Abstract
Previous studies proved that biochar provides potential solutions for hexavalent chromium (Cr(VI)) detoxification. However, the roles of inorganic constituents in addition to the organic carbon matrix still need to be verified. Besides, the interferences with environmental electron donors, including organic reductants like low molecular weight organic acids (LMWOAs) and inorganic reductants like Fe2+, also needs to be clearly elucidated. In this study, two typical kinds of biochar were compared for their performances and mechanisms in removing Cr(VI). The responses to exogenous electron donors were also examined. The removal of Cr(VI) by the biochar derived from maize straw, which had fewer inorganic content (Fe content < 0.1%, at%), was largely associated with the activity of the organic groups and the amount of persistent free radicals. While for the biochar derived from Fe-rich sludge (Fe content > 1%, at%), the Cr(VI) reduction was predominately contributed by the inorganic reducing component, i.e. Fe-containing fractions. For the exogenous reductants, the organic reductant LMWOAs (removal rate improved from 41 to 62% (p < 0.01)) were relative weaker than the inorganic reductant Fe2+ (removal rate improved to 76% (p < 0.01)). The better reduction by Fe than the organic molecules could be mainly contributed to the redox activity as well as the improved electron cycling with the biochar matrix. Besides, the precipitated Fe(III) after redox reaction on the biochar could further enhance the adsorption of Cr(VI) and reinforce the immobilization of Cr(III). These findings would help to develop highly cost-effective Fe-modified biochar based strategies for Cr(VI) detoxification.
Graphical abstract

Highlights
-
MBC with few ashes is more dependent on the organic functional groups.
-
Endogenous Fe in ash-rich SBC plays crucial roles in Cr(VI) removal.
-
LMWOAs enhance the reduction but hinder the adsorption.
-
Exogenous Fe2+ largely improves the reduction and reinforces the immobilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is one of the most concerned elements in heavy metal pollution control. Anthropogenic pollution from electroplating, metallurgy and dyeing industries (McNeill et al. 2012) causes serious water and soil pollutions if not treated properly (Zhang et al. 2017). Generally, Cr in environment stably occurs in the forms of Cr(VI) or Cr(III) (McNeill et al. 2012), whereas Cr(VI) holds much higher toxicity and mobility than Cr(III) (Lyu et al. 2018). Exposure to Cr(VI) would cause dermatotoxicity, neurotoxicity, genotoxicity, and immunotoxicity hazards, especially various malignancies and chromosomal damages (Mortada et al. 2023). Thus, one of the most commonly adopted treating strategies is to reduce Cr(VI) to Cr(III) for detoxification and better immobilization (Sharma et al. 2008; Fei et al. 2022).
Biochar is a carbonaceous material obtained through the pyrolysis of biomass (Chen et al. 2019). It gains increasing attention for its role in carbon sequestration and the potential for applications as solid fuel, adsorbent, and soil amendment (Spokas et al. 2012; Conte et al. 2015). It is of great interest in soil applications for improving soil physical properties, returning nutrients to soils and increasing soil productivity (Nelson et al. 2011). It is featured with porous structure (Spokas et al. 2012), which results in great potential for the adsorption of pollutants. Numerous studies prove that biochar has great adsorptive and removal ability to various of heavy metals, including arsenic, cadmium and Cr (Chen et al. 2021; Qin et al. 2020; Sun et al. 2016; Zhu et al. 2020). Besides, abundant functional groups on biochar surface create adequate sites for redox activities, and the organic functional groups like phenols and hydroxyls on biochar surface are related to the redox interactions with Cr(VI) (Chen et al. 2019; Xu et al. 2020b). Biochar could act as electron shuttle between pollutants and other electron donors as well (Kappler et al. 2014; Sun et al. 2017; Xu et al. 2019a). Combining the multiple roles as adsorbent, reductant and electron shuttle, biochar is believed to be a promising material for Cr(VI) adsorption and reduction. Applications of biochar for Cr(VI) detoxification were reported for water or soil in literature (Agrafioti et al. 2014a; Chen et al. 2021; El-Naggar et al. 2022).
However, biochars derived from feedstocks may vary in their properties (Zhao et al. 2013, 2015) and the performances in treating Cr(VI). Comparisons between different deriving methods (i.e. pyrolysis and hydrothermal carbonization) (Chen et al. 2021) or different pyrolyzing temperatures (Xu et al. 2020b; Zhou et al. 2016) have been reported. Currently, most reports for Cr(VI) detoxification by biochars mainly focused on phytomass-based biochars and their organic functional groups (Dong et al. 2011; Hsu et al. 2009; Liu et al. 2020; Zhou et al. 2016). While the comparison between typical biochars with varied inherent elemental compositions due to varied feedstocks, i.e. plant straws and municipal sludge, needs more investigations. Due to the usages of Fe- or Al- rich reagents and the adsorption of metals during wastewater treatments, municipal sludge-derived biochar often consists higher content of ashes than the phytomass-derived biochars, and thus has distinct surface physio- and electro-chemical properties (Zhao et al. 2013, 2015). It is therefore reasonable to inquire whether the endogenous mineral fractions (i.e. inorganic redox active elements) rather than the organic functional groups (i.e. organic electron donating groups) play more important roles in Cr(VI) detoxification.
Additionally, when applied to the environment, the coexisting environmental constituents with reductive capacities would impose interferences to Cr(VI) related processes. For instance, low molecular weight organic acids (LMWOAs), which have a wide range of sources in natural and industrial environment, from root exudates of plants (Jones et al. 2003), to wastewater treating additives (Mumtaz et al. 2008), could be potential weak organic electron donors for Cr(VI) (Yang et al. 2013). The surface functional groups on biochar would also be changed by the coexistence of LMWOAs due to complexation (Sun et al. 2016; Xu et al. 2019b). Thus, it could be implied that the adsorption and redox process of Cr(VI) by biochar would be affected by LMWOAs. Though the influences of organic acids on Cr(VI) removal by soil have been studied (Tian et al. 2010; Zhong and Yang 2012), report on the interaction between LMWOAs and biochar with regards to Cr(VI) transformation and immobilization is limited. Xu et al (2019b) found that the effects by seven different organic acids on the peanut shell derived biochar for Cr(VI) reduction varied, depending on the featured behaviors of the biochar derived at different temperatures. For typical biochars with distinct inherent organic and inorganic electron donors, the interactions with LMWOAs still needs more investigations.
Likely, iron (Fe) is also widely found in the environment naturally, which is of special importance as an inorganic redox active element (Nozoe et al. 2001). Materials with Fe modifications are widely employed for pollution controls (Ran et al. 2021; Song et al. 2014), including that for better reduction and removal of Cr(VI) (Wen et al. 2022; Yang et al. 2021). While it was less focused on the interaction between free iron cations with biochar for Cr(VI) detoxification. Xu et al. (2020a) addressed that soluble Fe3+ induced surface oxidation and coverage of the biochar and thus decreased Cr(VI) reduction. However, the influence by the more reductive species, i.e. the typical electron donor Fe2+, on the reaction processes of Cr(VI) and biochar was rarely reported. Fe2+ could be generated during the weathering and microbial dissolution of Fe-bearing minerals, which would trigger significant redox cycling processes even at low concentration (Hua et al. 2022). It is interesting to speculate a possible alteration in the behavior during the Fe(II)–Fe(III) redox transformation. Cr(VI) reduction might be enhanced as the result of free Fe2+ as reductant. While later the precipitation of oxidized Fe(III) and the complexation with the organic groups on biochar surface would provide or block active sites, which would make the interaction for Cr(VI) detoxification more complex. So far, such phenomena had not been reported and the actual effects imposed by Fe2+ to the Cr(VI) detoxification as an environmental factor still needs more discussions.
Therefore, in the present study, the detoxification of Cr(VI) by two typical kinds of biochar with few and enriched ash contents, respectively, and their responses to the coexisting LMWOAs and Fe2+ were investigated, so as to identify the roles or influences of endogenous (i.e. the organic functional groups and inorganic ashes in the biochar) and exogenous organic and inorganic electron donors (i.e. the additional LMWOAs and Fe2+ in the surrounding solution). To be environmental relevant, the concentrations of additional LMWOAs and Fe2+ were set at low level (0–2 mmol/L). The results would provide mechanistic insights in the alterations on the detoxification of Cr(VI) imposed by typical environmental constituents like the LMWOAs and Fe2+, which would further help to develop high-efficient remediation approaches for Cr pollution.
Materials and Methods
Biochar Preparation
Two types of biochar were prepared by pyrolysis. Dewatered municipal wastewater sludge (with about 70% moisture) and maize straw were collected from a municipal wastewater treatment plant and farms in South China, respectively, in July, 2017, and were then dried at 60 °C, crushed and passed through a 4-mm sieve after transported to the laboratory. The feedstocks were then heated in a tubular furnace (GLS-1700X-80, Hefei Kejing, China) under N2 atmosphere (1 L/min) (Fig. S1, Supplementary Information (SI)). The temperature was increased to 500 °C at the rate of 10 °C/min and hold for 2 h. The obtained biochars, i.e. SBC (sludge derived biochar) and MBC (maize straw derived biochar), show distinctive elemental composition, ash content and surface properties (Table 1; Fig. S2). SBC is featured with considerable amounts of oxygen and inorganic minerals, especially Fe, while MBC consists of less contents of inorganic elements but much higher content of carbon. Both of two types of biochar were grounded to pass through 60-mesh sieve before use.
Batch Tests for Cr(VI) Removal
In each 50 mL polypropylene tube, 0.5 g of biochar was mixed with 40 mL of 100 mg/L Cr(VI) solution for reaction. Initial pH was adjusted to 2.0 by HCl, which was favored by the reaction as confirmed in our previous study (Fei et al. 2022). The batch tests were conducted at room temperature (25 ± 0.5 °C) on a shaker at 130 rpm. Upon completion after 24 h, the mixtures was centrifuged at 5000 rpm for 5 min. Supernatant solution was retrieved by passing through a polyether sulfone filter (0.45 µm, Jinlong, China) to examine the aqueous Cr(VI) and Cr(III) concentration. The residual solid was washed by de-ionized water for three times and was then extracted to measure the adsorbed Cr(VI) content.
LMWOAs was added to the initial solution in order to investigate the influence of LMWOAs on Cr(VI) detoxification. Four typical LMWOAs, i.e. acetic acid, oxalic acid, malic acid and citric acid were selected. The dosage was 1 mmol/L of carboxy group, that is 1 mmol/L acetic acid, 0.5 mmol/L oxalic acid, 0.5 mmol/L malic acid and 0.33 mmol/L citric acid. For comparison, control tests with SBC or MBC alone but none organic acid were conducted simultaneously. For MBC, impact of different malic acid concentrations in the range of 0–2 mmol/L was further surveyed. Similarly, the effect of Fe2+ on Cr(VI) detoxification by SBC or MBC was also investigated. Stock solution was made at the concentration of 200 mmol/L Fe2+ (pH = 2.0). By adding 0–0.4 mL to the 40 mL Cr(VI) solution (pH = 2.0), the final spiked Fe2+ concentration was in the range of 0–2 mmol/L.
Determination of Cr Concentrations
The concentration of aqueous Cr(VI) [Cr(VI)aq] was analyzed with the 1,5-diphenylcarbazide spectrophotometric method (Wen et al. 2022; Xu et al. 2020a). In brief, after 0.5 mL of (1 + 1) H2SO4 and 0.5 mL of (1 + 1) H3PO4 were mixed into 50 mL of the diluted sample, the chromogenic effect occurred on Cr(VI) in solution by adding the (1 + 1) acetone solution of diphenylcarbohydrazide. The Cr(VI) concentration was then determined under 540 nm wavelength using a ultraviolet–visible spectrophotometry spectrophotometer (UV-5200, Shanghai Yuanxi, China).
Reacted solid was extracted by 0.1 mol/L NaOH for 24 h on the shaker at 130 rpm under room temperature (25 ± 0.5 °C) (Fei et al. 2022) and then centrifuged at 5000 rpm for 5 min. The extract passed through a polyether sulfone filter (0.45 µm) and measured for the Cr(VI) concentration with the diphenylcarbazide spectrophotometric method, which was then employed to calculated the sorbed amount of Cr(VI) on the biochar [Cr(VI)s]. For mass balance estimation, Cr(VI)s content was conversed to the equivalent removed aqueous concentration (mg/L) that was caused by the sorption.
The total residual Cr concentration in the reacted solution [TCraq] was determined by an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 5300 DV, Perkin Elmer, USA). Concentration of aqueous Cr(III) [Cr(III)aq] and sorbed Cr(III) [Cr(III)s] were then calculated according to the mass balance (Eq. 1–2).
where, Cr(VI)0 refers to the initial concentration of Cr(VI) in the system, i.e. 100 mg/L in this study.
Biochar Characterization
Specific surface area (SSA) and pore volume (PV) of the pristine biochar were measured by a surface area and porosity analyzer (TriStar II 3020, Micromeritics, USA). pH value of biochar was determined by measuring the pH of the biochar suspension (1:20 w/v) with a pH meter (FE28, Mettler Toledo, Switzerland). Suspension containing 0.01 g biochar in 50 mL 0.01 mol/L NaCl solution was sonicated for 10 min and then adjusted to varied pH to determine the ζ-potential (Zhao et al. 2013) by a particle analyzer (NanoBrook 90PlusPLAS, Brookhaven, U.S.A). The isoelectric pH (pHiep) was then estimated according to the plotting of ζ-potential versus the working pH. Cation exchange capacity (CEC) was determined using modified protocol of AOAC (Association of Official Analytical Chemists) method 973.09 (Kharel et al. 2019). Total acidity of biochar was determined using the modified Boehm titration method (Uchimiya et al. 2012).
For elemental composition, X-ray photoelectron spectra (XPS) were recorded on an X-ray photoelectron spectrometer (K-alpha, Themo Scientific, USA) with monochromatic Al Kα X-fay source (hυ = 1486.6 eV). High resolution of C 1s, Cr 2p and Fe 2p was further recorded to analyze the involvement of functional groups or active species. Fourier transform infrared spectroscopy (FTIR, Tensor II, Bruker, Germany) was performed in the 4000 to 400 cm−1 region using KBr pellet technique. The sample powder was mixed with KBr at the ratio of 1:100 (m:m). Electron paramagnetic resonance (EPR, EMXplus, Bruker, Germany) was also measured with a sweep width of 100 G, a modulation amplitude of 1.00 G, a modulation frequency of 100 kHz and a microwave frequency of 9.8 GHz. For solid particles, the EPR microwave power was set specifically to 35 dB and the sweep time was 60 ms.
Data Processing and Statistical Analysis
All the treatments and analysis were conducted in triplicates, and the data were processed and analyzed by Microsoft Excel (2019) and Origin 8.5. Statistical tests (i.e. t test for two groups comparison or one-way ANOVA and LSD-test for the multiple groups comparison) were applied when necessary using SPSS 25.0.
Results and Discussion
Cr(VI) Detoxification by Biochars
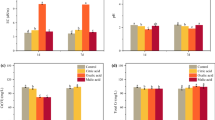
The results of Cr(VI) removal are summarized in Fig. 1a. At pH 2.0, SBC and MBC removed 7.33% and 31.92% of Cr(VI)aq from the bulk solution within 24 h, respectively. As suggested by Cr(VI)s, only 0.85% and 0.86% of the initial Cr(VI) was removed through sorption by MBC and SBC, respectively. All other Cr(VI) was reduced to Cr(III), majority of which was retained on biochar as Cr(III)s, whilst only 3.21% was released to the bulk solution as Cr(III)aq by MBC. This observation revealed that the Cr(VI) removal by both MBC and SBC were dominated by reduction, and the generated Cr(III) could be efficiently immobilized. This is consistent with previous findings that biochar could efficiently reduce Cr(VI) to Cr(III) and immobilize the reduced Cr(III) (Sun et al. 2016), while limited adsorption of Cr(VI) might be the determining factor that limited the overall removal rate (Fei et al. 2022).
In comparison, MBC showed better adsorptive and reductive ability than SBC. It was often considered that abundant surface accessibility would be beneficial to the adsorption of ions. Researches on modified biochar showed significant increasement on SSA of biochar would partly lead to enhanced contaminant adsorption (Ding et al. 2021). However, the Cr(VI) removal was inconsistent with the SSA or PV result in this study. As noted, SBC has higher SSA and PV than MBC (Table 1), but showed very limited adsorption and removal of Cr(VI)aq. This suggested that other factors, like surface charge and reactive functional groups, may play more crucial roles in Cr(VI) detoxification. Remarkably, the ζ-potential of MBC at pH 2.0 was higher than that of SBC, which would be much favorable for the electrostatic attraction to the anion of Cr(VI).
Besides of the distinctive elemental composition between SBC and MBC (Table 1), FTIR spectra also suggested significant differences in activities of functional groups (Fig. 2). The characteristic peak at around 3400 cm−1 indicated the presence of -OH (Sun et al. 2017). This broad peak was found to be one of the biggest two peaks on the spectra of intact SBC, suggesting the significance of –OH on SBC’s surface. Redox capability was often associated with the number of hydroxyls (Xu et al. 2020b). However, absorption at this peak of SBC nearly stayed the same after the reaction, while peak of MBC was weakened, representing that certain amount of -OH on MBC surface might be consumed during the reaction with Cr(VI). Similarly, peaks for –C = O (1600 cm−1), –OH (1438 cm−1), –C–O–C– (1090 cm−1) (Xu et al. 2019b) in MBC’s spectrum were also significantly weakened after the reaction, indicating the possible involvements of those functional groups in the interaction with Cr(VI). On SBC, only the -OH at the peak of 1438 cm−1 was attenuated, whilst other major peaks did not show significant change, suggesting that the organic skeleton of SBC participated in less extent in the reactions.
Persistent free radicals (PFRs) are often associated with the redox activity of biochars (Kappler et al. 2014; Luo et al. 2021). As suggested by EPR spectra summarized in Fig. 3, the g-factor of SBC and MBC before and after the reaction all located around 2.003–2.005, which are characteristic of O-centered free radicals, including semiquinone-type radicals (Fang et al. 2015). Compared to MBC, the intensity of SBC showed lower value, which was consisted with the analysis above that MBC had higher reactivity apparently than SBC. After the reaction, the signal of SBC and MBC both slightly decreased, implying that the Cr(VI) reduction consumed part of these free radicals. It is reported that the transformation of Cr(VI) on biochar was controlled by surface reaction in which available PFRs, i.e. O-centered radicals and semiquinone-type PFRs, were the key electron donors (Zhao et al. 2018; Sun et al. 2017; Zhu et al. 2020). It is predictable that during the Cr(VI) reduction, the O-centered PFRs like phenolic and semiquinone-type groups were consumed for the redox which led to the lowered signals in EPRs (Luo et al. 2021).
The surface element states of Cr, C and Fe of SBC and MBC before and after the reaction were revealed by XPS (Fig. 4). On both the SBC and MBC surface after reaction, the Cr 2p doublets corresponding to Cr 2p3/2 and Cr 2p1/2 orbitals were observed (Fig. 4), which matched well with the Cr(III) binding energies, indicating that majority of the Cr sorbed on biochar was reduced to Cr(III). This is consistent with the chemical analysis that Cr(VI)s was few and ignorable.
For C 1 s spectrum, the peaks were deconvoluted into C–C, C–O, O=C and carbonates (Fei et al. 2022). The fitting results of SBC showed insignificant difference before and after the reaction (Fig. 4), indicating slight surface status change occurred on SBC, which was accordant with the FTIR results that organic functional groups may be less involved in Cr(VI) reaction with SBC. For MBC, the characteristic peaks of C–O and O=C didn’t show a perceptible difference, whilst an obvious vanishment of peak of carbonate was observed after the reaction. For the limited Cr amount compared to the carbon matrix of biochar, the changes of carbon status due to the redox reaction may be lower than to be detectable. While, the loss of carbonate from MBC surface could be explained by the ion exchange between CO32− and CrO42− during the adsorption of Cr(VI) (Agrafioti et al. 2013), which was not notably observed on SBC surface. Though the reasons need more investigation and validation, it might be implied that SBC and MBC showed differences in the accessibility and activity of the organic functional groups to Cr(VI)-related adsorption and redox reactions.
The studied SBC was consisted of considerable content of Fe, whose doublet was detected by XPS (Fig. 4). According to the spectrum deconvolution, the average valence of Fe in the pristine SBC was about + 1.70 (32.9% of Fe(0), 31.3% of Fe(II), and 35.7% of Fe(III)). After reacted with Cr(VI), the average valence of Fe in SBC became + 1.78, and an increase of Fe(II) and Fe(III) as well as a decrease of Fe(0) was observed, indicating the oxidation of Fe occurred when Cr(VI) was reduced. Compared with the redox reactions between Cr and organic functional groups, the electron transfer between Cr and Fe would be faster and more favored (Fei et al. 2022).
Detoxification of Cr(VI) with the Presence of LWMOAs
The influence of typical exogenous organic electron donors, i.e. LMWOAs, on the detoxification of Cr(VI) by SBC or MBC was summarized in Fig. 1b and c. For SBC, little change was brought by acetic acid (from 6.38% to 6.39%, p > 0.05), oxalic acid made a slight increase (to 8.01%, p > 0.05), and the other two acids showed significant enhancement to Cr(VI)aq removal, i.e. to 8.77% by malic acid (p < 0.01) and 9.10% by citric acid (p < 0.01), respectively. The sorbed Cr(VI), i.e. Cr(VI)s, was not increased by the presence of LMWOAs, while the reduced Cr(III) was significantly higher in system, mostly of which were readily immobilized on SBC. Likely, for MBC, little change was brought by oxalic acid (from 34.70% to 34.93%, p > 0.05), but the other three LMWOAs enhanced the Cr(VI)aq removal by MBC significantly, i.e. to 37.59% (by acetic acid, p < 0.01), 40.71% (by malic acid, p < 0.01) and 40.13% (by citric acid, p < 0.01). Generally, the reduction was also significantly enhanced, resulting in more Cr(III). However, it was observed in MBC groups that certain percentage of Cr(III) was mobilized into the bulk solution by the additional LMWOAs, as indicated by Cr(III)aq. The raising of Cr(III)aq might be due to the saturated Cr(III) adsorption on biochar as for more Cr(III) was generated, or due to the coordinating effect of organic acids which may help to dissolute Cr(III) to the solution (Sun et al. 2016).
The FTIR results summarized in Fig. 2 showed the influences of the studied organic acids to the reaction. For SBC, the significantly attenuated peak at 1438 cm−1 by Cr(VI) reduction was slightly remedied by citric acid and malic acid. Likely, the consumption of -OH on MBC at the peaks of 3600 cm−1 and 3400 cm−1 were also relieved by adding LMWOAs, as well as –C = O (1600 cm−1), –OH (1438 cm−1) and –C–O–C– (1090 cm−1), which was especially more obviously noted when adding oxalic acid and acetic acid. The EPR analysis indicated that with the presence of LMWOAs (i.e. malic acid), the signals for PFRs on biochars became higher (Fig. 3), which was possibly associated the introduced hydroxyl or carboxyl groups (Luo et al. 2021). After reacted with Cr(VI), the signal intensities dropped but remained higher than the reacted biochar without organic acid. These results were all consistent with the statement that the coexisting LMWOAs could provide with reductive groups for Cr(VI) reaction and thus alleviate the consumption of the active groups from biochar (Xu et al. 2019b; Fei et al. 2022).
The concentration-dependent effects of LMWOAs on Cr(VI) reduction and Cr(III) immobilization by biochar was further investigated on malic acid and MBC. As summarized in Fig. 1d, with the concentration of malic acid elevated from 0 to 2.0 mmol/L, the Cr(VI) removal efficiency, which was similarly to the Cr(VI) reduction ratio, constantly raised from 40.96% to 62.15% (p < 0.01), confirming the reinforced reducing ability by the coexisting malic acid. Whilst, the immobilization of the generated Cr(III) declined with the increased concentration of malic acid, as suggested by the increased percentage of Cr(III)aq from 2.97% to 35.59% (p < 0.01). The generally declined percentage of Cr(III)s (from 37.08% to 25.65%, p < 0.01) excluded the possible reason of adsorption saturation of Cr(III), otherwise Cr(III)s should not be declined when the total Cr(III) became higher. Thus the less efficient immobilization of Cr(III) would be more probably due to the presence of malic acid. Malic acid may compete with Cr(III) for surface adsorption sites (Rivera-Utrilla et al. 2003), which would lead to the declined Cr(III) adsorption. Additionally, malic acid may coordinate with Cr(III) and form a more soluble complex (Büker et al. 2020). These two reasons would then synergistically increase the dissolution of Cr(III) and hence abate the immobilization of Cr(III).
Detoxification of Cr(VI) with the Presence of Fe2+
Varied concentration of Fe2+ from 0.25 to 2.0 mmol/L was added to the Cr(VI)-biochar system to explore their influences (Fig. 1e, f). Generally, the additional Fe2+ significantly enhanced Cr(VI)aq removal from 7.05% to 12.9% to 47.7% (p < 0.01) by SBC, and from 35.4% to 40.4% to 75.5% (p < 0.01) by MBC, respectively. Reduction of Cr(VI) to Cr(III) remained the major contribution to the Cr(VI)aq removal. This increment of reduction ability was predictable from the redox ability of Fe2+. Though other study may expect a lowered pH environment by the hydrolysis reaction of higher concentration of Fe2+, which would be more favorable for Cr(VI) adsorption and reduction (Ding et al. 2021), this may not be the crucial factor in our experiment, since the pH after reaction did not differed too much with or without additional Fe2+ (Fig. S3, SI).
The solutions after reaction were measured for aqueous Fe2+ and Fe3+ concentration but found both were undetectable, suggesting Fe2+ was possibly oxidized and precipitated as Fe(OH)3. Valence alteration of added Fe2+ was then identified via the analysis of high-resolution spectra of XPS of the solids (Fig. 4). For SBC, increased percentage of Fe(II) could be expected as introduced by the additional Fe2+. However, the results indicated much more increased Fe(III) after reacted with Cr(VI) than that without the presence of Fe2+, revealing the oxidation of added Fe(II) to Fe(III) during the interaction. For MBC without Fe2+ addition, no peak of Fe was observed, while with the presence of Fe2+, the doublets of both Fe(II) and Fe(III) was observed after the reaction, indicating the redox transformation of the added Fe(II) to newly formed Fe(III) during the reaction with Cr(VI), too. Suggested by the EPR signals, the Fe2+ addition contributed to the PFRs amount in the reaction system (Fig. 3). It is possible that adding certain amount of transition metal could enhance the concentration of PFRs, which would be beneficial for the electron transfer between Cr(VI) and surface components on biochar(Fang et al. 2015).
The improved detoxification of Cr(VI) by biochars with the presence of Fe2+ was also contributed by the increased adsorption ability of SBC and MBC, as significantly more sorbed Cr(VI)s and Cr(III)s was observed, as well as the decrease of Cr(III)aq. Fe may form Fe-BC complex on surface of biochar, which might cover the biochar surface (Yang et al. 2016), whilst the Fe-BC complex could provide new adsorption site for Cr(VI) (Xu et al. 2020a). At lower dosage of Fe2+, surplus Cr(III)aq that was not adsorbed by the biochars was observed, which may be associated with the covered surfaces by Fe-BC complex (Yang et al. 2016). Precipitation of Fe on the biochar surface may also decrease the negative charge, and thus weaken the electrostatic affinity of Cr(III) (Xu et al. 2020a). However, when Fe2+ concentration raised, the Cr(III)aq in reaction system gradually reduced to undetected level while Cr(III)s continuously increased, indicating better immobilization of Cr(III). If the concentration of Fe2+ continued to raise, the coordinate precipitation of Fe(III)–Cr(III) might be formed, which could make up the adsorption competition on biochar surface (Xia et al. 2022). Due to the low dosage of Fe and Cr in the system, Fe or Fe–Cr precipitates on the biochar after reaction was not identified. Advanced surface analysis may be employed to obtain more direct evidence in the future.
Discussion
Roles of Endogenous Electron Donors in Cr(VI) Reduction by Biochars
Biochar is favored in heavy metal removal for its high efficiency in adsorption (Spokas et al. 2012). Biochars derived from various feedstocks are confirmed to be capable for Cr(VI) removal (e.g. Hsu et al. 2009; Dong et al. 2011; Choppala et al. 2012). In details, the detoxification of Cr(VI) by biochar involved sorption of Cr(VI), reduction of Cr(VI) to Cr(III), and then the further immobilization of Cr(III) (Liu et al. 2020; Yang et al. 2018; Zhou et al. 2016). Our previous study confirmed this pathway of sorption-reduction-immobilization on SBC (Fei et al. 2022). In most reports, adsorption was often considered to be the main process, coupled with partial reduction to Cr(III) (Dobrzynska et al. 2022). However, in the present study, it was addressed that almost all the removed Cr(VI)aq were transformed to Cr(III), majority of which were fixed on biochar as Cr(III)s (Fig. 1a), indicating that the reduction process was crucial for the Cr(VI) removal by the studied biochars. Transforming Cr(VI) to Cr(III) would benefit the detoxification and better immobilization (Sharma et al. 2008).
Surface organic functional groups, e.g. phenolic and carboxylic groups, were mostly focused when discussing Cr(VI) reduction previously (Hsu et al. 2009; Liu et al. 2020; El-Naggar et al. 2022). PFRs in biochar were also considered to be contributive to the Cr(VI) reduction (Zhao et al. 2018; Zhu et al. 2020). Such roles of those organic electron donors were confirmed by this study, as consumption of functional groups evidenced by FTIR spectra (Fig. 2) and PFRs examined by EPR analysis (Fig. 3) was observed on the studied MBC and SBC. Nevertheless, the involvement of those organic groups and PFRs in SBC was not so significant as that in MBC.
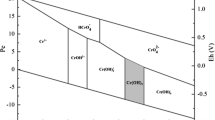
On the other side, the redox element in the ash of SBC, i.e. Fe, contributed more significantly to the Cr(VI) reduction. Valent change of Fe fractions after reacted with Cr(VI) was observed (Fig. 4). Based on a rough estimation (Text S1, SI), the electron transferred from Fe in SBC was much higher than the Cr(VI) reduction required. In the acidic environment (i.e. pH = 1–3), dissolved oxygen was not possible to oxidize Fe when there were residual Cr(VI) in the solution (Eary and Rai 1988, 1991). This suggested that Fe in SBC was probable to be the more important electron donor for Cr(VI) reduction than the organic groups on SBC surface. Compared with the organic groups, the electron transfer between Cr(VI) and inorganic donor Fe2+ would be easier and faster. Thus, a schematic mechanism could be summarized in Fig. 5, that the interaction between Cr(VI) and carbon-rich MBC was more dependent on the organic groups while the inorganic Fe-containing fractions played more significant roles in Fe-rich SBC.
Influences of Exogenous Electron Donors on Cr(VI) Reduction by Biochars
Upon application to the real environment, biochar would inevitably interplay with the water or soil constituents. The redox reaction between biochar and Cr(VI) would then be expected to be influenced by typical environmental reductants, like the organic electron donor LMWOAs and the inorganic redox active element Fe2+. Among the tested four organic acids, malic acid and citric acid consistently showed significant enhancement to the Cr(VI) detoxification by biochars (Fig. 1b, c). It was suggested that α-OH was among the most active groups for Cr(VI) reduction (Tian et al. 2010). Malic acid and citric acid both have α-OH, i.e. the hydroxyl group substituting on the carbon atom adjacent to the carboxyl, which can explain their advantages to the other two studied acids without α-OH. Generally, these exogenous organic electron donors could enhance the reduction of Cr(VI) to Cr(III) either in the bulk solution or on the sorbed surface (Fig. 5).
The other typical environmental electron donor, i.e. free Fe2+ ion, behaved much stronger and brought much more significant improvement to the Cr(VI) removal by the two biochars (Fig. 1e, f). The responses to additional Fe2+ by SBC and MBC were similar. Different with Fe3+ which could not enhance the biochars’ Cr(VI) removal ability due to surface oxidation and passivation (Agrafioti et al. 2014b; Xu et al. 2020a), the reductive Fe2+ largely increased the Cr(VI) reduction. Besides of the direct contribution of Fe2+ as electron donor, the enhancement may also be partially contributed by the recycled electron transfer within the biochar matrix (Fig. 5). As previously reported, biochar could play its role as electron shuttle, which would help to enhance the efficiency of Cr(VI) reduction by other electron donors (Kappler et al. 2014; Xu et al. 2020a). According to the stochiometric relationship estimated from this study (Fig. S4, SI), 0.316 mol of Cr(III) was generated from the reduction of Cr(VI) by each 1 mol of Fe2+ when without biochar. This was a little lower than the theoretically estimated 1/3 mol (as each 1 mol of Fe2+ could only donate 1 mol of electrons while each 1 mol of Cr(VI) needs 3 mol of electrons when transforming to Cr(III)), possibly due to the passivation effect induced by the precipitation of Fe(OH)3 or Fe(III)-Cr(III) precipitates (Xu et al. 2020a). When biochar coexisted in the system, the linear relationship between Fe2+ and Cr(III) had intercepts which was associated with the Cr(VI) reduction by the biochar itself, while the slope became higher (i.e. 0.319 and 0.329 for SBC and MBC, respectively) than that without biochar, indicating that more Cr(VI) was reduced and more Cr(III) was produced by each 1 mol of Fe2+. Despite that this enhancement was relatively slight (1% and 4% by SBC and MBC, respectively) due to the low dosage of Fe2+, it suggested the reinforced reduction by Fe2+ upon the combined effects with the contribution of biochar. With higher activity of organic groups as discussed above, MBC showed more significant enhancement of the stochiometric coefficient than SBC, as for the electron shuttling effects were usually dependent on the organic matrix of biochar (Kappler et al. 2014).
Influences of Exogenous Constituents on Cr Sorption by Biochars
Although reduction of Cr(VI) to Cr(III) was the most crucial step for Cr(VI) detoxification, the sorption processes were also important. As we proved previously, limited adsorption of Cr(VI) might be the determining factor that limited the overall removal rate (Fei et al. 2022). Comparing LMWOAs and Fe2+, the former would impose unfavored influences to Cr(VI) sorption. Due to the complexation with biochar (Sun et al. 2016; Xu et al. 2019b), LMWOAs may impose competitive adsorption with Cr(VI) (Liu et al. 2018), and thus restrain the overall removal of Cr(VI). Compared with MBC, the response to LMWOAs by SBC was much less notable (Fig. 1b, c). Although it was reported that Fe fraction could help to accelerate the reduction of Cr(VI) by malic acid (Zhong and Yang 2012), this was not observed in this studied Fe-rich SBC. As discussed above, SBC was less surface active than MBC originally. Since the LMWOAs would decrease the surface accessibility to Cr(VI), the limited removal by SBC may not be enhanced too much.
The immobilization of generated Cr(III), as the final step of the removal processes, should also be of great importance in reinforcing the Cr(VI) treating efficiency. As observed, the coexistence of LMWOAs decreased the immobilization of Cr(III) (Fig. 1c), and such negative impact escalated along with increased concentration of LMWOAs (Fig. 1d). Besides of possible surface competition due to organic acids complexation on the biochar (Rivera-Utrilla et al. 2003), LMWOAs may coordinate with Cr(III) (Büker et al. 2020), thus increased the dissolution of Cr(III) and hence abated the immobilization of Cr(III). Similar negative impact to Cr(III) sorption may also occur in the co-presence of Fe2+ or the oxidized product Fe3+. Fe(III) was found to block adsorption sites on carbon materials (Agrafioti et al. 2014b; Dobrzynska et al. 2022), which could explain that lower concentration of Fe3+ competed with Cr(III), making the immobilized Cr(III)s declined (Fig. 1e, f). Nevertheless, Fe could also contribute to Cr(III) adsorption through co-precipitation (Agrafioti et al. 2014a). The influence would be concentration dependent, as observed in this study that only higher concentrations led to better Cr(III) immobilization instead (Fig. 1e, f). For SBC, which already had a certain content of Fe originally, such a beneficial influence of Fe–Cr co-precipitation could be achieved at a relative lower concentration than MBC.
To sum up, compared with LMWOAs, the detoxification of Cr by biochar was more benefited from Fe2+, with regards to the much more significantly enhanced Cr(VI) reduction as well as the improved Cr(III) immobilization. It should be noted that the actual influences by the environmental electron donors and other co-existing constituents would be more complex than the experimental study in solution. More investigations simulating soil processes are required in the future.
Conclusions
In this study, biochar derived from municipal sludge and maize straw, i.e. SBC and MBC, with high and little content of ash content, respectively, was examined for the roles of organic and inorganic electron donors. These two biochars showed varied detoxification ability and chemical mechanisms of Cr(VI). For MBC, the higher surface charge as well as higher activity of functional groups and O-centered PFRs contributed to its advantages in Cr(VI) adsorption and reduction. While, with considerable content of Fe, SBC was featured with the more important participation of the inorganic reductant.
Influences of exogenous electron donors, including organic reductant LMWOAs and inorganic reductant Fe2+, were also examined. The organic acids, i.e. acetic acid, oxalic acid, malic acid and citric acid, enhanced the Cr(VI) detoxification by the studied biochars. The organic acids could act as organic electron donors for the transformation of Cr(VI) to Cr(III). However, they also compete with the adsorption of Cr(VI) or assist to dissolve the generated Cr(III), thus restricted the overall enhancement of Cr detoxification.
The other studied inorganic electron donor, i.e. Fe2+, contributed more significantly to the Cr(VI) reduction by biochars. Besides of directly donating electrons for Cr(VI) reduction, additional Fe2+ also stimulated more PFRs for the reaction and benefitted from the electron shuttling route with the support of biochar matrix. Furthermore, the improved adsorption of Cr(VI) and immobilization of Cr(III) further reinforced the overall detoxification of Cr from the polluted solution.
Data availability
All data generated or analysed during this study are included in this published article.
References
Agrafioti E, Bouras G, Kalderis D, Diamadopoulos E (2013) Biochar production by sewage sludge pyrolysis. J Anal Appl Pyrol 101:72–78. https://doi.org/10.1016/j.jaap.2013.02.010
Agrafioti E, Kalderis D, Diamadopoulos E (2014a) Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J Environ Manag 133:309–314. https://doi.org/10.1016/j.jenvman.2013.12.007
Agrafioti E, Kalderis D, Diamadopoulos E (2014b) Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J Environ Manag 146:444–450. https://doi.org/10.1016/j.jenvman.2014.07.029
Büker L, Dickbreder R, Böttcher R, Sadowski S, Bund A (2020) Investigation of the reaction kinetics of chromium(III) ions with carboxylic acids in aqueous solutions and the associated effects on chromium deposition. J Electrochem Soc 167(16):162509. https://doi.org/10.1149/1945-7111/abd1f4
Chen W, Meng J, Han X, Lan Y, Zhang W (2019) Past, present, and future of biochar. Biochar 1(1):75–87. https://doi.org/10.1007/s42773-019-00008-3
Chen N, Cao S, Zhang L, Peng X, Wang X, Ai Z, Zhang L (2021) Structural dependent Cr(VI) adsorption and reduction of biochar: hydrochar versus pyrochar. Sci Total Environ 783:147084. https://doi.org/10.1016/j.scitotenv.2021.147084
Choppala GK, Bolan NS, Megharaj M, Chen Z, Naidu R (2012) The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J Environ Qual 41:1175–1184. https://doi.org/10.2134/jeq2011.0145
Conte P, Schmidt HP, Cimo G (2015) Research and application of biochar in Europe. In: Guo M (ed) Agricultural and environmental applications of biochar: advances and barriers. Soil Science Society of America, Madison, pp 409–422
Ding K, Zhou X, Hadiatullah H, Lu Y, Zhao G, Jia S, Zhang R, Yao Y (2021) Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J Hazard Mater 420:126551. https://doi.org/10.1016/j.jhazmat.2021.126551
Dobrzynska J, Wysokinska A, Olchowsk R (2022) Raspberry stalks-derived biochar, magnetic biochar and urea modified magnetic biochar: synthesis, characterization and application for As(V) and Cr(VI) removal from river water. J Environ Manag 316:115260. https://doi.org/10.1016/j.jenvman.2022.115260
Dong X, Ma L, Li Y (2011) Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J Hazard Mater 190:909–915. https://doi.org/10.1016/j.jhazmat.2011.04.008
Eary L, Rai D (1988) Chromate removal from aqueous wastes by reduction with ferrous ion. Environ Sci Technol 22:972–977. https://doi.org/10.1021/es00173a018
Eary L, Rai D (1991) Chromate reduction by subsurface soil under acidic conditions. Soil Sci Soc Am J 55:676–683. https://doi.org/10.2136/sssaj1991.03615995005500030007x
El-Naggar A, Mosa A, Ahmed N, Niazi NK, Yousaf B, Sarkar B, Rinklebe J, Cai Y, Chang SX (2022) Modified and pristine biochars for remediation of chromium contamination in soil and aquatic systems. Chemosphere 303:134942. https://doi.org/10.1016/j.chemosphere.2022.134942
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49(9):5645–5653. https://doi.org/10.1021/es5061512
Fei Y, Li M, Ye Z, Guan J, Huang Z, Xiao T, Zhang P (2022) The pH-sensitive sorption governed reduction of Cr(VI) by sludge derived biochar and the accelerating effect of organic acids. J Hazard Mater 423:127205. https://doi.org/10.1016/j.jhazmat.2021.127205
Hsu N, Wang S, Lin Y, Sheng G, Lee J (2009) Reduction of Cr(VI) by crop-residue-derived black carbon. Environ Sci Technol 43:8801–8806. https://doi.org/10.1021/es901872x
Hua J, Fei Y, Feng C, Liu C, Liang S, Wang S, Wu F (2022) Anoxic oxidation of As(III) during Fe(II)-induced goethite recrystallization: evidence and importance of Fe(IV) intermediate. J Hazard Mater 421:126806. https://doi.org/10.1016/j.jhazmat.2021.126806
Jones DL, Dennis PG, Owen AG, Van Hees PAW (2003) Organic acid behavior in soils: misconceptions and knowledge gaps. Plant Soil 248(1–2):31–41. https://doi.org/10.1023/A:1022304332313
Kantar C, Ari C, Keskin S (2015) Comparison of different chelating agents to enhance reductive Cr(VI) removal by pyrite treatment procedure. Water Res 76:66–75. https://doi.org/10.1016/j.watres.2015.02.058
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ Sci Technol Lett 1(8):339–344. https://doi.org/10.1021/ez5002209
Kharel G, Sacko O, Feng X, Morris JR, Phillips CL, Trippe K, Kumar S, Lee JW (2019) Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain Chem Eng 7:16410–16418. https://doi.org/10.1021/acssuschemeng.9b03536
Liu X, Dong H, Yang X, Kovarik L, Chen Y, Zheng Q (2018) Effects of citrate on hexavalent chromium reduction by structural Fe(II) in nontronite. J Hazard Mater 343:245–254. https://doi.org/10.1016/j.jhazmat.2017.09.038
Liu N, Zhang Y, Xu C, Liu P, Lv J, Liu Y, Wang Q (2020) Removal mechanisms of aqueous Cr(VI) using apple wood biochar: a spectroscopic study. J Hazard Mater 384:121371. https://doi.org/10.1016/j.jhazmat.2019.121371
Luo K, Pang Y, Wang D, Li X, Wang L, Lei M, Huang Q, Yang Q (2021) A critical review on the application of biochar in environmental pollution remediation: role of persistent free radicals (PFRs). J Environ Sci 108:201–216. https://doi.org/10.1016/j.jes.2021.02.021
Lyu HH, Zhao H, Tang JC, Gong YY, Huang Y, Wu QH, Gao B (2018) Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 194:360–369. https://doi.org/10.1016/j.chemosphere.2017.11.182
McNeill LS, McLean JE, Parks JL, Edwards MA (2012) Hexavalent chromium review, part 2: chemistry, occurrence, and treatment. J Am Water Works Ass 104(7):395–405. https://doi.org/10.5942/jawwa.2012.104.0092
Mortada WI, El-Naggar A, Mosa A, Palansooriya KN, Yousaf B, Tang R, Wang S, Cai Y, Chang SX (2023) Biogeochemical behaviour and toxicology of chromium in the soil-water-human nexus: a review. Chemosphere 331:138804. https://doi.org/10.1016/j.chemosphere.2023.138804
Mumtaz T, Abd-Aziz S, Rahman NA, Phang LY, Shirai Y, Hassan MA (2008) Pilot-scale recovery of low molecular weight organic acids from anaerobically treated palm oil mill effluent (POME) with energy integrated system. Afr J Biotechnol 7(21):3900–3905. https://doi.org/10.4065/72.1.92-b
Nelson NO, Agudelo SC, Yuan W, Gan J (2011) Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci 176:218–226. https://doi.org/10.1097/SS.0b013e3182171eac
Nozoe T, Sekiguchi T, Inoue T (2001) Effects of the addition of Fe(0) on the reduction of Fe(III) in submerged rice soil. Soil Sci Plant Nutr 47(1):123–130. https://doi.org/10.1080/00380768.2001.10408374
Qin J, Li Q, Liu Y, Niu A, Lin C (2020) Biochar-driven reduction of As(V) and Cr(VI): effects of pyrolysis temperature and low-molecular-weight organic acids. Ecotox Environ Safe 201:110873. https://doi.org/10.1016/j.ecoenv.2020.110873
Ran B, Liu L, Hua C (2021) Comprehensive investigation and mechanisms of drilling waste sludge dewaterability by Fe(II)/persulfate pretreatment. J Environ Chem Eng 9(6):106751. https://doi.org/10.1016/j.jece.2021.106751
Rivera-Utrilla J, Sánchez-Polo M, Carrasco-Marín F (2003) Adsorption of 1,3,6-naphthalenetrisulfonic acid on activated carbon in the presence of Cd(II), Cr(III), and Hg(II): importance of electrostatic interactions. Langmuir 19(26):10857–10861. https://doi.org/10.1021/la0350103
Sharma SK, Petrusevski B, Amy G (2008) Chromium removal from water: a review. J Water Supply Res Technol-Aqua 57(8):541–553. https://doi.org/10.2166/aqua.2008.080
Song XJ, Qin ZQ, Yang F, Fang QL, Hu HM, Song ZJ (2014) Photocatalytic degradation using MWCNTs modified with Fe(III)-doped ZnO for water treatment. Asian J Chem 26(5):1497–1500. https://doi.org/10.14233/ajchem.2014.17270
Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, Boateng AA, Lima IM, Lamb MC, McAloon AJ, Lentz RD, Nichols KA (2012) Biochar: a synthesis of its agronomic impact beyond carbon sequestration. J Environ Qual 41(4):973–989. https://doi.org/10.2134/jeq2011.0069
Sun B, Lian F, Bao Q, Liu Z, Song Z, Zhu L (2016) Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole. Environ Pollut 214:142–148. https://doi.org/10.1016/j.envpol.2016.04.017
Sun T, Levin BDA, Guzman JJL, Enders A, Muller DA, Angenent LT, Lehmann J (2017) Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun 8:1–12. https://doi.org/10.1038/ncomms14873
Tian X, Gao X, Yang F, Lan Y, Mao J, Zhou L (2010) Catalytic role of soils in the transformation of Cr(VI) to Cr(III) in the presence of organic acids containing α-OH groups. Geoderma 159:270–275. https://doi.org/10.1016/j.geoderma.2010.07.019
Uchimiya M, Bannon DI, Wartelle LH (2012) Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J Agr Food Chem 60:1798–1809. https://doi.org/10.1021/jf2047898
Wen J, Xue Z, Yin X, Wang X (2022) Insights into aqueous reduction of Cr(VI) by biochar and its iron-modified counterpart in the presence of organic acids. Chemosphere 286:131918. https://doi.org/10.1016/j.chemosphere.2021.131918
Xia X, Wang J, Hu Y, Liu J, Darma AI, Jin L, Han H, He C, Yang J (2022) Molecular insights into roles of dissolved organic matter in Cr(III) immobilization by coprecipitation with Fe(III) probed by STXM-ptychography and XANES spectroscopy. Environ Sci Technol 56(4):2432–2442. https://doi.org/10.1021/acs.est.1c07528
Xu X, Huang H, Zhang Y, Xu Z, Cao X (2019a) Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ Pollut 244:423–430. https://doi.org/10.1016/j.envpol.2018.10.068
Xu Z, Xu X, Tao X, Yao C, Tsang DCW, Cao X (2019b) Interaction with low molecular weight organic acids affects the electron shuttling of biochar for Cr(VI) reduction. J Hazard Mater 378:120705. https://doi.org/10.1016/j.jhazmat.2019.05.098
Xu Z, Xu X, Tsang DCW, Yang F, Zhao L, Qiu H, Cao X (2020a) Participation of soil active components in the reduction of Cr(VI) by biochar: differing effects of iron mineral alone and its combination with organic acid. J Hazard Mater 384:121455. https://doi.org/10.1016/j.jhazmat.2019.121455
Xu Z, Xu X, Zhang Y, Yu Y, Cao X (2020b) Pyrolysis-temperature depended electron donating and mediating mechanisms of biochar for Cr(VI) reduction. J Hazard Mater 388:121794. https://doi.org/10.1016/j.jhazmat.2019.121794
Yang J, Zhong L, Liu L (2013) Coupling of tartaric acid-promoted soil dissolution and Cr(VI) reduction in an Oxisol. J Geochem Explor 125:138–143. https://doi.org/10.1016/j.gexplo.2012.12.003
Yang F, Zhao L, Gao B, Xu X, Cao X (2016) The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ Sci Technol 50(5):2264–2271. https://doi.org/10.1021/acs.est.5b03656
Yang Y, Chen N, Feng C, Li M, Gao Y (2018) Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloid Surface A 556:201–209. https://doi.org/10.1016/j.colsurfa.2018.08.035
Yang C, Ge C, Li X, Li L, Wang B, Lin A, Yang W (2021) Does soluble starch improve the removal of Cr(VI) by nZVI loaded on biochar? Ecotox Environ Safe 208:111552. https://doi.org/10.1016/j.ecoenv.2020.111552
Zhang Q, Chang G, Gao T, Sun H, Chen Y, Yue B, Tai X, Li W (2017) Review of chromium residue and chromium-containing waste water treatment. In: Proc 6th Int Conf Energy Environ Protect (ICEEP 2017). pp 420–425. https://doi.org/10.2991/iceep-17.2017.75
Zhao L, Cao X, Masek O, Zimmerman A (2013) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater 256–257:1–9. https://doi.org/10.1016/j.jhazmat.2013.04.015
Zhao L, Cao X, Zheng W, Wang Q, Yang F (2015) Endogenous minerals have influences on surface electrochemistry and ion exchange properties of biochar. Chemosphere 136:133–139. https://doi.org/10.1016/j.chemosphere.2015.04.053
Zhao N, Yin Z, Liu F, Zhang M, Lv Y, Hao Z, Pan G (2018) Environmentally persistent free radicals mediated removal of Cr (VI) from highly saline water by corn straw biochars. Bioresour Technol 260:294–301. https://doi.org/10.1016/j.biortech.2018.03.116
Zhong L, Yang J (2012) Reduction of Cr(VI) by malic acid in aqueous Fe-rich soil suspensions. Chemosphere 86:973–978. https://doi.org/10.1016/j.chemosphere.2011.11.025
Zhou L, Liu Y, Liu S, Yin Y, Zeng G, Tan X, Hu X, Hu X, Jiang L, Ding Y, Liu S, Huang X (2016) Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol 218:351–359. https://doi.org/10.1016/j.biortech.2016.06.102
Zhu S, Huang X, Yang X, Peng P, Li Z, Jin C (2020) Enhanced transformation of Cr(VI) by heterocyclic-N within nitrogen-doped biochar: Impact of surface modulatory persistent free radicals (PFRs). Environ Sci Technol 54(13):8123–8132. https://doi.org/10.1021/acs.est.0c02713
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41907119), the Natural Science Foundation of Guangdong Province (No. 2019A1515011617), and Guangzhou Municipal Science and Technology Project (No. 202201010735).
Funding
National Natural Science Foundation of China, 41907119, Ying-heng Fei, Natural Science Foundation of Guangdong Province, 2019A1515011617, Ying-heng Fei, Guangzhou Municipal Science and Technology Project, 202201010735, Ying-heng Fei.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Z., Fei, Yh., Li, M. et al. Hexavalent Chromium Detoxification by Biochars: Influences of Organic and Inorganic Electron Donors. Int J Environ Res 18, 71 (2024). https://doi.org/10.1007/s41742-024-00623-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00623-4