Abstract
The biocompatibility of clinoptilolite/alumina/bovine hydroxyapatite (Cp - A12O3 - BHA) composite, at different ratio obtained by powder pressing process, were investigated studying the behavior of osteosarcoma (SAOS-2) cells. The biocompatibility was examined by means of cytotoxicity and cytocompatibility tests. The structure and morphology of bioceramic composites were studied by scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) technique. The results showed that these materials have no toxic effects. The natural composite that fabricated in this study may be a promising approach for bone-engineering applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroxyapatite (HA) {Ca10(PO4)6(OH)2} is one of the most well-known phosphates in the biologically active phosphate ceramic family [1]. Due to the poor mechanical properties of pure HA [2, 3], usually, HA-based composite materials are preferred for load-bearing biomedical applications and HA must be mixed with biocompatible materials [4, 5].
When considered in terms of biocompatibility properties, ceramic bone graft substitutes including HA, tricalcium phosphate (TCP), or their combinations have excellent biocompatibility and have been used experimentally and clinically for filling bone defects [6, 7]. Other materials such as phosphate ceramics and in these different bioglass compositions have been used as sintering aids to evaluate in sintering of HA or as a component of composite to enhance material and bioactivity properties [8].
Clinoptilolite (Cp) is also a member of the Zeolite family that is defined with the following basal formulations (Na,K,Ca)4Al6Si30O72.24H2O [9]. Zeolite in the form of Cp has an increasing potential in medical and industrial field [10, 11]. It’s medical application fields are increasing rapidly. In vitro studies, showing similar behaviors like bioglass, draws attention for hard tissue applications. It has been also reported by Pavelić et al. that aluminosilicate particulates relates with specific cells and modifies their pathways. These reaction leads regulating of the gene expression. Especially in some in vivo studies on dogs and rats, it was reported that several zeolite compounds had inhibited the growth of some cancer cells [10, 11]. Moreover, Cp is a natural material, which includes trace elements that the silicon and low amounts containing zeolite was observed to improve bone formation [10]. Trace minerals are known to generally play a vital role in the human body homeostasis.
Alumina (A12O3) is one of the most widely reported particular biomaterial combinations used, which is used as an ideal reinforcement oxide [6]. It is also known that Al2O3 caused also chemical inertness that makes it very biocompatible, having almost no chemical impact on the body. Adding Al2O3 between 5 and 25% to HA and sample containing 15 wt% alumina reveals the maximum value for compressive strength and Rockwell hardness [12].
The addition of A12O3 to HA leads to a solid-state reaction between the alumina and CaO, which occurred at elevated temperatures (since HA only decomposes to CaO at high temperatures). Calcium aluminates are subsequently fabricated by the solid-state reaction. Calcium aluminates have a different sintering rate from that of the calcium phosphate matrix and therefore influence the sintering behavior of HA. The different sintering temperature rates between calcium aluminate and calcium phosphate phases result in the need for higher sintering temperatures of over ∼1400 in order to accomplish the complete densification of HA with 20 wt.% of A12O3 [13].
In this study, 5 and 15 wt% Cp, and 5 and 15 wt% Al2O3 were mixed with bovine hydroxyapatite (BHA). Using the above compositions, samples were prepared and sintered at 1300 °C. Furthermore, Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) studies were performed for the composites which resulted from optimum cytotoxicity tests. The aim of this study is to obtain the proper biological composites for future biomedical engineering applications.
Materials and methods
Materials
BHA source femoral parts of bovine were studied. Bovine bones were collected from a chain store from an international abattoir (CarrefourSA Haramidere, Istanbul, Turkey). The BHA used in this study was fabricated by calcination at 850 °C and milling, according to a method of Oktar et al. [14]. The calcined BHA parts were ground into smaller particles with wet ball milling and then dried. BHA powders were sieved through a 75 μm sieve. Cp was supplied by “Rota Madencilik” - “Rota Mining”, Gordes, Izmir. The purity of mineral is about 90–95% (<40 μm). The chemical analysis of the Cp is shown at [1]. Pure HA (commercial) and Al2O3 were supplied by Alfa Aeser 42572 (<1 μm).
Preparation of composites
Powdered raw materials were mixed with two different ratios. First is 5 wt% Cp, 5 wt% Al2O3 – BHA and second is 15 wt% Cp, 15 wt% Al2O3 – BHA.
These mixed combinations were unaxial pressed (at 350 MPa) to cylindrical pellets (2 mm of height and 15 mm in diameter). The pressed BHA-composites green bodies (Fig. 1a) were sintered 1300 °C for 4 h (Fig. 1b). Pellets to be used in MTT assays were powdered for reaction, 2 g of pure sample powders then all powdered and pellets were sterilized for 1 h at 180 °C prior to use.
Sample characterization
The presence of functional groups in the BHA-Cp-alumina composites was identified by Fourier transform infrared spectroscopy (FTIR) on a Bruker/Alpha-T using the classic KBr powder technique. The spectra were recorded over the 400–4000 cm−1 region with a scan rate of 4 cm−1 and spectra were recorded as a function of time in 1 min intervals at 25 °C. (Fig. 2). The size and morphology of the samples were studied by using a FEI, Quanta FEG450 scanning electron microscope (SEM). The elements present in each sample were analyzed the same microscope’s energy-dispersive X-ray spectroscopy (EDX) function (Fig. 3).
Biocompatibility evaluation
Biocompatibility of composites was evaluated by culturing with SAOS-2 cells provided by American Type Culture Collection (ATTC). The cells were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM) medium (cell culture grade; Sigma) supplemented with 10% volume fraction of calf serum (cell culture grade; Gibco, Rockville, MD, USA), 1% penicillin, streptomycin, and L-glutamine. Cells were subcultured once a week using trypsin and maintained at 37 °C in a humidified incubator with 95% air and 5% CO2. The medium was changed every 3 days. The confluent cells were used in cytotoxicity tests and SEM investigations.
Cytotoxicity tests: MTT assay
Firstly, conditioned medium or simulated body fluid (SBF) was prepared to understand any possible toxic effect leach-out of composites in the medium. For aim, 10 ml fresh medium was added in tubes and powder (2 g) and waited 72 h in the incubator to get medium extraction. Later, these conditioned medium was used for cytotoxicity tests which is the supernatant [15].
The SAOS-2 cells (about 105 cells per well) were seeded onto the plastic plate (98-well cell); the cells were cultured with conditioner medium which called supernatant (used conducting cytotoxicity tests) in a humidified incubator for 3 days. Only SAOS-2 cells were also cultured in DMEM as the control. Cell viability was measured by determining mitochondrial NADH/NADHP-dependent dehydrogenase activity, which resulted in the cellular conversion of the 3-(4,5-dimethylthiazol-2-gl)-5-(3-carboxymethoxylphenyl)-2-(4-sulfophenyl-2H) tetrazolium salt into a soluble formazan dye. After 3 days, supernatants were removed, and 10 μl 3-{4,5-dimethylthiazol-2yl}-2,5-diphenyl-2H-tetrazolium-bromide (MTT- 5 mg/ml- Sigma) solution was added to each well, following incubation at 37 °C for 72 h in humidified atmosphere at 5% CO2 in air [16]. MTT was taken up by live cells and reduced in the mitochondria to an insoluble purple formazan granule. Subsequently, 50 μl of acidified supernatant was discarded, and the precipitated formazan was dissolved in dimethyl sulfoxide (150 μl per well), and optical density of the solution was evaluated using a microplate spectrophotometer (Rayto RT-2100C, AB Medikal, Istanbul, Turkey) at a wavelength of 570 nm. Cytotoxicity assays were conducted in triplicate and at least three experiments. [16, 17]. A Student’s t test was performed to determine the statistical significance between experimental groups. A value of p < 0.05 was considered to be statistically significant (Fig. 4).
SEM investigations
Sterile pellets were used to evaluate cell adhesion. After washing composites with medium, they were put in a well with 5 ml of fresh medium (without cells) overnight in an incubator. SAOS-2 cells were seeded on the top of prewetted specimens (105 cells per specimen). The specimens were then placed in the wells of plastic dishes (6-well cell culture plate in an incubator for 3 h to allow the cells to attach, and additional 1 ml culture medium was added into each well. The cell where on composite constructs were cultured in a humidified incubator at 37 °C with 95% air and 5% CO2. After 3 days, the media were removed, and specimens were fixed with 3% volume fraction of glutaraldehyde, subjected to graded (50–100%) alcohol dehydration, rinsed with isoamyl acetate, and observed with FEI, Quanta FEG450 scanning electron microscope (SEM) at 10 kV (Fig. 5) [16].
Results and discussion
In the present study, calcium silicate ceramics may be potential candidates as bioactive and degradable scaffolds for hard tissue repair and tissue engineering applications.
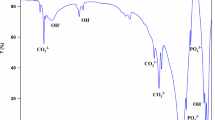
FTIR spectra of the starting pure (control samples) Al2O3, Cp, BHA, and BHA composites doped with 15 wt% Cp- 15 wt% Al2O3 and 5 wt% Cp- 5 wt% Al2O3 are revealed in Fig. 2. Most of the bands due to the phosphate vibrations of HA have hugely increased in intensity after sintering at 1300 °C. This behavior has been described by other researchers [18]. Band positions and relative intensity showed that other phases were mainly Ca3(PO4)2 and some amount of Ca2P2O7, showing the loss of −OH groups as the sintering temperature was increased, indicating the occurrence and development of dehydroxylation (Fig. 2) [19]. Bands corresponding to OH groups and to PO4 3− and also asymmetric stretching vibration modes of T-O bonds TO4 tetrahedra (T=Si and Al) were still present when sintering temperature increased to 1300 °C. Moreover, sintering temperature was increased at 1300 °C. Even though the material had been sintered at 1300 °C, a small amount of OH was still present, as indicated by indicating that small quantity of OH is still present as attested by the presence of small bands above 1040 cm−1 (1057, 1251, and 1395 cm−1) assigned to Al-OH or O-H in Fig. 2a, b. Figure 2c shows the characteristic peaks of HA (at 560, 609, 962, and ∼1000 cm−1 due to phosphate vibrations) [18]. The crystalline powder produces two characteristic stretching modes of O-H bands at about 560 cm−1, which are appeared in Fig. 2c spectra of BHA. The positions of the main adsorption bands for the Cp were listed as follows: 473, 1000, 1097, 1252, 1394, and 3686 cm−1 (in Fig. 2d). The presence of small bands at 3686 and 1394 cm−1 are assigned to water molecules (OH group) related to Na and Ca in the networks and structures in the Cp [20, 21]. The band at 1252 cm−1 is attributed to external TO4 [22]. One thousand and 1097 cm−1 band corresponds to asymmetric stretching vibration modes of internal T-O bonds TO4 tetrahedra (T=Si and Al) [20]. They are very strong and broad bands with fine structure [22]. The band at 473 cm−1 is attributed to Si-O-Si bending flexural vibration mode [20, 23]. The position of the mean adsorption bands for the Al2O3 was recorded as follows (in Fig. 2e): 457, 559 and 644 cm−1. The bands at 457 and 649 cm−1 are attributed to the presence of the AlO6 octahedra condensates, which are building blocks of the α-Al2O3 structure [24]. A small quantity of OH is still present as proved by the presence of small bands above 1000 cm−1 (1057 cm−1) assigned to Al-OH or O-H [25].
The SEM image in Fig. 3a shows the surface where EDS measurement have been performed for the 5 wt% Cp, 5 wt% Al2O3-BHA. On the surface, the composition mainly consists of calcium (44.7 at.%), oxygen (27.35 at.%), phosphorous (18.47 at.%), and aluminum (6.82 at.%) and also a low amount of silicon (2.82 at.%) (Fig. 3b). The 15 wt% Cp, 15 wt% Al2O3 -BHA composite samples (Fig. 3a) has also performed EDS measurement, which has seen calcium (37.32 at.%), oxygen (27.76 at.%), phosphorous (14.69 at.%), aluminum (11.57.82 at.%), and silicon (8.67 at.%) (Fig. 3b).
In Fig. 4, MTT assay results showing the SAOS-2 cells proliferation on the pure BHA, pure HA(commercial), pure Al2O3, 5 wt% Cp, 5 wt% Al2O3-BHA and 15 wt% Cp, 15 wt% Al2O3-BHA composite samples after 3 days of culture. The data are presented as mean 7SD and values with P40.05 were considered as statistically non-significant [11].
In vitro biocompatibility of the BHA, Al2O3, HA (commercial HA) and two Cp–Al2O3–HA composites was investigated in SAOS-2 cells. The cell proliferation was evaluated after 3 days. As it is shown in Fig. 4, osteoblast cells was better growing than control and alumina on BHA, HA, and composites.
The effect of the composites on cell proliferation was observed to be dose-dependent, which increased with the increasing zeolite and alumina content in 15 wt% compared to 5 wt%, which could have affected the cell adherence property. It was determined that composites zeolites and alumina were not toxic and did not affect the morphology and viability of osteoblast cells.
These results suggested that lower concentration of zeolite could stimulate cell proliferation and attachment. These findings are also in accordance with those reported in previous studies, indicating that zeolite-based materials can be considered for bone tissue engineering applications.
Figure 5 shows SEM images revealing SAOS-2 cells morphology at 3 days of cell morphology on the pure BHA, pure HA, pure Al2O3, 5 wt% Cp, 5 wt% Al2O3-BHA, and 15 wt% Cp, 15 wt% Al2O3-BHA composite samples. The low (column with cell) and high magnification (column with cell and no cell) SEM images shown that the cells had increase well on the surface of all pure samples and composite surfaces. Quite similar cell morphology was indicated on both type of HA, Al2O3, 5 wt% Cp, 5 wt% Al2O3-BHA, and 15 wt% Cp, 15 wt% Al2O3-BHA composites sintered at 1300 °C. Interestingly, in the case of these samples, more cells have involved per unit of area, being also much better attach in comparison to the varying composite samples sintered at 1300 °C. In addition, the SAOS-2 cells appear to have involved very well in the composites surface being able to connection the micropores with their lamellipodia.
Conclusions
A new biocompatible material was prepared by powder pressing followed by sintering at 1300 °C. It can be seen that composite sample’s biocompatibility behavior was higher than that of the pure HA, pure BHA, and pure Al2O3. Composite samples have revealed a significant potential for biomedical applications such as bone scaffolds.
References
Kalkanadelen, C., Gunduz, O., Akan, A., Oktar, F.N.: Part 1: Clinoptilolite - Alumina - Hydroxyapatite Composites for Biomedical Engineering. Aust Ceram. (2016)
O’Brien, W.J.: Dental Materials and Their Selection 3rd ed. Edited by William J. O’Brien, PhD, FADM. Quintessence Pub. Co. Inc., Chicago (2002)
Erkmen, Z.E., Genc, Y., Oktar, F.N.: Microstructual and mechanical properties of hydroxyapatite—zirconia composites. Jurnal American Society. 90, 2885–2892 (2007)
Oktar, F.N., Goller, G.: Sintering effects on mechanical properties of glass—reinforced hydroxyapatite composites. Ceram Int. 28, 617–621 (2002)
Oktar, F.N., Agathopoulo, S., Ozyegin, L.S., Gunduz, O., Demirkol, N., Bozkurt, Y., Salman, S.: Mechanical properties of bovine hydroxyapatite (BHA) of composites doped with SiO2, MgO, Al2O3, and ZrO2. J Mater Sci Mater Med. 18, 2137–2143 (2007)
Welch, R.D., Hudson, B., Crawford, K., Zhang, H., Zobitz, M., Bronson, D., Krishnan, S.: Subchondral defects in caprine femora augmented with in situ setting hydroxyapatite cement, polymethylmethacrylate, or autogenous bone graft: biomechanical and histomorphological analysis after two-years. J Orthop Res. 20, 464472 (2002)
Fielding, G., Bose, S.: SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 9, 9137–9148 (2013)
Sampaio, B.V., Göller, G., Oktar, F.N., Valério, P., Goes, A., Leite, M.F.: Evaluation of osteoblast viability, alkaline phosphatase production and collagen secretion in the presence of hydroxyapatite reinforced with oxide glasses. Key Eng Mater. 284-286, 635–638 (2005)
Kowalczyk, P., Sprynskyy, M., Terzyk, A.P., Lebedynets, M., Namieśnik, J., Buszewski, B.: Porous structure of natural and modified clinoptilolites. J Colloid Interface Sci. 297, 77–85 (2006)
Pavelić, K., Hadžija, M., Bedrica, L., Pavelić, J., Diki, I., Katić, M., Kralj, M., Bosnar, M.H., Kapitanović, S., Poljak-Blaži, M., Križanac, Š., Stojković, R., Jurin, M., Subotić, B., Čolić, M.: Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med. 78, 708–720 (2001)
Iqbal, N., Kadir, M.R.A., Mahmood, N.H.B., Yusoff, M.F.M., Siddique, J.A., Salim, N., Froemming, G.R.A., Sarian, M.N., Raghavendran, H.R.B., Kamarul, T.: Microwave synthesis, characterization, bioactivity and in vitro biocompatibility of zeolite–hydroxyapatite (Zeo–HA) composite for bone tissue engineering applications. Ceram Int. 40, 16091–16097 (2014)
Hannora, A.E.: Preparation and characterization of hydroxyapatite/alumina nanocomposites by high-energy vibratory ball milling. J Ceramic Science and Technology. 05(04), 293–298 (2014)
Ji, H., Marquis, P.M.: Sintering behaviour of hydroxyapatite reinforced with 20 wt% AI203. Journal of Materıals Science. 28, 1941–1945 (1993)
Oktar, F.N., Kesenci, K., Piskin, E.: Artif Cell, Blood Sub Immobil Biotechnology. 27(4), 367–379 (1999)
Komur, B., Lohse, T., Can, H.M., Khalilova, G., Geçimli, Z.N., Aydoğdu, M.O., Kalkandelen, C., Stan, G.E., Sahin, Y.M., Sengil, A.Z., Suleymanoglu, M., Kuruca, S.E., Oktar, F.N., Salman, S., Ekren, N., Ficaiand, A., Gunduz, O.: Fabrication of natural pumice/hydroxyapatite composite for biomedical engineering. BioMedEngOnLine. 15, 81 (2016). doi:10.1186/s12938-016-0203-0
Deniz, D.Y., Kahraman, M.V., Kuruca, S.E., Suleymanoglu, M., Gungor, A.: 4-vinylbenzene boronic acid—hydroxy apatite/polyvinyl alcohol based nanofiber scaffold synthesized by UV-activated reactive electrospinning. International Journal of Polymeric Materials and Polymeric Biomaterials. 64, 727–732. doi:10.1080/00914037.2014.1002130
Mosmann, T.: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 65, 55–63 (1983)
Figueiredo, M., Fernando, A., Martins, G., Freitas, J., Judas, F., Figueiredo, H.: Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram Int. 36, 2383–2393 (2010)
Pattanayak, D.K., Divya, P., Upadhyay, S., Prasad, R.C., Rao, B.T., Mohan, T.R.M.: Synthesis and evaluation of hydroxyapatite ceramics. Trends Biomater Artif Organs. 18(2), 87–92 (2005)
Mansouri, N., Rikhtegar, N., Panahi, H.A., Atabi, F., Shahraki, B.K.: Porosity, characterization and structural properties of natural zeolite—clinoptilolite—as a sorbent. Environ Prot Eng. 39, 139–152 (2013)
Zhan, Y., Lin, J., Li, J.: Preparation and characterization of surfactant-modified hydroxyapatite/zeolite composite and its adsorption behavior toward humic acid and copper (II). Environ Sci Pollut Res. 20, 2512–2526 (2013)
Pechar, F., Rykl, D.: Infrared spectra of natural zeolites of the stilbite group. Chem zvesti. 35(2), 189–202 (1981)
Lin, H., Liu, Q., Dong, Y., Chen, Y., Huo, H., Liu, S.: Study on channel features and mechanism of clinoptilolite modified by LaCl3. Journal of Materials Science Research. 2(4), 37–44 (2013)
Sanches, E.A., de Souza, S.M., Carvalho, A.P.L., Trovati, G., Fernandes, E.G.R., Mascarenhas, Y.P.: Nanocomposite based on polyaniline emeraldine-base and α-Al2O3: a structural characterization. International Int J Mater Res. 106(10), (2015) formerly Z. Metallkd. doi:10.3139/146.111280
Boumaza, A., Favaro, L., Lédion, J., Sattonnay, G., Brubach, J.B., Berthet, P., Huntz, A.M., Roy, P., Tétot, R.: Transition alumina phases induced by heat treatment of boehmite: an X-ray diffraction and infrared spectroscopy study. J Solid State Chem. 182, 1171–1176 (2009)
Acknowledgements
This study was supported by Istanbul University, Scientific Research Projects Coordination Unit, Project No: 29071.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalkandelen, C., Suleymanoglu, M., Kuruca, S.E. et al. Part 2: biocompatibility evaluation of hydroxyapatite-based clinoptilolite and Al2O3 composites. J Aust Ceram Soc 53, 217–223 (2017). https://doi.org/10.1007/s41779-017-0027-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-017-0027-9