [12, 18, 20], one in Australia [19], three in Greece [10, 21,22,23,24,25,26,27], one in Cyprus [21], and one in China [28]. The samples considered were small (23–118 patients), with only one article enrolling more than 100 patients [18]. The BPs investigated included zoledronate (n = 4) [19, 22, 23, 25], alendronate (n = 2) [12, 21], pamidronate (n = 2) [21, 28], clodronate (n = 2) [12, 20, 22], and neridronate (n = 1) [18]. From the aforementioned studies, five were not RCTs [1, 26, 27, 29, 30], while nine were [12, 18,19,20,21,22,23,24,25]. Dosage applied for zoledronate had a median of 4 mg (min = 1 mg, max = 4 mg); for alendronate the sole dosage was 10 mg administered either daily (10 mg/day) or weekly (70 mg/week); for pamidronate the median dosage was 60 mg (min = 22.50, max = 90 mg); for clodronate the median dosage was 100 mg (min = 10 mg, max = 300 mg), and for neridronate the median and sole dosage was 100 mg. Of note, the dosage of zoledronate was not the one currently used for osteoporosis treatment. The studies were comparable with respect to demographic and clinical characteristics of the study populations included. Subsequently, all patients received complementary treatment, which included calcium (median dosage 500 mcg/day, min = 500 mcg, max = 1250 mcg) and Vitamin D (median and sole dosage 10 mcg/day, min = 0.25 mcg/day, max = 25 mcg/day). In addition, all patients received chelation therapy, which included desferrioxamine (75 mg/kg/day) and deferiprone (75 mg/kg/day). Three studies did not report chelation therapy [19, 23, 27]. In all studies, patients were maintained on regular transfusion programs to guarantee pre-transfusional hemoglobin levels (9.0–9.5 g/dL), while subjects presenting with hypogonadism received sex hormone replacement therapy.
Assessment of risk of bias
The results of the RoB assessment are presented in Table 2. The median “risk of bias” score of the nine RCTs was 5 (interquartile range [IQR] = 5–6), indicating a moderate quality of the included studies.
The effect of biphosphonates on BMD
In the studies under consideration, it appeared that zoledronate [22, 23] manifested a tendency for the best performance as compared to neridronate [22] and the placebo effect, with respect to femoral neck BMD in g/cm2 units (Fig. 2a) (QBP = 1.02, df = 3, p = 0.24, I2 = 0%) [19]Footnote 1. At the same time, the total effect of treatment, which included both medications (zoledronate and neridronate), was increased as compared to placebo effect or the control group that received no treatment (Fig. 2a) (QPlacebo = 3.37, df = 4, p = 0.15, I2 = 0%)Footnote 2. At the same time, total effect of treatment, with respect to the T score, was higher in patients who received treatment as compared to placebo (Fig. 2b) (QBP = 7.19, df = 3, p = 0.03, I2 = 58.32%, while QPlacebo = 17.37, df = 1, p = 1.61 × 10−5, I2 = 94.24%). Interestingly, in terms of T score, in the study of Morabito et al. (2002), alendronate [12] demonstrated a better effect than zoledronate [22].
Effect of bisphosphonate therapy on bone mineral density of the Femoral Neck (BMD FN) expressed g/cm2 (a) as well as T score units (b). A SMD < 0 implies a negative effect with respect to time, whereas SMD > 0 implies a positive effect with respect to time (SMD standardized mean difference, BMD FN bone mineral density femoral neck, BP biphosphonates. Each black box, with the respective error bars, corresponds to a study. Close to the error bar we report the drug used and in the superscript the respective study as reported in the “References” section. In particular, the works of Gilfillan et al. [19], Voskaridou et. al. [23], Voskaridou et al. [22], Morabito et al. [12], and Forni et al. [18] are presented)
Similar results were observed in the case of the lumbar spine in terms of BMD in g/cm2. In particular, all studies showed a stronger effect in patients treated with zoledronate and alendronate than those treated with neridronate and placebo (Fig. 3a) (QBP = 100.31, df = 4, p = 4.13 × 10−21, I2 = 96.01%, while QPlacebo = 733.52, df = 3, p = 5.63 × 10−15, I2 = 99.59%). Moreover, the mean effect of BP in all studies was higher as compared to the control or the placebo groups. Additionally, in terms of T score, similar results were obtained including the effect of pamidronate in the study of Voskaridou et al. (2003) (Fig. 3b) [27] (QBP = 0.199, df = 4, p = 0.04, I2 = 0%, while QPlacebo = 64.54, df = 3, p = 3.1 × 10−14, I2 = 95.35%).
Effect of bisphosphonate therapy on bone mineral density of the lumbar spine (BMD LS) expressed as g/cm2 (a) as well as T score units (b). A SMD < 0 implies a negative effect with respect to time, whereas SMD > 0 implies a positive effect with respect to time (SMD standardized mean difference, BMD LS bone mineral density lumbar spine, BP biphosphonates). Each black box, with the respective error bars, corresponds to a study. Close to the error bar we report the drug used and in the superscript the respective study as reported in the “References” section. In particular, the works of Gilfillan et al. (2006) [19, 21], Voskaridou et. al. (2008a) [23, 25], Voskaridou et al. (2006) [22, 24], Morabito et al. (2002) [12, 14], Forni et al. (2012) [18, 20], and Pennisi et al. (2003) [20, 22] are presented)
Finally, with respect to BMD, in the total hip zoledronate showed a positive effect as compared to placebo in the study of Gilfillan et al. (2006) [19] (Fig. 4a). Similar results were obtained with respect to total body BMD, where zoledronate demonstrated a positive effect as compared to placebo and control groups (Fig. 4b). It is however worth mentioning that in the cases of total body and hip BMD, the available data did not allow a more extended comparison with respect to treatment effects. All results regarding the effect of BP treatment on BMD are summarized in Table 3.
Effect of bisphosphonate therapy on bone mineral density of the total hip (BMD Total Hip) expressed as g/cm2, as well as T score (a) and effect of bisphosphonate therapy on bone mineral density of total body (BMD Total Body) expressed as g/cm2 as well as T score units (b). SMD < 0 implies a negative effect with respect to time, whereas SMD > 0 implies a positive effect with respect to time (SMD standardized mean difference, BMD LS bone mineral density lumbar spine, BP biphosphonates). Each black box, with the respective error bars, corresponds to a study. Close to the error bar we report the drug used and in the superscript the respective study as reported in the “References” section. In particular, the works of Gilfillan et al. (2006) [19, 21] and Pennisi et al. (2003) [20, 22] are presented)
The effect of bisphosphonates on calcium homeostasis and bone turnover markers
In the case of bone metabolic factors, there was no consistent record in the studies under consideration. In the study of Gilfillan et al. (2006), alkaline phosphatase (ALP) was not different between treated patients with zoledronate (SMD = 0.00) and the placebo group (SMD = 0.00) (Table 4). In the case of bone alkaline phosphatase (bALP), data from the available studies showed that treatment yielded a more pronounced decreasing total effect (SMD = − 0.02), as anticipated, than in the placebo group (SMD = 0.00) and a more pronounced increasing effect than in the control group (SMD = − 0.03) (Table 4). Furthermore, C-telopeptide of collagen Type I (CTX) was decreased in the treated group (SMD = − 0.43) compared to placebo (SMD = 0.26) and control (SMD = 0.02) groups (Table 4). Notably, the effect reported was for treatment with zoledronate only. With regard to parathyroid hormone (PTH),Footnote 3 zoledronate (SMD = 0.00) had no effect on its levels2, while clodronate had a positive effect on PTH (SMD = 0.37) [12] and, respectively, alendronate (SMD = − 0.40) had a negative effect on PTH compared to the placebo effect (SMD = − 1.35, QBP = 0.31, df = 3, p = 0.19, I2 = 0%, while QPlacebo = 1.19, df = 2, p = 0.27, I2 = 0%) in the study by Morabito et al. (2002) [12]. Phosphorus was affected by BP treatment with lower levels in response to clodronate (SMD = − 0.45) [12], followed by alendronate (SMD = − 0.18) [12]. Compared to the placebo group, clodronate had similar results (SMD = − 0.44) [12], while alendronate was associated with higher levels of phosphorus (SMD = − 0.18) [12].
Discussion
In this study, we have reviewed the literature with regard to the effects of bisphosphonates in the treatment of osteoporosis in patients with thalassemia. We found that bisphosphonates are effective over time and as compared to placebo and control subjects. In particular, BPs, and especially zoledronate, are effective in the treatment of osteoporosis, as they improve the femoral neck (FN) BMD, although a significant difference was not observed. The fact that we did not find a significant difference in the treatment of FN BMD indicates that all BPs could be equally effective with respect to the BMD of this region. Furthermore, the heterogeneity observed was very low, indicating that the differences observed were related to chance or were due to the wide range of interventions and outcomes assessed in the analysis. BPs were effective in increasing lumbar spine (LS) BMD. In particular, zoledronate was more effective than neridronate or placebo. Interestingly, LS BMD results were significant despite high heterogeneity, with respect to g/cm2, though non-significant, when estimated in terms of T score.
To our knowledge, this is the first study that has investigated the effects of bisphosphonates in such a systematic manner over time and across different outcomes, while also quantifying the magnitude of the observed effects.
Previous studies have demonstrated the effect of BPs in BMD, T score, and bone turnover markers as compared to placebo and control subjects. Our study attempted to demonstrate the efficacy of therapy for osteoporosis in TM patients over time. The main purpose was not to make a direct comparison among BPs, even though we found that zoledronate was more effective, followed by alendronate, an observation that is in agreement with previous studies. Through our observations, we were able to conclude that a medical pharmaceutical treatment for osteoporosis had a positive effect as compared to placebo, or to the absence of treatment. The more positive effect of zoledronate, as mentioned above, is in agreement with previous reports regarding the efficacy of the drug. In addition, the better efficacy of zoledronate has been demonstrated in other forms of osteoporosis in the general population. The study of Byun et al. (2017) showed that zoledronate was more effective in reducing vertebral fractures and fracture risk than the other BPs [32].
It is well documented that thalassemia is closely associated with increased risk of fracture, a risk that was particularly severe before the advances in transfusion and chelation treatments, but which is today declining. Thus, while in earlier studies, fracture rates were reported to vary from 30 to 50% [33], in recent studies fracture rates have been reported to range between 12.1 and 38.8% depending on the study population and method of data collection [33]. Of note, thalassemia may be related to high fracture risk because of the increase in life expectancy. Therefore, given the present-day lengthening of life expectancy, treatment of thalassemia-related osteoporosis is of increased importance, while it is moreover closely linked both to patients’ quality of life as well as more generally speaking to the public health problem of thalassemia.
In a previous meta-analysis, it was shown that zoledronate improved BMD in TM patients, this being in agreement with our meta-analysis. Biochemical markers of bone metabolism diminished after treatment, indicating that the most feasible therapeutic scenario was reduction of bone turnover. Interestingly, zoledronate continues to improve BMD, even after its cessation [34, 35]. Today, osteoporosis has become a topic of increasing concern for thalassemic patients given that, during the last decade, the presence of osteopenia or osteoporosis in well-treated TM patients has been reported as having a frequency of approximately 40–50% in the populations studied [36]. The decrement in bone density in thalassemic patients appears to be a consequence of a higher bone remodeling rate accompanied by higher bone resorption over bone formation, resulting in net bone loss [27, 37].
We have found that, overall, zoledronate was more effective than the other medications employed, as well as than placebo and control groups. Alendronate followed by clodronate and neridronate were similarly effective. Our findings are in agreement with a recent meta-analytic report in which bisphosphonates were effective in the same order as compared to placebo and control groups [33]. In the case of bALP, it is expected that treatment of osteoporosis should reduce its levels, a phenomenon shown by the reviewed studies. In particular, zoledronate reduced bALP levels as compared to placebo and control groups. In the case of CTX, zoledronate treatment manifested a decreased effect compared to the placebo and control groups. Because of its high potency, only small doses are required to inhibit bone resorption [38]. It should be noted that the effects of bisphosphonates on bone are long-acting and remain long after the treatment has been administered, reaching a plateau after 3 years [38]. However, the dosage of zoledronate used in these studies was much higher than the dosage currently approved for osteoporosis. In particular, in the work of Gilfillan et al. 2006 4 mg of zoledronate were administered for 2 years every 3 months. In fact, most studies reported an administration of 4 mg every 3–4 months with the exception of some studies reporting 1 mg administration. This means that results could be different with the BPs dose, approved for postmenopausal osteoporosis.
Study limitations
The number of RCTs is quite limited as well as the quality of most studies. One of the main obstacles we faced was the fact that the variables were not consistent in all studies or at all time points. In particular, in many studies biochemical bone metabolic factors were not evaluated at all time points or were evaluated only at the time of study entry.
Conclusions
In this meta-analysis, we have provided significant evidence that BPs, especially zoledronate, remain a front-line treatment of osteoporosis in TM. However, larger, more complete RCTs with the approved dosage for BPs should be conducted to provide stronger and more complete evidence for the use and efficacy of BP in TM.
Notes
QBP Q value of Cochran’s heterogeneity statistic in samples treated with BP, QPlacebo Q value of Cochran’s heterogeneity statistic in samples received placebo, df degrees of freedom, I2 percentage of total variation across studies
See footnote 1
In all studies, patients and controls received calcium and vitamin D supplementation. Also, in the studies under consideration, only in the study of Morabito et al. (2002) were three patients mentioned with hypoparathyroidism. In the other studies, there was no mention of the presence of hypoparathyroidism in the patient cohort. This is indeed an important aspect, since in the case of parathyroid dysfunction, calcium and vitamin D supplementation is mandatory [31]. Basha NK, Shetty B, Shenoy UV, 2014 Prevalence of Hypoparathyroidism (HPT) in Beta Thalassemia Major. Journal of clinical and diagnostic research: JCDR 8(2): 24–6.
References
Otrock ZK, Azar ST, Shamseddeen WA et al (2006) Intravenous zoledronic acid treatment in thalassemia-induced osteoporosis: results of a phase II clinical trial. Ann Hematol 85(9):605–609
Musallam KM, Taher AT, Rachmilewitz EA (2012) Beta-thalassemia intermedia: a clinical perspective. Cold Spring Harb Perspect Med 2(7):a013482
Schnedl WJ, Schenk M, Lackner S, Holasek SJ, Mangge H (2017) Beta-thalassemia minor, carbohydrate malabsorption and histamine intolerance. J Community Hosp Intern Med Perspect 7(4):227–229
Coley TB (1925) Series of cases of sp lenomegaly in children with anemia and peculiar bone changes. Trans Am Pediat Soc 47:29
Vogiatzi MG, Macklin EA, Fung EB et al (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res Off J Am Soc Bone Miner Res 24(3):543–557
Terpos E, Voskaridou E (2010) Treatment options for thalassemia patients with osteoporosis. Ann N Y Acad Sci 1202:237–243
Carmina E, Di Fede G, Napoli N et al (2004) Hypogonadism and hormone replacement therapy on bone mass of adult women with thalassemia major. Calcif Tissue Int 74(1):68–71
Hatori M, Sparkman J, Teixeira CC et al (1995) Effects of deferoximine on chondrocyte alkaline phosphatase activity: proxidant role of deferoximine in thalassemia. Calcif Tissue Int 57(3):229–236
Lasco A, Morabito N, Gaudio A et al (2001) Effects of hormonal replacement therapy on bone metabolism in young adults with beta-thalassemia major. Osteoporos Int 12(7):570–575
Voskaridou E, Terpos E (2005) Osteoprotegerin to soluble receptor activator of nuclear factor kappa-B ligand ratio is reduced in patients with thalassaemia-related osteoporosis who receive vitamin D3. Eur J Haematol 74(4):359–361
Anapliotou ML, Kastanias IT, Psara P et al (1995) The contribution of hypogonadism to the development of osteoporosis in thalassaemia major: new therapeutic approaches. Clin Endocrinol 42(3):279–287
Morabito N, Lasco A, Gaudio A et al (2002) Bisphosphonates in the treatment of thalassemia-induced osteoporosis. Osteoporos Int 13(8):644–649
Lindsay R (1993) Prevention and treatment of osteoporosis. Lancet 341(8848):801–805
Papapoulos SE (2011) Use of bisphosphonates in the management of postmenopausal osteoporosis. Ann N Y Acad Sci 1218:15–32
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Dragioti E, Dimoliatis I, Evangelou E (2015) Disclosure of researcher allegiance in meta-analyses and randomised controlled trials of psychotherapy: a systematic appraisal. BMJ Open 5(6):e007206
Dragioti E, Dimoliatis I, Fountoulakis KN, Evangelou E (2015) A systematic appraisal of allegiance effect in randomized controlled trials of psychotherapy. Ann General Psychiatry 14:25
Forni GL, Perrotta S, Giusti A et al (2012) Neridronate improves bone mineral density and reduces back pain in beta-thalassaemia patients with osteoporosis: results from a phase 2, randomized, parallel-arm, open-label study. Br J Haematol 158(2):274–282
Gilfillan CP, Strauss BJ, Rodda CP et al (2006) A randomized, double-blind, placebo-controlled trial of intravenous zoledronic acid in the treatment of thalassemia-associated osteopenia. Calcif Tissue Int 79(3):138–144
Pennisi P, Pizzarelli G, Spina M, Riccobene S, Fiore CE (2003) Quantitative ultrasound of bone and clodronate effects in thalassemia-induced osteoporosis. J Bone Miner Metab 21(6):402–408
Skordis N, Ioannou YS, Kyriakou A et al (2008) Effect of bisphosphonate treatment on bone mineral density in patients with thalassaemia major. Pediatr Endocrinol Rev 6(Suppl 1):144–148
Voskaridou E, Anagnostopoulos A, Konstantopoulos K et al (2006) Zoledronic acid for the treatment of osteoporosis in patients with beta-thalassemia: results from a single-center, randomized, placebo-controlled trial. Haematologica 91(9):1193–1202
Voskaridou E, Christoulas D, Antoniadou L, Terpos E (2008) Continuous increase in erythropoietic activity despite the improvement in bone mineral density by zoledronic acid in patients with thalassemia intermedia-induced osteoporosis. Acta Haematol 119(1):40–44
Voskaridou E, Christoulas D, Konstantinidou M et al (2008) Continuous improvement of bone mineral density two years post zoledronic acid discontinuation in patients with thalassemia-induced osteoporosis: long-term follow-up of a randomized, placebo-controlled trial. Haematologica 93(10):1588–1590
Voskaridou E, Christoulas D, Xirakia C et al (2009) Serum Dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis. Reduction post-zoledronic acid administration. Haematologica 94(5):725–728
Perifanis V, Vyzantiadis T, Tziomalos K et al (2007) Effect of zoledronic acid on markers of bone turnover and mineral density in osteoporotic patients with beta-thalassaemia. Ann Hematol 86(1):23–30
Voskaridou E, Terpos E, Spina G et al (2003) Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol 123(4):730–737
Leung TF, Chu Y, Lee V et al (2009) Long-term effects of pamidronate in thalassemic patients with severe bone mineral density deficits. Hemoglobin 33(5):361–369
Chatterjee R, Shah FT, Davis BA et al (2012) Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to pamidronate in beta-thalassaemia presenting with osteopenia-osteoporosis syndrome. Br J Haematol 159(4):462–471
Perifanis V, Vyzantiadis T, Vakalopoulou S et al (2004) Treatment of beta-thalassaemia-associated osteoporosis with zoledronic acid. Br J Haematol 125(1):91–92 author reply 3-4
Basha NK, Shetty B, Shenoy UV (2014) Prevalence of hypoparathyroidism (HPT) in Beta thalassemia major. J Clin Diagn Res 8(2):24–26
Byun JH, Jang S, Lee S et al (2017) The efficacy of bisphosphonates for prevention of osteoporotic fracture: an update meta-analysis. J Bone Metab 24(1):37–49
Dede AD, Trovas G, Chronopoulos E et al (2016) Thalassemia-associated osteoporosis: a systematic review on treatment and brief overview of the disease. Osteoporos Int 27(12):3409–3425
Brown JE, Ellis SP, Lester JE et al (2007) Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res 13(18 Pt 1):5406–5410
Bhardwaj A, Swe KM, Sinha NK, Osunkwo I (2016) Treatment for osteoporosis in people with ss-thalassaemia. Cochrane Database Syst Rev 3:CD010429
Shamshirsaz AA, Bekheirnia MR, Kamgar M et al (2003) Metabolic and endocrinologic complications in beta-thalassemia major: a multicenter study in Tehran. BMC Endocr Disord 3(1):4
Voskaridou E, Kyrtsonis MC, Terpos E et al (2001) Bone resorption is increased in young adults with thalassaemia major. Br J Haematol 112(1):36–41
Reid IR, Brown JP, Burckhardt P et al (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346(9):653–661
Author information
Authors and Affiliations
Contributions
ANT: Data collection, review of the literature, primary reviewer, statistical analysis, drafted the manuscript, GIL: Review of the literature, secondary reviewer, statistical analysis, meta-analysis, drafted the manuscript, DT: Statistical analysis, CS: Data collection, review of the literature, third reviewer, MK: Review of the literature, ET: Review of the literature, CK: Review of the literature, critical reading, GPC: Review of the literature, critical reading, proof-edited the manuscript, gave final permission for submission, AT: Critical reading.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study does not contain any original human or animal study performed by the authors.
Informed consent
Not applicable
Electronic supplementary material
ESM 1
(XLSX 3021 kb)
Rights and permissions
About this article
Cite this article
Tsartsalis, A.N., Lambrou, G.I., Tsartsalis, D. et al. The role of biphosphonates in the management of thalassemia-induced osteoporosis: a systematic review and meta-analysis. Hormones 17, 153–166 (2018). https://doi.org/10.1007/s42000-018-0019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0019-3