Key summary points
This study aimed to compare postural control without or with dual task, in patients over 65 years,depending on the degree of arterial stiffness, measured using the carotid-femoral pulse.
AbstractSection FindingsSway path traveled and surface covered by the center of foot pressure were measured on a force platform in different postural conditions, i.e. eyes open, eyes closed and eyes open with a dual task, allowing to calculate the length function of surface (LFS), this ratio providing information about the precision (surface) of postural control and the effort made (length) by the participant. After an age-adjustment, LFS was higher in participants with high pulse wave velocity in different postural conditions especially dual task condition.
AbstractSection MessageThe difficulties in maintaining the equilibrium under a dual task condition being more pronounced in people with increased arterial stiffness, it is necessary to avoid or to limit its aggravation to reduce the number of falls, one of the main causes of mortality in the elderly.
Abstract
Purpose
Arterial stiffness generates vascular alterations that may cause balance disorders and falls. This study aimed to investigate the possible link between arterial stiffness and postural control under different sensorial conditions in patients over 65 years.
Methods
Carotid-femoral pulse wave velocity (PWV) was measured in 47 participants aged over 65 years to evaluate their arterial stiffness (high PWV). Twenty-seven participants (mean age = 70.52 ± 4.02 years, 22 females) had a normal PWV (< 10 m s−1) and 20 participants (mean age = 75.93 ± 6.11 years; 15 females) had a high PWV (≥ 10 m s−1). Postural control was evaluated using a force platform in four postural conditions: eyes open (EO) 1, eyes closed (EC), eyes open with a dual task (DT) and eyes open again (EO2). Using sway path traveled and surface covered by the center of foot pressure, we calculate the length function of surface (LFS). This ratio provides information about the precision (surface) of postural control and the effort made (length) by the subjects.
Results
After an age-adjustment, LFS was lower in EO than in EC and DT in both groups (p ≤ 0.001). LFS was higher in participants with high PWV both in eyes open and eyes closed conditions (p < 0.05). LFS increased when PWV increased in EO (p < 0.01) and EC conditions (p < 0.001) but not when a dual task was performed.
Conclusion
Difficulties in maintaining equilibrium under a dual-task condition are more pronounced in people with increased arterial stiffness. These data suggest that understanding of the influence of the arterial stiffness level on specific balance control parameters could contribute to propose better balance-oriented rehabilitation programs in older adults in an attempt to prevent fall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physiological aging is accompanied by deterioration in the quality of movement [1] and impairment in postural control causing loss of balance [2]. Thus, all these age-related changes, without any pathology, put older adults in a precarious situation in walking, gradually resulting in a loss of autonomy in addition to an increased risk of falling [3].
Clinical practice shows that balance disorders which could promote falls are frequent in older patients [4, 5]. Indeed, older patients with systolic hypertension, which is a clinical manifestation of the arterial stiffness, are particularly susceptible to the risk of variability in blood pressure and to orthostatic hypotension which can result in falls [6, 7].
Moreover, arterial stiffness is a recognized risk factor of subcortical vascular lesions that may promote the occurrence of dementia syndromes [8,9,10,11,12]. Falls and postural disorders can be related to cerebrovascular attacks [13]. Arterial stiffness can also cause vascular alterations of the vestibular system, generating vertigo, dizziness and falls. Finally, arterial stiffness can be responsible for balance disorders through proprioceptive disorders secondary to atherosclerosis. However, few studies in the literature have addressed the direct association of arterial stiffness and balance control impairment [4, 5]. Due to this, we decided to evaluate the possible impairment of postural control using sensitive tests, such as the dual-task situations in older adults according to arterial stiffness. Balance evaluation in hypertensive older patients subjected to multiple tasks is even more interesting because arterial stiffness can lead an alteration of central information processing through the nervous system lesions it causes.
Several studies examined the effect of a cognitive task on posture, which compared the motor task posture stabilization during a cognitive task between non-hypertensive older subjects and to younger subjects. Most studies have shown that an additional cognitive task significantly worsens a balance disorder, evaluated by the increase in the movement of the center of foot pressure in the older adults [14, 15]. These findings are possibly consistent with electromyography data which showed a significant decrease in muscle activity, in the older adults, during this same dual task [16]. However, the other authors either found no effect or showed a positive effect of cognitive task on the balance of presumed healthy older adults depending on the type of cognitive task [17].
Arterial stiffness could can be caused by lesions in the white subcortical matter, or through dizziness associated with postural hypotension or vascular alterations of the vestibular system, balance disorders and walking. Stiffness can occur without clinically overt cerebral vascular disease. A cognitive task can be a destabilizing factor which will become even more important in the context of accelerated aging that can cause a cognitive weakness. This can result in balance disorders, which can cause impaired central processing information through the central nervous system.
This study aimed to compare the postural control without or with dual task, in patients over 65 years, depending on the degree of arterial stiffness, measured using the carotid-femoral [Pulse Wave Velocity (PWV)].
Materials and methods
Participants
Forty-seven volunteers over the age of 65 years participated in this study. The exclusion criteria were: musculoskeletal disorder, chronic neurological disorder (dementia, stroke, Parkinson’s disease, and diabetic neuropathy), uncorrected visual pathology, vestibular pathology, progressive organic pathology, psychotropic drug use, opposing personality, low motivation or any other emotional or intellectual problem which may invalidate the consent or limit the possibilities of the participant to cooperate with the protocol requirements. Subjects were assigned into two groups function of degree of arterial stiffness, measured using the carotid-femoral PWV. The PWV was considered as normal (≤ 10 m s−1) in the first group (Gr1), composed of 27 patients (mean age = 70.52 ± 4.02 years; 22 females). The PWV was considered as high (> 10 m s−1) in the second group (Gr2), composed of 20 patients (mean age = 75.93 ± 6.11 years; 15 females). Age, gender, and body mass index were recorded in each group.
All participants gave their written informed consent before the study, which was approved by the French Medical Ethical Committee (Comité de Protection des Personnes Est III de Lorraine).
Measurement of carotid-femoral pulse wave velocity
Carotid-femoral PWV was measured in the supine position after lying for 10 min. The PulsePen device (Diatecne, Milan, Italy) was used for measuring carotid-femoral PWV [18]. The delay between pulse waves was determined by a single high-fidelity applanation tonometer to obtain the carotid and femoral pulse recorded sequentially in highly rapid succession, using the ECG trace as reference [19]. Carotid-femoral PWV was calculated as the distance divided by the time delay measured between pressure upstroke at each site. Distance was determined using a sliding caliper subtracting the distance from the carotid location to the sternal notch from the distance between the sternal notch and the femoral site of measurement. The time delay was measured between the feet of the femoral and carotid waveforms. The foot of the wave was defined at the end of diastole, at the onset of the steep rise of the wavefront for the PulsePen.
A study group on behalf of the Artery Society, the European Society of Hypertension Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries suggested a critical cutoff value of carotid-femoral PWV of 10 m s−1 [20, 21]. The same cutoff value has also adopted by the European Society of Hypertension guidelines for hypertension management [22]. This cutoff value has been estimated while considering the additive value of arterial stiffness beyond traditional risk factors to predict the occurrence of a cardiovascular event.
Cognitive assessment
The Folstein Mini-Mental State Examination (MMSE) was used to assess the patient’s level of general cognitive domains (temporal and spatial orientation, learning ability and recall, attention, language and visual-constructive praxis) [23]. A score higher than 25 was considered as normal [24].
The short form of the Geriatric Depression Scale (GDS) [25] was used to rule out depression, which can affect the cognitive state. A score equal or higher than 10 was considered to be a sign of depression, in which case patients were not included in the study.
The evaluation of autonomy was assessed by the activities of daily living (ADL) scale [26] and the instrumental activities of daily living (IADL) scale [27]. For both scales, the higher the score is, the higher the dependency.
Posturography
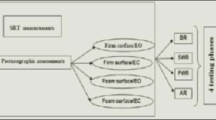
A vertical force platform, fitted with three strain-gauge force transducers (Satel, Blagnac, France) was used to perform posturography and to provide a measurement of the body sway in terms of displacement of the center of foot pressure (CoP) in a two-dimensional horizontal plane (recording time: 25.6 s, acquisition frequency: 40 Hz). Each participant was asked to stand upright on the platform, barefoot, feet abducted at 30°, heels separated by 3 cm, arms along the body, remaining as stable as possible and breathing normally [28, 29]. Sway path traveled and area covered by the CoP were used to calculate the LFS (length function of surface).
The LFS, providing information about the precision of postural control and the effort made (efficiency), is calculated using the following equation: \({\text{LFS}}\; = \;{\text{sway}}\;{\text{path/}}396\; \times \;\exp^{{\left( {0.0008\; \times \;{\text{area}}} \right)}}\) [30,31,32].
The analysis was carried out under four conditions, alternating with periods of rest for 1 min. In the third condition (the “dual task” condition), a cognitive task was included. This task consisted of serial subtraction by 7 from a random number between 100 and 200.
The four posturographic conditions consisted of:
-
Eyes open 1 condition (EO1);
-
Eyes closed condition (EC);
-
Eyes open with dual task (DT); the number and precision of the answer were recorded.
-
The eyes open condition was repeated at the end of the sequence (EO2) to assess possible learning or fatigue effects.
A difference between the LFS obtained in each condition was also calculated (Δ LFS) to show the improvement or deterioration of the postural control between each condition.
Statistical analysis
The SPSS statistical software package (version 23.0) was used in this study for data analysis. Qualitative data were expressed as number (n) and percentage (%) and were compared using χ2 test. Quantitative data were expressed as mean with standard deviation (SD) and non-parametric Mann–Whitney test were performed to compare characteristics of patients (age, sex, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, MMSE, ADL, IADL, and GSD) and the LFS in the four postural conditions of the two groups. Statistically significant differences were accepted for a probability level of p ≤ 0.05. Comparisons between the four conditions (EO1, EC, DT, and EO2) were produced using a non-parametric Wilcoxon test. The Bonferroni–Dunn procedure was applied to pairwise comparisons by adjusting “family-wise alpha” to a significance level of p ≤ 0.05/6 = 0.0083 in accordance with the six possible comparisons. A Student T test was used to evaluate the impact of the gap between eyes open and eyes closed or PWV on LFS, a highest T absolute value indicating the parameters having a higher impact on LFS.
For the comparison between the two groups with adjustment to age, a Mann–Whitney test was used. This test was also used to compare the Δ LFS between the two groups. Statistically significant differences were accepted for a probability level of p ≤ 0.05.
The relationship between PWV and LFS, without and with adjustment to age, was tested using a linear regression model. Statistically significant relationships were accepted for a probability level of p < 0.05.
The effect size of PWS on LFS can be estimated using two coefficients: the coefficient of determination, square of the Pearson correlation R (R2 < 0.2 as weak effect; 0.2 < R2 < 0.5 as moderate effect; R2 > 0.5 as strong effect), and the Cohen’s d measuring the standardized difference between the two means of LFS in normal PWV and high PWV.
Results
Participants with a high PWV were older than participants with normal PWV (p = 0.001) (Table 1). Due to this significant difference, an adjustment to age was made before comparing the characteristics of the populations. No other baseline characteristic was different between the two groups except for the resting heart rate which was higher in the participant with high PWV (p < 0.001). Participants were autonomous and did not have depression.
The LFS was significantly higher in participants with high PWV than in participants with normal PWV in EO1 condition (p = 0.031) and in EC condition (p = 0.017) (Fig. 1). No difference between the two groups was observed in EO with DT condition and EO2 condition. In both groups, LFS was higher in eyes closed condition than in both eyes open conditions (p < 0.0083). LFS in EO with DT was higher than in both EO1 conditions in participants with normal PWV (p < 0.0083) but was only higher than in EO2 condition in participants with high PWV (p < 0.0083). For the intra-group difference in LFS between YO and YC, evaluated by the paired Student T between these groups, we observed t = 6.84. For the inter-group difference in LFS between the two PWV groups, estimated by the usual T, we observed t = 2.41 in EO condition and t = 2.55 in EC condition. Therefore, we can conclude that the difference between EO and EC is larger than the difference between the two PWV groups.
Δ LFS between EC and DT condition was higher in participants with high PWV than in participants with normal PWV (p = 0.014).
Δ LFS between EC and EO2 condition was higher in participants with high PWV than in participants with normal PWV (p = 0.007).
Figure 2 shows the linear regression models for the relationship between LFS and PWV. The LFS increased when the PWV increased in EO1 condition (p < 0.001), EC condition (p < 0.001), EO with DT condition (p = 0.003), EO2 condition (p = 0.001). The LFS increased when the age-adjusted PWV increased in EO1 condition (p < 0.001), EC condition (p < 0.001), EO2 condition (p = 0.01). PWV has a medium effect on LFS. After age-adjustment, we observed r2 = 0.269 in EO condition and r2 = 0.445 in EC condition. For the Cohen’s d measuring the standardized difference between the two means of LFS in normal PWV and high PWV, we observed d = 0.31 in EO condition and d = 0.51 in EC condition.
Discussion
This study showed higher values of LFS in postural performances for patients with increased arterial stiffness. Depending on the postural condition, the values of the LFS varied in the same way in patients with normal or higher arterial stiffness. LFS values increased in the following order: (1) eyes open on firm support, (2) dual tasking in eyes open and (3) eyes closed. LFS was lower when condition with eyes open has been reproduced at the end of the evaluation, indicating a learning effect in both groups. The LFS increased when the PWV increased in eyes open and eyes closed.
During the fulfillment of a cognitive task or in eyes closed condition, the LFS increased, indicating more energetic expenditure requirement and/or lower precision of balance [31]. Jamet et al. (2004) [14] showed that the achievement of a cognitive task could induce postural perturbations. A cognitive task requires attention to maintain balance control and needs to share attentional resources between counting task and postural control [14]. In this context and depending on the limited attentional resources available, subjects could be forced to grade their mental activities and focus their attention on relevant information [14]. To perform a backward counting task, the subject mentally uses visual mental images of the calculation and so is partly disconnected from external visual landmarks, reducing the visual anchorage required to control balance [14]. Older adults are more dependent in visual information to maintain postural control [29, 33]. This loss of external visual anchorage when older adults perform a cognitive task can explain the balance perturbation observed [14].
A significant poorer postural stability in eyes closed conditions compared with eyes open confirms the importance of the visual system for body balance [34, 35]. Vision facilitates head stabilization in older adults to compensate for age-related decrease in vestibular and proprioceptive systems involved in maintenance of postural control [36]. The modulation of the somatosensory and visual pathway by eye closure diminishes with age and the dominance of the visual system is more pronounced in the aging brain [37].
Higher LFS was observed in participants with increased arterial stiffness compared to the participants with lower values of PWV. PWV has a moderate effect on LFS in eyes open (first condition) and eyes closed and a weak effect on LFS in dual task and eyes open (last condition). The gap between eyes open and eyes closed has a higher impact on LFS than PWV. During the fulfillment of eyes open or closed conditions, more energetic expenditure is required for patients with arterial stiffness to maintain balance and its precision compared to participants with normal wave velocity. Arterial stiffness may be explained by gradual loss of the cushioning capacity of the aorta and early wave reflection from the periphery, resulting in lesion of small vessels and thus, a lower irrigation of various organs [38]. Wong et al., (2004) [39] showed that multiorganic and multisystemic manifestations arising from arterial stiffness could increase the risk of falls through impaired cognitive function and postural control or peripheral neurological changes. In older adults, aortic stiffness may be detrimental to mobility.
This study has several limitations. Limited sample size of mostly female population needs to be highlighted. There are no data available on the fitness state or muscle mass of the population. It is well known that exercise capacity and muscle mass play a significant role in modulating arterial stiffness [40]. We do not have information on the extent to which participants practice physical and sport activities other than ADL and IADL questionnaire responses. In particular, combined aerobic and low-intensity resistance exercise training increases basal nitric oxide production and could have a beneficial effect on arterial stiffness in older adults [41].
Conclusion
Postural control efficiency is better in people with normal pulse wave velocity than in patients with higher pulse wave velocity. The impact of arterial stiffness on different organs, more particularly through a deficit in irrigation, can increase the risk of falls through impaired cognitive function and postural control. More energetic expenditure is required for patients with arterial stiffness to maintain balance and its precision. These data suggest that understanding of the influence of the arterial stiffness level on specific balance control parameters could contribute to propose better balance-oriented rehabilitation programs in older adults in an attempt to prevent fall.
References
Salthouse TA, Somberg BL (1982) Time-accuracy relationships in young and old adults. J Gerontol 37:449–453
Mourey F, Pozzo T, Rouhier-Marcer I, Didier JP (1998) A kinematic comparison between elderly and young subjects standing up from and sitting down in a chair. Age ageing 27:137–146
Ungar A, Rivasi G, Petrovic M et al (2020) Toward a geriatric approach to patients with advanced age and cardiovascular diseases: position statement of the EuGMS special interest group on cardiovascular medicine. Eur Geriatr Med 11(1):179–184. https://doi.org/10.1007/s41999-019-00267-0
Hausdorff JM, Herman T, Baltadjieva R, Gurevich T, Giladi N (2003) Balance and gait in older adults with systemic hypertension. Am J Cardiol 91:643–645
Lipsitz LA (1985) Abnormalities in blood pressure homeostasis that contribute to falls in the elderly. Clin Geriatr Med 1:637–648
Matsubayashi K, Okumya K, Wada T, Osaki Y, Fujisawa M, Doi Y, Ozama T (1997) Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke 28:2169–2173
Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ (1991) Prevalence of postural hypotension at baseline in the systolic hypertension in the elderly program (SHEP) cohort. J Am Geriatr Soc 39:1057–1064
Van Swieten JC, Geyskes GG, Derix MM, Peeck BM, Ramos LM, van Latum JC, van Gijn J (1991) Hypertension in the elderly is associated with white matter lesions and cognitive decline. Ann Neurol 30:825–830. https://doi.org/10.1002/ana.410300612
Schmidt R, Fazekas F, Koch M, Kapeller P, Augustin M, Offenbacher H, Fazekas G, Lechner H (1995) Magnetic resonance imaging cerebral abnormalities and neuropsychologic test performance in elderly hypertensive subjects. Arch Neurol 52:905–910
Scheinberg P (1988) Dementia due vascular disease: a multifactorial disorder. Stroke 19:1291–1299
Ylikoski R, Ylikoski A, Raininko R, Keskivaara P, Sulkava R, Tilvis R, Erkinjuntti T (2000) Cardiovascular disease, health status, brain imaging findings and neuropsychological functioning in neurologically healthy elderly individuals. Arch Gerontol Geriatr 30:115–130
Pfitzenmeyer P, Martin-Hunyadi C, Mourey F, d’Athis P, Baudouin N, Mischis-Troussard C (2002) Cardiovascular characteristics and cerebral CT findings in elderly subjects with psychomotor disadaptation syndrome. Aging Clin Exp Res 14:100–107
Kuo HK, Lipsitz LA (2004) Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci 59:818–826
Jamet M, Deviterne D, Gauchard GC, Vançon G, Perrin P (2004) Higher visual dependency increases balance control perturbation during cognitive task in the elderly people. Neurosci Lett 25:179–184. https://doi.org/10.1016/j.neulet.2004.02.010
Jamet M, Deviterne D, Gauchard GC, Vançon G, Perrin P (2007) Age-related part taken by attentional cognitive processes in standing postural control in dual-task context. Gait Posture 25:179–184. https://doi.org/10.1016/j.gaitpost.2006.03.006
Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA (2000) Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A boil Sci Med Sci 55:MI112-119
Deviterne D, Gauchard GC, Jamet M, Vançon G, Perrin P (2005) Added cognitive load through rotary auditory stimulation can improve the quality of postural control in the elderly. Brain Res Bull 64:157–162. https://doi.org/10.1016/j.brainresbull.2004.10.007
Salvi P, Giuseppe L, Labat C, Ricci E, Pannier B, Benetos A (2004) Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens 22(12):2285–2293. https://doi.org/10.1097/00004872-200412000-00010
Salvi P, Magnani E, Valbusa F, Agnoletti D, Alecu C, Joly L, Benetos A (2008) Comparison study of methodologies for pulse wave velocity estimation. J Hum Hypertens 22:669–677
Butlin M, Qasem A (2017) Large artery stiffness assessment using SphygmoCor technology. Pulse 4:180–192
Van Bortel L.M., Laurent S., Boutouyrie P., Chowienczyk P., Cruickshank J.K., De Backer T., Filipovsky J., Huybrechts S., Mattace-Raso F.U.S., Protogerou A.D., Schillaci G., Segers P., Vermeersch S., Weber T., Artery Society, European Society of Hypertension Working Group on Vascular Structure and Function, European Network for Noninvasive Investigation of Large Arteries (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens 30:445–448
Williams B, Mancia G, Spiering W, Agabiti RE, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I (2018) Practice guidelines for the management of arterial hypertension of the European society of hypertension (ESH) and the European society of cardiology (ESC). Blood Press 27:314–340
Folstein MF, Folstein SE, McHugh PR (1975) « Mini-mental state ». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Zuliani G, Polastri M, Romagnoli T, Marabini L, Seripa D, Cervellati C, Zurlo A, Passaro A, Brombo G (2020) Clinical and demographic parameters predict the progression from mild cognitive impairment to dementia in elderly patients. Aging Clin Exp Res. https://doi.org/10.1007/s40520-020-01697-8
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17(1):37–49. https://doi.org/10.1016/0022-3956(82)90033-4
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA 185:914–919. https://doi.org/10.1001/jama.1963.03060120024016
Kuo HK, Lipsitz LA (2004) Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci 59(8):818–826. https://doi.org/10.1093/gerona/59.8.m818
Lion A, Spada RS, Bosser G, Gauchard GC, Anello G, Bosco P et al (2013) Biological determinants of postural disorders in elderly women. Int J Neurosci 123(1):24–30. https://doi.org/10.3109/00207454.2012.722570
Perrin P, Jeandel C, Perrin CA, Béné MC (1997) Influence of visual control, conduction, and central integration on static and dynamic balance in healthy older adults. Gerontology 43(4):223–231. https://doi.org/10.1159/000213854
Bizzo G, Guillet N, Patat A, Gagey PM (1985) Specifications for building a vertical force platform designed for clinical stabilometry. Med Biol Eng Comput 23:474–476. https://doi.org/10.1007/BF02448937
Lion A, Spada RS, Bosser G, Gauchard GG, Anello G, Bosco P, Calabrese S, Iero A, Stella G, Elia M, Perrin P (2014) “Postural first” principle when balance is challenged in elderly people. Int J Neurosci 124(8):558–566. https://doi.org/10.3109/00207454.2013.864288
Peultier-Celli L, Audouin M, Beyaert C, Perrin P (2020) Postural control in lyric singers. J Voice 23:S0892-1997(20)30154–5. https://doi.org/10.1016/j.jvoice.2020.04.019
Maire R, Mallinson A, Ceyte H, Caudron S, Van Nechel C, Bisdorff A, Magnusson M, Petersen H, Kingma H, Perrin P (2017) Discussion about visual dependence in balance control: European society for clinical evaluation of balance disorders. J Int Adv Otol 13(3):404–406. https://doi.org/10.5152/iao.2017.4344
Brooke-Wavell K, Perrett LK, Howarth PA, Haslam RA (2002) Influence of the visual environment on the postural stability in healthy older women. Gerontology 48(5):293–297. https://doi.org/10.1159/000065252
Perrin P, Gauchard GC, Perrot C, Jeandel C (1999) Effects of physical and sporting activities on balance control in elderly people. Br J Sports Med 33(2):121–126
Cromwell RL, Newton RA, Forrest G (2002) Influence of vision on head stabilization strategies in older adults during walking. J Gerontol A Biol Sci Med Sci 57(7):M442-448
Brodoehl S, Klinger C, Witte OW (2016) Age-dependant modulation of the somatosensory network upon eye closure. Behav Brain Res 298:52–56
Watson NL, Sutton-Tyrell K, Youk AO, Boudreau RM, Mackey RH, Simonsick EM, Rosano C, Hardy SE, Windham BG, Harris TB, Najjar SS, Lakatta EG, Atkinson HH, Johnson KC, Bauer DC, Newman AB (2011) Arterial stiffness and gait speed in older adults with and without peripheral arterial disease. Am J Hypertens 24(1):90–95
Wong AK, Lord SR, Trollor JN, Sturnieks DL, Delbaere K, Menant J, Brodaty H, Sachdev PS, Close JC (2004) Hight arterial pulse wave velocity is a risk factors for falls in community-dwelling older people. J Am Geriatr Soc 62(8):1534–1539
Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksäss A (2015) Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: a review. Eur J Sport Sci 15(5):443–457. https://doi.org/10.1080/17461391.2014.955129
Otsuki T, Namatame H, Yoshikawa T, Zempo-Miyaki A (2020) Combined aerobic and low-intensity resistance exercise training increases basal nitric oxide production and decreases arterial stiffness in healthy older adults. J Clin Biochem Nutr 66(1):62–66. https://doi.org/10.3164/jcbn.19-81
Acknowledgements
The authors acknowledge Dr. Art Mallinson (Vancouver, British Columbia, CA) for his helpful advice in the final read-through of the manuscript.
Funding
This study was promoted by the Nancy University Hospital, France, which, as promoter, according to the law, paid for the study’s insurance coverage.
Author information
Authors and Affiliations
Contributions
All the authors contribute to the study concept and design. LP-C, AL and SB realized the posturography recordings. GW and AB realized the cardiovascular investigations determining the assignment in a given group. Statistical analyses were made under the supervision of RG, statistician. PhP is principal investigator of the study. All the authors contribute to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the French Medical Ethical Committee (Comité de Protection des Personnes Est III de Lorraine) and by the National Agency for Medicines and Health Products Safety.
Informed consent
All the participants gave their written informed consent prior to the study.
Consent for publication
All the authors consent for publication.
Availability of data and material
The Direction for Research of the Nancy University Hospital, as study Promoter (which is not Investigator of the study), is the owner of the data and may allow their transmission according to the terms of an agreement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peultier-Celli, L., Lion, A., Buatois, S. et al. Relation of arterial stiffness with postural control in older people. Eur Geriatr Med 12, 871–879 (2021). https://doi.org/10.1007/s41999-021-00468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-021-00468-6