Abstract
This paper analyses the composition of the foraminiferal assemblages recorded in two different depositional contexts located in Iberia for the lower Aalenian–lower Bajocian stratigraphical interval: the Talveila section, corresponding to proximal facies within the Iberian Basin (NE Spain), and the Murtinheira section representing distal facies within the Lusitanian Basin (Western Portugal). The obtained specimens (10,736 in total) correspond to 6 suborders, 10 superfamilies, 16 families and 30 genera in Talveila, and 6 suborders, 9 superfamilies, 14 families and 26 genera in Murtinheira. Several biostratigraphic units based on the foraminiferal record and accurately calibrated with the ammonite record were recognized in both sections. Moreover, bioevents based on the first or last appearances of a taxon, significant changes in the abundance of one or several taxa or noticeable changes in diversity of the assemblages were also identified. The palaeoecological analysis shows that the foraminiferal assemblages from both sections were developed in a well-oxygenated and normal salinity shelf environment. The application of diversity indexes indicates that the paleoenvironmental conditions did not remain constant throughout the studied stratigraphic interval; changes recognized in both sections are similar, coeval and correspond to three intervals representing paleoenvironmental conditions more or less favourable for the development of the foraminiferal assemblages. As so, despite the different paleogeographical locations, the development of the assemblages in both sections during the Early Aalenian–Early Bajocian seem to have been conditioned by environmental changes of regional scale, which affected at the same time both the Iberian and the Lusitanian basins.
Resumen
En este trabajo se analiza la composición de las asociaciones de foraminíferos registradas en dos contextos deposicionales diferentes, localizados en Iberia, para el intervalo estratigráfico Aaleniense inferior- Bajociense inferior. La sección de Talveila corresponde a facies proximales de la Cuenca Ibérica (NE España), y la sección de Murtinheira representa facies distales de la Cuenca Lusitánica (O de Portugal). Los ejemplares obtenidos (10736 en total) corresponden a 6 subórdenes, 10 superfamilias, 16 familias y 30 géneros en Talveila y 6 subórdenes, 9 superfamilias, 14 familias y 26 géneros en Murtinheira. Se han reconocido en ambas secciones algunas unidades bioestratigráficas basadas en el registro foraminíferos, y calibradas con precisión con el registro de ammonoideos. Además, se han identificado bioeventos basados en la primera o la última aparición de un taxón, cambios significativos en la abundancia de uno o varios taxones o cambios significativos en la diversidad de las asociaciones. El análisis paleoecológico realizado pone de manifiesto que en ambas secciones las asociaciones de foraminíferos se desarrollaron en un ambiente de plataforma bien oxigenada y con salinidad normal. Los resultados obtenidos tras la aplicación de varios índices de diversidad indican que las condiciones paleoambientales no fueron constantes durante todo el intervalo estratigráfico analizado; los cambios reconocidos en ambas secciones son similares y se corresponden con tres intervalos que representan condiciones paleoambientales más o menos favorables para el desarrollo de las asociaciones de foraminíferos. Por lo tanto, a pesar de las diferentes ubicaciones paleogeográficas de las secciones estudiadas, el desarrollo de las asociaciones en ambas durante el Aaleniense inferior - Bajociense inferior parece haber estado condicionado por cambios ambientales de escala regional, que afectaron tanto a la Cuenca Ibérica como a la Cuenca Lusitánica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In this work the benthic foraminiferal assemblages of the lower Aalenian–lower Bajocian stratigraphical interval from the Talveila (Iberian Basin, Spain) and Murtinheira (Lusitanian Basin, Portugal) sections are analyzed and compared. The taxonomical composition of the foraminiferal assemblages obtained from the two sections seems to be homogeneous and similar to others recognized in coeval stratigraphic intervals of other basins (namely at the Iberian Plate). However, notable differences can be found between them depending on their paleonvironmental position within the carbonate platform (Canales et al. 2014). Therefore, the comparison between the foraminiferal assemblages of Talveila and Murtinheira sections, located in different paleonvironments within the platform (proximal in the case of the Talveila section and distal in the case of the Murtinheira section), may contribute to establish differences in the taxonomical composition of benthic foraminiferal assemblages assigned to particular paleoenvironmental conditions, and represent a useful tool in the reconstruction of depositional environments.
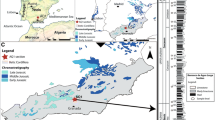
The Talveila section is located in the Iberian Range (Spain) (Fig. 1). The lower Aalenian–lower Bajocian interval was previously the subject of several ammonite-based studies (Ureta and Goy 1986; García-Frank 2006), and more recently concerning its benthic foraminiferal record (Hernández 2015).

(after Hernández 2015)
Map of the Iberian Peninsula representing the Jurassic record and the location of the Talveila (TL) and the Murtinheira (M) sections
The Murtinheira section (also known as Cabo Mondego), is located in the Lusitanian Basin (Portugal) (Fig. 1). Its lower Aalenian–lower Bajocian interval includes the Bajocian Global Stratotype Section and Point (GSSP), the first stratotype defined for the Jurassic System (Pavia and Enay 1997), and it has been subject of several studies on ammonites (Henriques 1992), brachiopods (Andrade 2004, 2006), calcareous nannofossils (De Kaenel and Bergen 1993; De Kaenel et al. 1996; Perilli et al. 2002a, b; Neto 2010; Henriques et al. 2010; López-Otálvaro et al. 2012; Giraud et al. 2016) and foraminifers (Canales et al. 2000; Canales and Henriques 2007, 2008, 2013; Henriques et al. 2008, 2010, 2016; Magno et al. 2008; Canales et al. 2014).
The aim of this paper is: to analyse from taxonomical, biostratigraphical and paleoecological points of views, the lower Aalenian–lower Bajocian foraminiferal assemblages in both sections; to improve the zonal scale based on foraminifera established for the Iberian Peninsula; to characterize the paleoenvironments on which these assemblages were developed during the Early Aalenian–Early Bajocian; and to compare our findings with results from coeval sections of the Iberian Peninsula.
2 Geological setting
The Talveila and the Murtinheira sections include marly carbonate sediments deposited in marine platforms developed in an extensional context during Jurassic times around the western and eastern margins of the Iberian Plate, respectively. Thus, during the Lower and Middle Jurassic on the Iberian Plate occurs the first post-rifting phase in which a regional thermal subsidence was registered, as well as small variations in sea level. Consequently, a sedimentation of alternating limestones and marls, typical of an epicontinental carbonate platform and high energy took place (Sánchez-Moya and Sopeña 2004).
2.1 Talveila section
The Talveila section is located in the northeastern edge of the Iberian Range (Spain) at about 0.5 km to the South of the Talveila village (Soria) (UTM coordinates: X = 502.591 m; Y = 4.625.346 m) (Fig. 1). The analysed section is composed of 6.63 m of thin beds of limestones, marly limestones and marls (Fig. 2a). These materials are organized in sedimentary sequences separated by discontinuities, indicating vertical increase in the energy level, and deposited in a shallow marine platform environment (Ureta and Goy 1986; García-Frank 2006). They correspond to the Turmiel Formation of the Chelva Group (Gómez et al. 2003; Gómez and Fernández-López 2004; García-Frank 2006; García-Frank et al. 2008). A previous biostratigraphic detailed framework allowed the recognition of the following standard ammonite biozones and subzones of the lower Aalenian–lower Bajocian interval: Opalinum Biozone (Comptum Subzone), Murchisonae Biozone, Bradfordensis Biozone, Concavum Biozone (Concavum and Limitatum subzones), Discites Biozone and Laeviuscula Biozone (García-Frank 2006); these units are accurately correlatable with other coeval sections located around the Iberian Plate.
2.2 Murtinheira section
The Murtinheira section is located in the northern sector of the Lusitanian Basin (western edge of the Iberian Peninsula, Portugal), at about 200 km north of Lisbon and 40 km west of Coimbra village (UTM coordinates: 512,065 mE; 4,450,058 mN) (Fig. 1). The section is composed by 70 m of an alternation of limestones and marls (Fig. 2b), deposited in a distal ramp established throughout the Toarcian (Duarte 1997; Azerêdo et al. 2003; Canales and Henriques 2008), which correspond to the lower part of the Cabo Mondego Formation (Azerêdo et al. 2003; Canales and Henriques 2008; Henriques et al. 2008; Canales et al. 2014). The biostratigraphical units for the Aalenian–Bajocian interval were established by Fernández López et al. (1988) and Henriques (1992, 1995) based on the ammonite record, and they include the Opalinum Biozone (Comptum Subzone), the Bradfordensis Biozone (Bradfordensis and Gigantea subzones), the Concavum Biozone (Concavum and Limitatum subzones), the Discites Biozone and the Laeviuscula Biozone (Ovalis Subzone), also correlatable with other coeval sections located around the Iberian Plate.
3 Materials and methods
A total of 37 samples were collected (13 from the Talveila section and 24 from the Murtinheira section) to be processed and analyzed. Sampling in both sections was based on previous biostratigraphic data provided by the ammonoid record (Ureta and Goy 1986; Fernández López et al. 1988; Henriques 1992, 1995; García-Frank 2006), the thickness of the biozones and subzones and the lithological features. The collected marly samples were identified as TL (for the samples from the Talveila section) and M (for the samples from the Murtinheira section) followed by the bed number of the level of the corresponding stratigraphic section.
Approximately 300 g of each sample were processed using a mixture of 400 ml of water, 400 ml of hydrogen peroxide and eight tablets of sodium hydroxide to eliminate the organic matter and clay. Subsequently, the samples were washed with water, in order to remove the mixture of chemical and clay fraction separated by this treatment, in a column of mesh sieves (1, 0.5, 0.25, 0.125 and 0.063 mm).
The foraminifers were picked and studied using a binocular microscope (Wild M-8). The specimens were classified following the classification proposed by Loeblich and Tappan (1987) for the suprageneric and generic levels and for the specific determinations the Ellis and Messina Catalogue of Foraminifera (1940–1990) was consulted.
A JEOL-JSM 6400 electron microscope, located in the Centro Nacional de Microscopía Electrónica at the Complutense University of Madrid (Spain), was used for more detailed observations and to photograph specimens. The specimens and residues of the Talveila section are stored at the Department of Geodynamic, Stratigraphy and Paleontology, Faculty of Geological Sciences, Complutense University of Madrid (Spain). All sample residues and specimens of the Murtinheira section are stored at the Laboratório de Geologia Sedimentar e Registo Fóssil, located in the Geosciences Centre and Department of Earth Sciences, University of Coimbra (Portugal).
For the paleoecological analysis of the obtained foraminiferal assemblages, several usual indexes were applied: the species richness (number of specimens present in each sample), two diversity indexes (Fisher’s α index and Margalef’s richness index) and some indexes based on the proportional abundance of species (Simpson index, Shannon–Wiener index and Pielou’s equitability). These indexes were calculated using the computer program PAST (Hammer et al. 2001), and Microsoft Excel.
The Fisher’s α index relates the number of species and the number of specimens, and its mathematical expression is S = α ln(1 + n∕α), where S is the number of species and n is the sample size (Hammer and Harper 2006). The richness index of Margalef relates the total number of species with the number of specimens in each sample, and it is defined by S = S−1/ln S, where S is the number of species and N is the number of specimens (Magurran 1988).
The Simpson index expresses the dominance of a species over the total number of species. It indicates the probability that two specimens randomly picked in an assemblage belong to the same species. Its mathematical expression is λ = ∑pi2 where pi = ni/n, being ni the number of specimens of each species and n is the total number of specimens (Hammer and Harper 2006). The higher value indicates lower diversity, so that usually the value of this index is shown as (1 − λ) (Buzas 1979; Canales 2001), being (1 − λ) high when diversity is high. In this work the value of the Simpson index will be also presented as (1 − λ). The Shannon–Wiener index measures the degree of uncertainty in predicting the species that belong to one specimen randomly picked. Its mathematical expression is H’ = − Σpilnpi, being pi = ni/n, where ni is the number of specimens of each species, and n is the total number of specimens (Hammer and Harper 2006). The lowest value of this index (H′ = 0) corresponds to a single taxa in the assemblage and the highest value indicates a diverse assemblage. Pielou’s equitability indicates how the specimens belonging to an assemblage are distributed and its mathematical expression is J = H′/ln S, where H′ is the Shannon–Wiener index and S is the total number of species (Hammer and Harper 2006). If J = 1, all the species have the same number of specimens, and this means that there is no dominance of any species.
4 Results
A total of 5109 foraminifers were obtained from the studied samples in the Talveila section, showing in most of cases only a relatively good preservation, but 20.2% of them could not be classified at the specific level (Hernández 2015). Taphonomic processes affecting the recovered specimens include breakages, sedimentary infillings of the chambers, dissolution (partial or even total, as is the case for some representatives of the Ceratobuliminidae Family), distortion and recrystallization comparable with those described by Herrero and Canales (2002). However, no evidence of any alteration of the assemblages´composition can be inferred.
The foraminiferal assemblages are characteristic of Jurassic carbonate platforms of the Boreal Atlantic Realm. Most of the assemblages are abundant and diverse and contain high numbers of specimens and taxa. Their composition is variable throughout the studied interval, which records the last ocurrences (LOs) of some typical species of the Lower Jurassic and the first occurrences (FOs) of some characteristic Middle Jurassic foraminiferal species.
In the Murtinheira section a total of 5626 foraminifers were obtained, most of them very well preserved, and only 3.75% of the specimens could not be assigned to any species (Canales and Henriques 2013). Some specimens show the same taphonomic features recognized in the material from Talveila, but again changes in the composition of the assemblages are considered as negligible (Canales and Henriques 2008). Like in the Talveila section, most assemblages from the Murtinheira section are abundant and diverse, showing variable composition throughout the studied interval and are characteristic of the Jurassic carbonate platforms of the Boreal Atlantic Realm.
4.1 Taxonomical index
Seventy-four foraminiferal taxa were recognized in the Talveila section (Figs. 3, 4), corresponding to 6 suborders, 10 superfamilies, 16 families and 30 genera (Hernández 2015). A table containing the number of specimens of each identified species is shown in Supplementary Appendix 1, where the number of specimens corresponding to each identified species can be found. In the following taxonomic list they are represented by (TL). In the Murtinheira section, 61 foraminiferal taxa were identified, corresponding to 6 suborders, 9 superfamilies, 14 families and 26 genera (Canales and Henriques 2008, 2013). In the taxonomic list they are represented by (M). Selected specimens of the recognized species from the Talveila section are illustrated in Figs. 5, 6 and 7, and their stratigraphic position is located in Figs. 3 and 4. The specimens from Murtinheira referred to in this index were illustrated in previous publications (Canales and Henriques 2008, 2013; Henriques et al. 2016).
Selected foraminifers from the Talveila section belonging to the Suborders Textulariina, Spirillinina, Miliolina and Lagenina. Scale bars = 100 μm. a Thurammina jurensis, TL-81.1.1, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. b Ammovertella liassica, TL-47.2.2, lower Aalenian, Opalinum Biozone, Comptum Subzone. c Tolypammina flagella, TL-77.3.3, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. d Glomospira bulbifera, TL-73.8.10, middle Aalenian, Bradfordensis Biozone. e Glomospira perplexa, TL-51.10.12, lower Aalenian, Opalinum Biozone, Comptum Subzone. f Haplophragmoides bartensteini, TL-51.5.6, lower Aalenian, Opalinum Biozone, Comptum Subzone. g Haplophragmoides infracalloviensis, TL-51.6.7 lower Aalenian, Opalinum Biozone, Comptum Subzone. h Haplophragmoides kingakensis, TL-51.6.8, lower Aalenian, Opalinum Biozone, Comptum Subzone. i Ammobaculites fontinensis, TL-87.78.94, lower Bajocian, Laeviuscula Biozone. j Trochammina canningensis: 1 dorsal view, TL-51.12.15; 2 ventral view, TL.51.13.16, lower Aalenian, Opalinum Biozone, Comptum Subzone. k Trochammina globoconica, lateral view, TL-77.14.17, upper Aalenian Concavum Biozone, Concavum/Limitatum subzones. l Conicospirillina sp. 1: 1 dorsal view, TL-76a.18.21; 2 lateral view, TL-76a.18.22, upper Aalenian Concavum Biozone, Concavum/Limitatum subzones. m Spirillina numismalis, TL-41.19.23, lower Aalenian, Opalinum Biozone, Comptum Subzone. n Spirillina orbicula, TL-51.20.24, lower Aalenian, Opalinum Biozone, Comptum Subzone. o Ophthalmidium concentricum, TL-41.21.25, lower Aalenian, Opalinum Biozone, Comptum Subzone. p Lingulonodosaria dentaliniformis, TL-81.22.26, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones
Selected foraminifers from the Talveila section belonging to the Suborder Lagenina. Scale bars = 100 μm. a Prodentalina intorta, TL-77.24.28, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. b Prodentalina pseudocommunis, TL-51.25.29, lower Aalenian, Opalinum Biozone, Comptum Subzone. c Prodentalina subsiliqua, TL-81.26.30, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. d Prodentalina cf. P. varians, TL-51.27.31, lower Aalenian, Opalinum Biozone, Comptum Subzone. e Falsopalmula jurensis, TL-51.28.32, lower Aalenian, Opalinum Biozone, Comptum Subzone. f Falsopalmula obliqua, TL-51.29.33, lower Aalenian, Opalinum Biozone, Comptum Subzone. g Nodosaria fontinensis, TL-51.31.39, lower Aalenian, Opalinum Biozone, Comptum Subzone. h Nodosaria hortensis, TL-51.32.40, lower Aalenian, Opalinum Biozone, Comptum Subzone. i Nodosaria liassica TL-41.33.41, lower Aalenian, Opalinum Biozone, Comptum Subzone. j Nodosaria opalini, TL-51.34.42, lower Aalenian, Opalinum Biozone, Comptum Subzone. k Nodosaria pseudoregularis, TL-81.35.43, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. l Nodosaria pulchra, TL-51.36.44, lower Aalenian, Opalinum Biozone, Comptum Subzone. m Nodosaria simoniana, TL-65.37.45, middle Aalenian, Murchisonae Biozone. n Pseudonodosaria vulgata, TL-41.38.46, lower Aalenian, Opalinum Biozone, Comptum Subzone. o Frondicularia oolithica, TL-51.39.47, lower Aalenian, Opalinum Biozone, Comptum Subzone. p Tristix oolithica, TL-47.40.48, lower Aalenian, Opalinum Biozone, Comptum Subzone. q Lenticulina constricta, TL-77.41.50, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. r Lenticulina exgaleata, TL-65.42.52, middle Aalenian, Murchisonae Biozone. S Lenticulina helios, TL-51.43.53, lower Aalenian, Opalinum Biozone, Comptum Subzone. t Lenticulina muensteri, TL-77.44.54, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. u Lenticulina polygonata, TL-51.45.55, lower Aalenian, Opalinum Biozone, Comptum Subzone. v Lenticulina quenstedti, TL-77.46.56, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. w Astacolus dorbignyi, TL-77.48.59, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. x Astacolus varians, TL-41.83.99, lower Aalenian, Opalinum Biozone, Comptum Subzone. y Marginulina cf. M. glabra, TL-87.84.100, lower Aalenian, Laeviuscula Biozone. z Marginulina scapha, TL-81.52.63, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones
Selected foraminifers from the Talveila section belonging to the Suborders Lagenina, Robertinina and Rotaliina. Scale bars = 100 μm. a Citharina clathrata, TL-77.53.73, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. b Citharina colliezi, TL-51.54.66, lower Aalenian, Opalinum Biozone, Comptum Subzone. c Citharina flabelloides, TL-41.55.67, lower Aalenian, Opalinum Biozone, Comptum Subzone. d Citharina ornithocephala, TL-81.56.68, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. e Planularia beierana, TL-51.57.69, lower Aalenian, Opalinum Biozone, Comptum Subzone. f Planularia cordiformis, TL-47.74.90, lower Aalenian, Opalinum Biozone, Comptum Subzone. g Planularia cf. P. eugenii, TL-51.59.71, lower Aalenian, Opalinum Biozone, Comptum Subzone. h Planularia protracta, TL-77.61.74, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. i Vaginulina herrerae, TL-81.62.75, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. j Vaginulina cf. V. legumen, TL-51.47.58, lower Aalenian, Opalinum Biozone, Comptum Subzone. k Eoguttulina bilocularis, TL-77.63.76, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. l Eoguttulina liassica, TL-77.64.77, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. m Eoguttulina oolithica, TL-51.65.79, lower Aalenian, Opalinum Biozone, Comptum Subzone. n Bullopora globulata, TL-83.66.80, lower Bajocian, Discites Subzone. o Ceratobuliminidae indet., dorsal view, TL-77.75.91, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones. p Reinholdella crebra, dorsal view, TL-51.70.85, lower Aalenian, Opalinum Biozone, Comptum Subzone. q Reinholdella dreheri, dorsal view, TL-51.68.82, lower Aalenian, Opalinum Biozone, Comptum Subzone. r Reinholdella quadrilocula: 1 dorsal view, TL-51.69.83; 2 lateral view, TL-51.69.84, lower Aalenian, Opalinum Biozone, Comptum Subzone. s Paalzowella goyi: 1 lateral view, TL-81.73.88; 2 ventral view, TL-81.73.89, upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones
Order Foraminiferida Eichwald 1830
Suborder Textulariina Delage and Hérouard 1896
Textulariina indet. (TL)
Superfamily Astrorhizacea Brady 1881
Family Saccamminidae Brady 1884
Subfamily Thurammininae Miklukho—Maklay 1963
Genus Thurammina Brady 1879
Thurammina jurensis (Franke 1936) (TL: Fig. 5a)
Superfamily Ammodiscacea Reuss 1862
Family Ammodiscidae Reuss 1862
Subfamily Tolypammininae Cushman 1928
Genus Ammovertella Cushman 1928
Ammovertella liassica Barnard 1950a (TL: Fig. 5b)
Ammovertella cf. A. liassica Barnard 1950a (TL; not illustrated)
Genus Tolypammina Rhumbler 1895
Tolypammina flagella (Terquem 1870b) (TL: Fig. 5c)
Tolypammina cf. T. flagella (Terquem 1870b) (TL; not illustrated)
Subfamily Ammovertellininae Saidova 1981
Genus Glomospira Rzehak 1885
Glomospira bulbifera Paalzow 1932 (TL: Fig. 5d)
Glomospira gordialis (Jones and Parker 1860) (TL: not illustrated; M: Fig. 3.1 in Canales and Henriques 2013)
Glomospira pattoni Tappan 1955 (TL; not illustrated)
Glomospira perplexa Franke 1936 (TL: Fig. 5e)
Glomospira cf. G. perplexa Franke 1936 (TL; not illustrated)
Superfamily Hormosinaceae Haeckel 1894
Family Hormosinidae Haeckel 1894
Subfamily Reophacinae Cushman 1910
Genus Reophax De Montfort 1808
Reophax agglutinans (Terquem 1870a) (M: Fig. 3.2 in Canales and Henriques 2013)
Superfamily Lituolacea de Blainville 1827
Family Haplophragmoididae Maync 1952
Genus Haplophragmoides Cushman 1910
Haplophragmoides bartensteini Kalantari 1969 (TL: Fig. 5f)
Haplophragmoides infracalloviensis Dain 1948 (TL: Fig. 5g)
Haplophragmoides kingakensis Tappan 1955 (TL: Fig. 5h)
Family Lituolidae de Blainville 1827
Subfamily Ammomarginulininae Podobina 1978
Genus Ammobaculites Cushman 1910
Ammobaculites coprolithiformis Cushman 1910 (M: Plate I, Fig. 4 in Canales and Henriques 2008)
Ammobaculites fontinensis (Terquem 1870b) (TL: Fig. 5i; M: Plate I, Fig. 4 in Canales and Henriques 2008, and Fig. 3.3 in Canales and Henriques 2013)
Superfamily Trochamminacea Schwager 1877
Family Trochamminidae Schwager 1877
Subfamily Trochammininae Schwager 1877
Genus Trochammina Parker and Jones 1859
Trochammina canningensis Tappan 1955 (TL: Fig. 5j1, j2)
Trochammina globoconica Tyszka and Kaminski 1995 (TL: Fig. 5k)
Trochammina praesquamata Myatlyuk 1939 (TL; not illustrated)
Trochammina sablei Tappan 1955 (M: Fig. 3.4 in Canales and Henriques 2013)
Trochammina squamata Jones and Parker 1860 (TL; not illustrated)
Trochammina squamataformis Kaptarenko-Chernousova 1959 (TL; not illustrated)
Superfamily Verneuilinacea Cushman 1911
Family Verneuilinidae Cushman 1911
Subfamily Verneuilinoidinae Suleymanov 1973
Genus Verneuilinoides Loeblich and Tappan 1949
Verneuilinoides sp. indet. (TL; not illustrated)
Suborder Spirillinina Hohenegger and Piller 1975
Family Spirillinidae Reuss and Fritsch 1861
Genus Conicospirillina Cushman 1927
Conicospirillina conoidea (Paalzow 1917) (M: Fig. 3.5 in Canales and Henriques 2013)
Conicospirillina sp. 1 Canales 2001 (TL: Fig. 5l1, l2; M: Plate 1, Fig. 6a, b in Canales and Henriques 2008, and Fig. 3.6 in Canales and Henriques 2013)
Genus Spirillina Ehrenberg 1843
Spirillina numismalis Terquem and Berthelin 1875 (TL: Fig. 5m; M: Plate I, Fig. 7 in Canales and Henriques 2008, and Fig. 3.7 in Canales and Henriques 2013)
Spirillina orbicula Terquem and Berthelin, 1875 (TL: Fig. 5n; M: Plate I, Fig. 8 in Canales and Henriques 2008, and Fig. 3.8 in Canales and Henriques 2013)
Spirillina sp. indet. (TL; not illustrated)
Suborder Miliolina Delage and Hérouard 1896
Superfamily Cornuspiracea Schultze 1854
Family Nubeculariidae Jones 1875
Subfamily Nubeculinellinae Avnimelech and Reiss 1954
Genus Vinelloidea Cushman 1930
Vinelloidea rosacea (Termirbekova 1979) (TL; not illustrated)
Vinelloidea cf. tibia (Terquem and Berthelin 1875) (M: Fig. 3.9 in Canales and Henriques 2013)
Family Ophthalmidiidae Wiesner 1920
Genus Ophthalmidium Kübler and Zwingli 1866
Ophthalmidium concentricum (Terquem and Berthelin 1875) (TL: Fig. 5o)
Ophthalmidium liasicum Kübler and Zwingli 1866 (M: Plate 1, Fig. 9 in Canales and Henriques 2008)
Ophthalmidium sp. indet (TL)
Family Miliolidae Ehrenberg 1839
Suborder Lagenina Delage and Hérouard 1896
Superfamily Robuloidacea Reiss 1963
Family Ichthyolariidae Loeblich and Tappan 1986
Genus Lingulonodosaria Silvestri 1903
Lingulonodosaria dentaliniformis (Terquem 1870b) (TL: Fig. 5p; M: Fig. 3.9 in Canales and Henriques 2013)
Genus Prodentalina Norling 1968
Prodentalina cf. P. guembeli (Schwager 1865) (M: not illustrated)
Prodentalina intorta (Terquem 1870b) (TL: Fig. 6a and M: not illustrated)
Prodentalina mucronata (Neugeboren 1856) (M: Fig. 3.10 in Canales and Henriques 2013)
Prodentalina cf. P. mucronata (Neugeboren 1856) (M: not illustrated)
Prodentalina propinqua (Terquem 1870b) (TL: not illustrated)
Prodentalina pseudocommunis (Franke 1936) (TL: Fig. 6b; M: Plate I, Fig. 11 in Canales and Henriques 2008, and Fig. 3.11 in Canales and Henriques 2013)
Prodentalina cf. P. pseudocommunis (Franke 1936) (TL: not illustrated)
Prodentalina subsiliqua (Franke 1936) (TL: Fig. 6c and M: Plate I, Fig. 12 in Canales and Henriques 2008)
Prodentalina cf. P. varians (Terquem 1866a) (TL: Fig. 6d)
Family Robuloididae Reiss 1936
Genus Falsopalmula Bartenstein 1948
Falsopalmula jurensis (Franke 1936) (TL: Fig. 6e; M: Plate I, Fig. 14 in Canales and Henriques 2008 and Fig. 3.12 in Canales and Henriques 2013)
Falsopalmula cf. F. jurensis (Franke 1936) (TL: not illustrated)
Falsopalmula obliqua (Terquem 1864) (TL: Fig. 6f; M: Fig. 3.13 in Canales and Henriques 2013)
Falsopalmula cf. F. obliqua (Terquem 1864) (TL: not illustrated)
Falsopalmula cf. F. sp. 1 Canales 2001 (TL: not illustrated)
Falsopalmula sp. indet. (TL: not illustrated)
Superfamily Nodosariacea Ehrenberg 1838
Family Nodosariidae Ehrenberg 1838
Subfamily Nodosariinae Ehrenberg 1838
Genus Nodosaria Lamarck 1812
Nodosaria fontinensis Terquem 1870b (TL: Fig. 6g; M: Plate I, Fig. 15 in Canales and Henriques 2008 and Fig. 3.14 in Canales and Henriques 2013)
Nodosaria hortensis Terquem 1866b (TL: Fig. 6h)
Nodosaria liassica Barnard 1950b (TL: Fig. 6i; M: Plate II, Fig. 1 in Canales and Henriques 2008 and Fig. 3.15 in Canales and Henriques 2013)
Nodosaria nuda Terquem 1886 (M: Fig. 3.16 in Canales and Henriques 2013)
Nodosaria opalini Bartenstein 1937 (TL: Fig. 6j; M: Plate II, Fig. 2a, b in Canales and Henriques 2008 and Fig. 3.17 in Canales and Henriques 2013)
Nodosaria plicatilis Wiśniowski 1890 (M: Fig. 3.18 in Canales and Henriques 2013)
Nodosaria pseudoregularis Canales 2001 (TL: Fig. 6k; M: Plate II, Fig. 3 in Canales and Henriques 2008 and Fig. 3.19 in Canales and Henriques 2013)
Nodosaria pulchra (Franke 1936) (TL: Fig. 6l; M: Plate II, Fig. 4 in Canales and Henriques 2008 and Fig. 3.20 in Canales and Henriques 2013)
Nodosaria cf. N. pulchra (Franke 1936) (TL: not illustrated)
Nodosaria simoniana d’Orbigny 1850 (TL: Fig. 6m)
Nodosaria sp. indet. (TL: not illustrated)
Genus Pseudonodosaria Boomgaart 1949
Pseudonodosaria vulgata (Bornemann 1854) (TL: Fig. 6n; M: Plate II, Figs. 5–6 in Canales and Henriques 2008 and Fig. 3.21 in Canales and Henriques 2013)
Subfamily Frondiculariinae Reus 1860
Genus Frondicularia Defrance 1826
Frondicularia oolithica Terquem 1870b (TL: Fig. 6o; M: Fig. 3.22 in Canales and Henriques 2013)
Genus Tristix Macfadyen 1941
Tristix oolithica (Terquem 1886) (TL: Fig. 6p)
Family Vaginulinidae Reuss 1860
Subfamily Lenticulininae Chapman, Parr and Collins 1934
Genus Lenticulina Lamarck 1804
Lenticulina constricta (Kaptarenko-Chernousova 1961) (TL: Fig. 6q; M: Plate II, Fig. 8 in Canales and Henriques 2008 and Fig. 4.1 in Canales and Henriques 2013)
Lenticulina cf. L. constricta (Kaptarenko-Chernousova 1961) (TL: not illustrated)
Lenticulina exgaleata Dieni 1985 (TL: Fig. 6r; M: Plate II, Fig. 9 in Canales and Henriques 2008 and Fig. 4.2 in Canales and Henriques 2013)
Lenticulina cf. L. exgaleata Dieni 1985 (TL: not illustrated)
Lenticulina helios (Terquem 1870a) (TL: Fig. 6s; M: Plate II, Fig. 10 in Canales and Henriques 2008 and Fig. 4.3 in Canales and Henriques 2013)
Lenticulina muensteri (Roemer 1839) (TL: Fig. 6t; M: Plate II, Fig. 11 in Canales and Henriques 2008 Fig. 4.4 in Canales and Henriques 2013 and Fig. 5.5 in Henriques et al. 2016)
Lenticulina polygonata (Franke 1936) (TL: Fig. 6u; M: Plate II, Fig. 12 in Canales and Henriques 2008 and Fig. 4.5 in Canales and Henriques 2013)
Lenticulina quenstedti (Gümbel 1862) (TL: Fig. 6v; M: Plate II, Fig. 13 in Canales and Henriques 2008 Fig. 4.5 in Canales and Henriques 2013, and Fig. 5.2 in Henriques et al. 2016)
Lenticulina cf. L. quenstedti (Gümbel 1862) (TL: not illustrated)
Lenticulina sp. indet. (TL and M; not illustrated)
Genus Saracenaria Defrance 1824
Saracenaria cornucopiae (Schwager 1865) (M: Fig. 4.7 in Canales and Henriques 2013)
Saracenaria sublaevis (Franke 1936) (TL: not illustrated)
Subfamily Marginulininae Wedekind 1937
Genus Astacolus De Montfort 1808
Astacolus cf. A. brevispirus (Wiśniowski 1890) (M: Fig. 4.8 in Canales and Henriques 2013)
Astacolus dorbignyi (Roemer 1839) (TL: Fig. 6w; M: Plate III, Fig. 3 in Canales and Henriques 2008 and Fig. 4.9 in Canales and Henriques 2013)
Astacolus scalptus (Franke 1936) (TL: not illustrated; M: Plate III, Fig. 4 in Canales and Henriques 2008 and Fig. 4.10 in Canales and Henriques 2013)
Astacolus cf. A. scalptus (Franke 1936) (TL: not illustrated)
Astacolus varians (Bornemann 1854) (TL: Fig. 6x; M: Plate III, Fig. 5 in Canales and Henriques 2008 and Fig. 4.11 in Canales and Henriques 2013)
Astacolus cf. A. varians (Bornemann 1854) (TL: not illustrated)
Astacolus vetustus (d’Orbigny 1849) (TL: not illustrated)
Astacolus sp. 1 Canales 2001 (M: Plate III, Fig. 6 in Canales and Henriques 2008 and Fig. 4.12 in Canales and Henriques 2013)
Astacolus sp. indet. (TL and M: not illustrated)
Genus Marginulina d’Orbigny 1826
Marginulina cf. M. glabra d’Orbigny 1826 (TL: Fig. 6y)
Marginulina scapha Lalicker 1950 (TL: Fig. 6z; M: Fig. 4.13 in Canales and Henriques 2013)
Subfamily Vaginulininae Reuss 1860
Genus Citharina d’Orbigny 1839
Citharina clathrata (Terquem 1863) (TL: Fig. 7a; M: Plate III, Fig. 7 in Canales and Henriques 2008)
Citharina colliezi (Terquem 1866a) (TL: Fig. 7b; M: Plate III, Fig. 8 in Canales and Henriques 2008 and Fig. 5.1 in Canales and Henriques 2013)
Citharina cf. C. colliezi (Terquem 1866a) (TL: not illustrated)
Citharina flabelloides (Terquem 1868) (TL: Fig. 7c)
Citharina ornithocephala (Wiśniowski 1890) (TL: Fig. 7d; M: Fig. 5.2 in Canales and Henriques 2013)
Citharina sagittiformis (Terquem 1868) (M: Fig. 5.3 in Canales and Henriques 2013)
Genus Planularia Defrance 1826
Planularia beierana (Gümbel 1862) (TL: Fig. 7e; M: Fig. 5.4 in Fig. 5.2 in Canales and Henriques 2013)
Planularia aff. P. beierana (Gümbel 1862) (M: Plate III, Fig. 9 in 2 in Canales and Henriques 2008)
Planularia cordiformis (Terquem 1863) (TL: Fig. 7f; M: Plate III, Fig. 10 in Fig. 5.2 in Canales and Henriques 2008 and Fig. 5.5 in Fig. 5.2 in Canales and Henriques 2013)
Planularia cf. P. eugenii (Terquem 1864) (TL: Fig. 7g and M: not illustrated)
Planularia protracta (Bornemann 1854) (TL: Fig. 7h; M: Plate III, Fig. 11 in Canales and Henriques 2008 and Fig. 5.6 in Canales and Henriques 2013)
Planularia sp. 1 Canales 2001 (TL: not illustrated)
Planularia sp. indet. (TL: not illustrated)
Genus Vaginulina d’Orbigny 1826
Vaginulina herrerae Canales 2001 (TL: Fig. 7i)
Vaginulina cf. V. herrerae Canales 2001 (M: Plate III, Fig. 12 in Canales and Henriques 2008)
Vaginulina cf. V. legumen (Linné 1758) (TL: Fig. 7j)
Family Polymorphinidae d’Orbigny 1839
Subfamily Polymorphininae d’Orbigny 1839
Genus Eoguttulina Cushman and Ozawa 1930
Eoguttulina bilocularis (Terquem 1864) (TL: Fig. 7k)
Eoguttulina liassica (Strickland 1846) (TL: Fig. 7l; M: Plate III, Fig. 13 in Canales and Henriques 2008 and Fig. 5.7 in Fig. 5.2 in Canales and Henriques 2013)
Eoguttulina oolithica (Terquem 1874) (TL: Fig. 7m; M: Fig. 5.8 in Canales and Henriques 2013)
Eoguttulina cf. E. oolithica (Terquem 1874) (M: not illustrated)
Subfamily Webbinellinae Rhumbler 1904
Genus Bullopora Quenstedt 1856
Bullopora globulata Barnard 1950a (TL: Fig. 7n)
Bullopora rostrata Quenstedt 1858 (TL: not illustrated; M: Fig. 5.9 in Canales and Henriques 2013)
Genus Ramulina Jones 1875
Ramulina spandeli Paalzow 1917 (M: Fig. 5.10 in Canales and Henriques 2013)
Suborder Robertinina Loeblich and Tappan 1984
Superfamily Ceratobuliminacea Cushman 1927
Family Ceratobuliminidae Cushman 1927
Ceratobuliminidae indet. (TL: Fig. 7o; M: Plate III, Figs. 14–15 in Canales and Henriques 2008 and Figs. 5.11–5.12 in Canales and Henriques 2013)
Subfamily Reinholdellinae Sieglie and Bermúdez 1965
Genus Lamarckella Kaptarenko-Chernousova 1956
Lamarckella inflecta Kaptarenko-Chernousova 1956 (M: not illustrated)
Genus Reinholdella Brotzen 1948
Reinholdella crebra Pazdro 1969 (TL: Fig. 7p)
Reinholdella dreheri (Bartenstein 1937) (TL: Fig. 7q and M: not illustrated)
Reinholdella cf. R. dreheri (Bartenstein 1937) (TL: not illustrated)
Reinholdella quadrilocula Subbotina and Datta 1960 (TL: Fig. 7r1, r2)
Reinholdella sp. indet. (TL: not illustrated)
Suborder Rotaliina Delage andHérouard 1896
Superfamily Discorbacea Ehrenberg 1838
Family Placentulinidae Kasimova, Poroshina and Geodakchan 1980
Subfamily Ashbrookinae Loeblich and Tappan 1984
Genus Paalzowella Cushman 1933
Paalzowella goyi Canales 2001 (TL: Fig. 7s1, s2; M: Fig. 5.13 in Canales and Henriques 2013)
4.2 Description of the Talveila assemblages
From the 13 samples studied in this section, the Suborder Lagenina is the most abundant, and the genus Lenticulina (except for the assemblage corresponding to the sample TL-87 in the lower Bajocian) is also the most abundant. The relative abundance of Lagenina representatives decreases at the Aalenian/Bajocian boundary, whereas the representatives of Textulariina, Spirillinina and Robertinina suborders show an increasing relative abundance across the Aalenian–Bajocian transition. The Miliolina and Rotaliina suborders are minor components throughout the Talveila section. The studied assemblages show apparently a homogeneous composition, but they display variations in abundance and diversity when analysed in detail. The main features of the foraminiferal assemblages are presented in the following sections.
4.2.1 Lower Aalenian, Opalinum Biozone, Comptum Subzone
The asssemblage obtained from sample TL-41, corresponding to the middle part of the Comptum Subzone, displays high abundance (for this and subsequent assemblages and species from the Talveila section, numerical data can be seen in Supplementary Appendix 1) and high diversity (36 taxa). Most of them belong to Lenticulina helios, Lenticulia muensteri and Astacolus scalptus. The species Ophthalmidium concentricum, Prodentalina propinqua and Citharina flabelloides were found only in this assemblage.
The assemblage from sample TL-47 (middle part of the Comptum Subzone) contains high number of specimens and also high diversity (31 taxa). Lenticulina is the most abundant genus, represented mainly by Lenticulina helios, Lenticulina muensteri and Lenticulina polygonata. The species Tristix oolithica, Planularia sp. 1, Bullopora rostrata and Haplophragmoides bartensteini were only recorded in this assemblage.
The overlying assemblage TL-51 shows high abundance and diversity (51 taxa). Lenticulina helios, Glomospira perplexa and Reinholdella dreheri are the most abundant species. This assemblage records an increase in the abundance and diversity of representaives of the Suborder Textulariina, an increase in the abundance of representatives of the Suborder Robertinina, and a slight decrease in the relative abundance of the representatives of the Suborder Lagenina. The species Planularia cf. P. eugenii, Prodentalina cf. P. varians, Nodosaria opalini and Vaginulina cf. V. legumen were identified only in this assemblage.
The last assemblage (sample TL-55) from this subzone (upper part of the Comptum Subzone) shows high foraminiferal abundance and diversity (29 taxa), being Lenticulina helios, Astacolus scalptus and Lenticulina exgaleata the most abundant species. Saracenaria sublaevis was only recorded in this assemblage.
4.2.2 Middle Aalenian, Murchisonae Biozone
The first assemblage corresponding to the Murchisonae Biozone was obtained from sample TL-59 and shows a decrease in abundance and diversity (14 taxa), when compared with the previous ones. Lenticulina is the most abundant genus, represented mainly by Lenticulina helios and Lenticulina muensteri followed by Astacolus scalptus.
The composition of the overlying assemblage TL-65 is very similar to the previous one, displaying relatively high foraminiferal abundance and diversity (20 taxa) being Lenticulina helios and Lenticulina muensteri followed by Astacolus scalptus the most abundant species.
4.2.3 Middle Aalenian, Bradfordensis Biozone
The only assemblage studied from the Bradfordensis Biozone (TL-73) shows low abundance but high diversity (30 taxa). Lenticulina helios, Astacolus scalptus and Paalzowella goyi are the most abundant species. The FOs of Thurammina jurensis, Lenticulina quenstedti and Paalzowella goyi are recorded in this biozone in the Talveila section. This assemblage also records the LOs of Glomospira bulbifera and Nodosaria simoniana, whose FO in this section occurs in the lower Aalenian (Opalinum Biozone, Comptum Subzone) and middle Aalenian (Murchisonae Biozone), respectively.
4.2.4 Upper Aalenian, Concavum Biozone, Concavum/Limitatum subzones
The assemblage from sample TL-76a (lower part of the Concavum Biozone) contains relatively high number of specimens and high diversity (33 taxa) being Lenticulina helios, Spirillina orbicula and Spirillina numismalis the most abundant species. This assemblage records an increase in the abundance of representatives of the Suborder Spirillinina and the LOs of Planularia beierana and Haplophragmoides kingakensis, whose FOs in this section occur in the lower Aalenian (Opalinum Biozone, Comptum Subzone).
Assemblage obtained from sample TL-77, from the middle part of the Concavum Biozone, shows high abundance and diversity (40 taxa). Most of the specimens belong to Lenticulina helios, Ammovertella cf. A. liassica and Lenticulina muensteri. Representatives of the Suborder Textulariina increase in abundance and diversity whereas representatives of the Suborder Robertinina increase only in abundance. This assemblage records the LOs of Trochammina globoconica, Prodentalina pseudocommunis, Nodosaria hortensis, Nodosaria pulchra, Planularia protracta and Eoguttulina oolithica, whose FO in this section occurs in the lower Aalenian (Opalinum Biozone, Comptum Subzone). The species Trochammina squamata, Prodentalina intorta and Eoguttulina bilocularis were recognized only in this assemblage.
The last studied assemblage from this subzone (sample TL-81) also displays high abundance and diversity (43 taxa). Lenticulina is the most abundant genus, represented mainly by Lenticulina helios, Lenticulina polygonata and Lenticulina muensteri. This assemblage records a high number of LOs (Thurammina jurensis, Glomospira gordialis, Glomospira pattoni, Glomospira perplexa, Lingulonodosaria dentaliniformis, Prodentalina subsiliqua, Falsopalmula cf. F. sp. 1, Nodosaria fontinensis, Lenticulina constricta, Lenticulina exgaleata, Astacolus dorbignyi, Astacolus varians, Citharina clathrata, Citharina colliezi and Eoguttulina liassica), whose FOs in this section are in the lower Aalenian (Opalinum Biozone, Comptum Subzone), except for the first species whose FO is in the middle Aalenian (Bradfordensis Biozone). This assemblage also includes some species only found here, such as Trochammina squamataformis, Astacolus vetustus, Marginulina scapha, Citharina ornithocephala and Vaginulina herrerae.
4.2.5 Lower Bajocian, Discites Biozone
The assemblage obtained from sample TL-83 shows low abundance but high diversity (20 taxa). The most abundant species are Ammovertella liassica, Spirillina orbicula, and Bullopora globulata. This assemblage displays a decrease in the abundance and diversity of representatives of the Suborder Lagenina, and Lenticulina helios, one of the most abundant taxa in the studied assemblages, was not found. LOs include Conicospirillina sp. 1, recorded for the first time in the lower part of the Concavum Biozone at Talveila, and Reinholdella crebra whose FO in this section takes place in the lower Aalenian (Opalinum Biozone, Comptum Subzone). Moreover, Vinelloidea rosacea was only recorded in this assemblage.
The last assemblage from this subzone (sample TL-85) displays low abundance but high diversity (21 taxa), being Ammovertella liassica, Spirillina orbicula and Bullopora globulata the most abundant species. This assemblage displays low abundance and diversity of representatives of the Suborder Lagenina and records the LOs of Haplophragmoides infracalloviensis, Trochammina canningensis and Trochammina praesquamata, all of them identified for the first time in the lower part of the Concavum Biozone in the Talveila section.
4.2.6 Lower Bajocian, Laeviuscula Biozone
The only assemblage studied of the Laeviuscula Biozone, TL-87, shows low abundance but relatively high diversity (20 taxa). Spirillina numismalis, Spirillina orbicula and Ammobaculites fontinensis are the most abundant species, and again the representatives of the Suborder Lagenina are scarce. Marginulina cf. M. glabra was recognized only in this assemblage.
4.3 Description of the Murtinheira assemblages
At the Murtinheira section, except for the assemblage corresponding to the sample M-269 (upper Aalenian, Concavum Biozone, Concavum Subzone), the Suborder Lagenina and the genus Lenticulina are the most abundant. The suborders Textulariina, Miliolina, Robertinina and Rotaliina are minor components throughout the studied stratigraphic interval in the Murtinheira section, where assemblages show variations in the abundance and diversity, but apparently their composition is quite homogeneous (Canales and Henriques 2008, 2013). The main features of the assemblages are described in the following sections.
4.3.1 Lower Aalenian, Opalinum Biozone, Comptum Subzone
From this subzone, four samples were studied and a variable number of specimens were recovered. The recorded assemblages show high abundance and high diversity (33 taxa). The Suborder Lagenina prevails, followed by Spirillinina and Textulariina, while Miliolina and Robertinina may be considered as minority suborders. M-103 is the only sample throughout the section where specimens of the five suborders were recognized (Canales and Henriques 2008). The largest number of specimens corresponds to different species of the genus Lenticulina.
4.3.2 Middle Aalenian, Bradfordensis Biozone, Bradfordensis Subzone
From the Bradfordensis Subzone 2 samples were studied. A total of 22 taxa were identified. The Lagenina continues to be the most abundant suborder throughout this subzone. Only the Suborder Robertinina was not recognized in M-124. Again, the best represented genus is Lenticulina, and Lenticulina muensteri is the most abundant species. The FO of miliolids occurs in this assemblage, but the taxonomic composition of this assemblage is very similar to those of the previous ones (Canales and Henriques 2008).
4.3.3 Middle Aalenian, Bradfordensis Biozone, Gigantea Subzone
From the Gigantea Subzone, 5 samples were studied, and a total of 35 taxa. In the first 3 samples, a relatively high number of specimens were obtained. Only in the first sample (M-142) all the suborders were recognized. All assemblages are mainly constituted by specimens of Lagenina, specifically of the genus Lenticulina, with Lenticulina muensteri the most abundant species. However, in M-185 Suborder Spirillinina attains a significant percentage, while Textulariina may be considered as a minority. Several FOs and LOs occur in this stratigraphic interval. Thus in M-142 the LO of Nodosaria liassica and miliolids are observed. In M-152 the LO of Ophthalmidium liasicum and Citharina clathrata and the FO of Saracenaria cornucopiae are recorded. The species Prodentalina cf. P. mucronata and Vaginulina cf. V. herrerae were only recognized in the assemblage corresponding to M-152, and Ammobaculites coprolithiformis, a typical Middle Jurassic species, was identified only in M-185. The FO of Lenticulina quenstedti is also recorded in M-241. This typical Middle Jurassic species plays an important role in the following assemblages. The number of specimens and taxa in the two assemblages corresponding to the upper part of the Gigantea Subzone decrease when compared to the previous ones (Canales and Henriques 2008).
4.3.4 Upper Aalenian, Concavum Biozone, Concavum Subzone
Within the Concavum Subzone 3 samples were collected, and a total of 30 taxa were identified. M-269 records the lowest number of specimens (3) and taxa (3) corresponding to the suborders Lagenina and Robertinina. In the following analyzed assemblage (M-273) a high number of specimens and taxa (26) were obtained, but all of them corresponding to the suborders Lagenina, Spirillinina and Textulariina. In the last studied sample (M-283), the number of specimens and taxa decrease again. In these assemblages, the prevalent suborder is Lagenina. At the specific level, Lenticulina muensteri continues to be the most abundant species. Some LOs were recorded: Prodentalina subsiliqua and Planularia cf. P. eugenii in M-273 and Pseudonodosaria vulgata and representatives of the Family Ceratobuliminidae in M-283. The FO of Conicospirillina sp. 1 and Nodosaria opalini were also recorded in M-273 (Canales and Henriques 2008).
4.3.5 Upper Aalenian, Concavum Biozone, Limitatum Subzone
In the Limitatum Subzone, 4 samples were studied and a total of 1060 specimens were obtained, corresponding to 26 species. In M-291 a relatively high number of specimens were recovered, but in the remaining analyzed assemblages (M-285, M-306 and M-330) abundance is relatively low. In all of these assemblages representatives of the suborders Lagenina and Spirillinina were recognized, while representatives of Textulariina and Robertinina suborders were only obtained in samples M-291 and M-306. In these assemblages, the representatives of the Suborder Lagenina continue to prevail, and the most abundant species is again Lenticulina muensteri. However, the species Spirillina orbicula attains its maximum percentage in sample M-330. The taxonomic composition of these assemblages is very similar to the preceding ones. However, some LOs were recorded in M-291: Astacolus dorbignyi, Astacolus varians, Nodosaria fontinensis, Citharina colliezi, Planularia cordiformis, Astacolus sp. 1, Conicospirillina sp. 1 and Nodosaria opalini. The only FO identified in this interval was Reophax agglutinans, also recorded in sample M-291. Assemblage M-306 records the LOs of Planularia aff. P. beierana (Canales and Henriques 2008, 2013).
4.3.6 Lower Bajocian, Discites Biozone
In the Discites Biozone, 5 samples were studied. A total of 759 specimens were recovered and 34 taxa were identified. The largest number of specimens corresponds to different species of the genus Lenticulina. From the analyzed samples, a variable number of specimens were recovered. The Suborder Lagenina prevails, followed by Spirillinina which attains high relative abundance, while Textulariina, Miliolina and Robertinina may be considered as minority components, when they are present. Hence, M-352 is the only sample throughout the section where specimens of the five suborders were recognized. At the specific level, the most abundant species is Spirillina orbicula followed by Lenticulina muensteri. Some FOs were recorded, such as, Frondicularia oolithica and Eoguttulina cf. E. oolithica (both restricted to this assemblage), Lingulonodosaria dentaliniformis, Paalzowella goyi, Lamarckella inflecta, Nodosaria plicatilis and Ramulina spandeli in M-352, Planularia beierana in M-355 and finally Prodentalina intorta, Eoguttulina oolithica, Trochammina sablei and Conicospirillina conoidea in M-386. Along this stratigraphic interval the LO of the species Nodosaria pulchra and Nodosaria pseudoregularis was recorded in M-363 and Lingulonodosaria dentaliniformis in M-386 (Canales and Henriques 2013).
4.3.7 Lower Bajocian, Laeviuscula Biozone
The only sample studied in the Laeviuscula Biozone, M-402, recorded the highest abundance and diversity (38 taxa) of all the analyzed assemblages from the Murtinheira section. Lagenina is the most abundant suborder and, at the specific level, Spirillina orbicula, Reophax agglutinans, Lenticulina helios and Lenticulina quenstedti are the most abundant (Canales and Henriques 2013).
4.4 Biostratigraphy
The best fossil group for dating Jurassic marine sedimentary rocks is the ammonites. However, these fossils are not always abundant, or their record may be impossible to study, as is the case with core and cutting samples. In these cases, alternative biostratigraphic scales, based on other fossil groups such foraminifers, are needed, but their range must be calibrated with accurate zonal scales based on good guide-fossils like ammonites, as is the case in both studied sections. In adition, it is necessary to ascertain that these foraminiferal-based zonal scales are useful in different sections located in the same basin, as well as in different basins.
Several Jurassic biostratigraphic scales based on foraminifers have been established in the northern hemisphere, some of them throughout the Aalenian–Bajocian boundary, and such biozones have been recognized in several basins of the Iberian Plate (Canales 2001; Figueiredo 2009; Canales and Henriques 2013; Silva 2013; Henriques et al. 2016) (Fig. 8). These alternative biostratigraphic scales are useful only when they are calibrated with biostratigraphic scales based on ammonites. In the case of benthic foraminifera, their stratigraphic ranges are relatively wide; its biostratigraphic usefulness can be improved when foraminiferal bioevents (such as notable changes in diversity or relative abundance and/or FO, LO of some taxa) are recognized. Such bioevents initially have local character, given the close relationship of benthic foraminifera with the paleoenvironmental conditions. However, detailed studies within the same basin or in other coeval basins may help to support the regional range of these foraminiferal bioevents (Canales and Henriques 2013). The main foraminiferal biozones and foraminiferal bioevents recognized in the Talveila and Murtinheira sections are reported in the following sections.
4.4.1 Foraminiferal Biozones
In the Iberian Plate, the stratigraphic distribution of foraminifers across the lower Aalenian–lower Bajocian interval allowed the establishment and/or recognition of three foraminiferal biozones: Astacolus dorbignyi Zone, Lenticulina quenstedti Zone and Ramulina spandeli Zone (Canales 2001; Canales and Henriques 2008, 2013; Silva et al. 2015a, b). The Astacolus dorbignyi Zone and the Lenticulina quenstedti Zone were both recognized in the Talveila and Murtinheira sections and the Ramulina spandeli Zone was only recognized in the last one (Canales and Henriques 2008, 2013; Hernández 2015) (Fig. 8).
The Astacolus dorbignyi Zone was recognized based on the relatively continuous and abundant record of the species Astacolus dorbignyi throughout the Aalenian. The lower boundary of this biozone is not known in the Talveila and Murtinheira sections but it must be recorded probably in the Mactra Subzone (upper Toarcian, Lower Jurassic). The upper boundary of this biozone coincides with the FO of Lenticulina quenstedti, the following index species. This foraminiferal biozone (Astacolus dorbignyi Zone) corresponds to the Opalinum (Comptum Subzone), and Murchisonae ammonite biozones in the Talveila section. In the Murtinheira section this biozone corresponds to the Opalinum and Bradfordensis ammonite biozones (Comptum, Bradfordensis and the lower part of Gigantea subzones, respectively) (Canales and Henriques 2008).
The Lenticulina quenstedti Zone was recognized from the middle Aalenian, coinciding with the FO of the index species, whose record is relatively continuous and abundant, until the upper part of the Aalenian. The lower boundary of this biozone coincides with the FO of the index species in both the Talveila and Murtinheira sections. However, the upper boundary of this biozone is not known in the Talveila section, whereas in the Murtinheira section it coincides whith the FO of Ramulina spandeli, the nominal index species of the overlying foraminiferal biozone established in the Bajocian GSSP (Canales and Henriques 2013). The Lenticulina quenstedti Zone corresponds to the Bradfordensis, Concavum, Discites and Laeviuscula ammonite biozones in Talveila, while in Murtinheira it corresponds to the Bradfordensis, Concavum, and the lower part of Discites ammonite biozones (upper part of Gigantea, Concavum and Limitatum subzones, respectively) (Canales and Henriques 2013).
Finally, the Ramulina spandeli Zone was established in the Murtinheira section by Canales and Henriques (2013) and, until now, it has not been recognized in the Talveila section. The lower boundary of this foraminiferal biozone, recorded in the lower Bajocian, is marked by the FO of the index species Ramulina spandeli; the upper boundary of this foraminiferal biozone is so far not known. This foraminiferal biozone spans from the middle part of the Discites ammonite biozone upwards (Canales and Henriques 2013).
4.4.2 Foraminiferal Bioevents
In both studied sections, some events including FO and LO, significant variations in the relative abundance of some taxa, as well as changes in the diversity recorded in some assemblages were recognized. These bioevents have initially local application, but when they are recognized in other sections, in the same or in other basins, it is possible to correlate them, hence increasing their geographical applicability (Canales and Henriques 2013). Bioevents recognized in the Talveila section, in the Bajocian Global Stratotype Section and Point (GSSP) (Murtinheira section) or in both sections are represented in Fig. 9.
In the Talveila section, throughout the analyzed stratigraphic interval the gradual disappearance of several typical Early Jurassic species occurs (i.e., Thurammina jurensis, Ophthalmidium concentricum, Prodentalina pseudocommunis, Nodosaria liassica, Citharina clathrata), as well as the progressive appearance of some typical Middle Jurassic species (i.e., Vinelloidea rosacea, Lingulonodosaria dentaliniformis, Lenticulina quenstedti, Vaginulina herrerae). A decrease in abundance and diversity in the foraminiferal assemblages between the Comptum Subzone and Murchisonae Biozone (with the disappearance of 13 taxa) and a recovery of the diversity in the upper Aalenian were also recorded. During this recovery, the FO of the representatives of the Suborder Rotaliina occurs. In the Concavum Biozone, the suborders Spirillinina, Robertinina and Textulariina increase their relative abundances, coinciding with a decrease in the relative abundance of representatives of Lagenina and the disappearance of 36 taxa. Moreover, a swift increase in foraminiferal abundance and diversity occurs in the Discites and Laeviuscula biozones assemblages, where the species Ammovertella liassica and Spirillina numismalis are predominant.
The Murtinheira assemblages also record a gradual disappearance of typical Early Jurassic species (i.e., Thurammina jurensis, Nodosaria liassica, Ophthalmidium liasicum, Citharina clathrata) and a progressive appearance of Middle Jurassic species (i.e., Lenticulina constricta, Nodosaria fontinensis, Planularia aff. P. beierana, Vaginulina cf. V. herrerae, Ammobaculites coprolithiformis, Lenticulina quenstedti, Nodosaria opalini) (Canales and Henriques 2008). Regarding the taxonomical composition of the assemblages, an increase in the relative abundance of the representatives of the Suborder Spirillinina is recorded in the Concavum Biozone, as well as a decrease in the abundance and diversity of the assemblages (Canales and Henriques 2008). Across the Aalenian–Bajocian transition a high decrease in abundance and diversity takes place with the disappearance of 15 taxa in the Murtinheira section, but a quick recovery occurs in the lower Laeviuscula Biozone (Canales and Henriques 2013).
4.5 Paleoecology
In the studied assemblages, the number of recovered specimens and identified species is variable and the percentage of the determinable specimens is high, being 79.8% in the Talveila section (Hernández 2015) and 98.9% in the Murtinheira section (Canales and Henriques 2008). The studied specimens present relatively good preservation; fragmentation, recrystallization, deformation and dissolution were observed only in a few shells. It is also remarkable that both sections provide relatively high number of specimens belonging to species displaying very thin shells; the dissolution of shells, just partially present, reinforces the original character of the assemblages’ composition. Moreover, the assemblages are composed by juvenile and adult specimens, thus supporting that the composition of these foraminiferal assemblages were not affected by the influence of transport. In conclusion, there is no evidence that the above mentioned taphonomic changes may have modified the composition of the foraminiferal assemblages. As so, alteration in the composition of the foraminiferal assemblages was caused by changes in paleoenvironmental factors, which can be inferred through interpretations based on paleoecological analysis.
The paleoecological interpretations were based on quantitative analysis of relative abundance and several statistical techniques were also used. The applied indexes in this work are the species richness (number of specimens present in each sample), two diversity indexes (Fisher’s α index and Margalef’s richness index) and some indexes based on the proportional abundance of species (Simpson index, Shannon–Wiener index and Pielou’s equitability).
4.5.1 Relative abundance
Relative abundance is the relation between suborder, genus or species ranks and the total number of specimes in each assemblage, expressed as a percentage. At the suborder rank, all assemblages show high relative abundance of the Suborder Lagenina, with percentages always above 56%, but reaching 90% in some assemblages from Murtinheira (Canales and Henriques 2008) as in M-83 (Comptum Subzone, lower Aalenian), M-152 (Gigantea Subzone, middle Aalenian) or M-336 (Discites Biozone), while in Talveila they exceed at least 38%, and in some assemblages they reach 98% (TL-59 and TL-65; Murchisonae Biozone, middle Aalenian).
Representatives of the Suborder Textulariina present high relative abundance in the assemblages TL-51 (21%), located in the Comptum Subzone, TL-83 (33%) and TL-85 (35%), both from the lower Bajocian, Discites Biozone of the Talveila section (see Supplementary Appendix 1), while in the Murtinheira section the highest relative abundance of this suborder is 17%, being a noticeable difference when taxonomical composition of the assemblages of both sections is compared.
In the same way, also the representatives of the Suborder Spirillinina were identified in both sections, showing differences in their relative abundances when compared. Thus, in the Talveila section they present relative abundances between 0.5 and 13%, with a peak in the last analyzed assemblage, where it displays a relative abundance of 40%. In contrast, in the Murtinheira section representatives of the Suborder Spirillinina present rather higher relative abundance, between 3 and 38%, being highest in the Discites and Laeviuscula biozones.
The specimens of Robertinina are in general scarce. However, their relative abundance increases considerably in samples with very small number of specimens or in assemblages displaying very high diversity in both sections. Finally, Miliolina and Rotaliina, which show a discontinuous record and low percentages, are considered minor constituent of the assemblages, both in Talveila and in Murtinheira.
At the generic level, it is observed a clear prevalence of Lenticulina in almost all the assemblages, with percentages from 24 until 93% in Talveila and percentages between 33 and 81% in Murtinheira. The only exception is the assemblage TL-87 (in Talveila), where the genus Spirillina presents the highest relative abundance (40%). Regarding the relative abundance of this last genus, it is noticeable that it increases in the Discites and the Laeviuscula biozones in both sections.
The species of the genera Lenticulina, Astacolus and Spirillina show the highest relative abundance values in both sections. Only in a few cases other species record high relative abundance, as is the case of Ammovertella liassica (TL-83 and TL-85) and Ammobaculites fontinensis (TL-85) in the Talveila section and Reophax agglutinans (M-402) in the Murtinheira section.
4.5.2 Diversity indexes
The number of species identified in each assemblage is variable in both sections. In the Talveila section it ranges from 12 to 48, while in the Murtinheira section varies between 3 and 39. The assemblages obtained in the Talveila section show a slightly decreasing tendency on diversity from the upper part of the Comptum Subzone to the Murchisonae Biozone, although the obtained diversity indexes values are still high. From the Murchisonae Biozone to the Aalenian–Bajocian boundary, diversity shows an increasing tendency and finally, the assemblages from lower Bajocian show a clear decreasing tendency. In the obtained assemblages from the upper part of the Comptum Subzone to the Limitatum Subzone in the Murtinheira section, the number of taxa generally increases and remains high; the assemblages around the Aalenian–Bajocian transition show a clear decreasing tendency (Canales and Henriques 2008). Finally, in the recorded assemblages of the lower Bajocian (Discites and Laeviuscula biozones) it is observed an increase in the number of identified taxa (Canales and Henriques 2015).
The values of the Fisher’s α (Fig. 10) index are different in both sections. In the Talveila section these values are variable, between 2.6 and 13.7, been relatively high. In this section the α values are < 5 only in the Murchisonae Biozone. In contrast, in the Murtinheira section they oscillate between 0 and 7.52, showing punctual variations throughout the studied stratigraphic interval and being lower than those recorded in Talveila. From the lower part of the Comptum Subzone to the lower part of the Limitatum Subzone the values are higher, being in most of the cases α > 5. In the assemblages of the Aalenian–Bajocian transition the values are low again, being α < 5. Finally, in the Bajocian assemblages, α > 5 (except in M-336).
The values of the Margalef’s richness index (Fig. 10) in the Talveila section range between 2 and 7.7, recording the highest values in the Comptum Subzone and the Concavum Biozone. In this section it is also recorded a decrease of the values in the Aalenian–Bajocian transition. In contrast, the values of the Margalef’s richness in the Murtinheira section range between 1.54 and 5.55. In general, they are homogeneous, especially between the upper part of the Comptum Subzone and the lower part of the Limitatum Subzone, where they range between 3 and 4, with a few exceptions. The obtained values decrease in the assemblages of the Aalenian–Bajocian transition and show an increase throughout the Bajocian assemblages, reaching the maximum of 5.55.
In the studied assemblages of the Talveila section, the Simpson index values (Fig. 11) vary between 0.5 and 0.9. They are high, being 1 − λ > 0.7 with the exception of the sample TL-59 (1 − λ = 0.5). In the assemblages of the Murtinheira section, the values of the Simpson index (Fig. 11) vary between 0.92 and 0.56. In most of the cases, they are high, i.e. 1 − λ > 0.7, being low only in some assemblages (Fig. 9).
The values of the Shannon–Wiener index (Fig. 11) obtained in the Talveila section are very similar, ranging between 1.2 and 3. Throughout the studied interval, they are constant and without significant variations. The values obtained in the Murtinheira section vary between 1.10 and 2.74. From the Comptum Subzone to the Limitatum Subzone they are relatively stable, ranging between 2 and 3. The assemblages of the Aalenian–Bajocian transition show values with a decreasing tendency, which tends to increase in the Bajocian assemblages.
The equitability values (Fig. 11) in Talveila are variable between 0.5 and 0.9, showing an increase in the assemblages of the Aalenian–Bajocian transition. In contrast, the equitability values vary between 0.71 and 1 in the Murtinheira section. Throughout the studied stratigraphic interval, these values remain quite constant, without significant variations.
5 Discussion
In the assemblages from the lower Aalenian–lower Bajocian condensed-shallow marine platform sediments of the Talveila section, the distribution of specimens is irregular throughout the studied stratigraphic interval but, in most cases, the number of 300 specimens in each assemblage was exceeded. In contrast, in the 24 foraminiferal assemblages from the lower Aalenian–lower Bajocian shelfal basin sediments of the Murtinheira section, a total of 5626 specimens were recovered. Their distribution throughout the section is irregular and the number of 300 specimens per assemblage was also exceeded in almost all the studied assemblages (Canales and Henriques 2008). In other coeval sections of epicontinental platforms located in Iberia, like the Fuentelsaz section (Aalenian GSSP, Iberian Range, Spain), Herrero and Canales (1997) obtained 10,234 specimens in 23 samples from the Upper Toarcian–Lower Aalenian, and Canales and Herrero (2000) recovered 9674 specimens in 25 samples from the Upper Toarcian–Middle Aalenian deposits from the Moyuela section (Iberian Range, Spain). In seven Upper Toarcian–Aalenian–Lower Bajocian sections of the Basque-Cantabrian Basin, Canales (2001) described more than 6000 specimens in each section. In the Betic Range (Barranco de Agua Larga section) Silva et al. (2015a, 2016, 2017) obtained 3139 foraminifers in 17 samples from the upper Aalenian–lower Bajocian interval. Comparable data are avaliable from different sections located in the Lusitanian Basin. Thus, in the Serra da Boa Viagem II section, Silva et al. (2014, 2015b) obtained a total of 2356 specimens in 16 samples for the upper Aalenian–lower Bajocian transition; Henriques and Canales (2013) in the São Gião section obtained 13,116 specimens in 25 samples for the upper Toarcian-lower Aalenian interval; in the Maria Pares section Figueiredo and Guterres (2012) described 15,273 foraminifers in 19 samples for the Toarcian–Aalenian transition, and finally in the Zambujal de Alcaria section Figueiredo et al. (2010, 2014) obtained 5291 specimens in 20 samples collected throughout the upper Toarcian-middle Aalenian. Comparing all the studied sections, located in differents basins around the Iberian Plate, it seems that the sections located in middle platform environments show the highest abundance of benthic foraminifera, whilst the sections that seem to have been located proximal or distal positions within the platform, such as Talveila (Iberian Range) and Murtinheira (Lusitanian Basin), display lower abundances.
According to Gordon (1970), Jurassic foraminiferal biogeography consists of 3 types of shelfal assemblages (Type-A) for the Boreal Realm assemblages, dominated by Nodosariidae, and 2 types of Tethyan assemblages (Type-B) for the Tethyan assemblages, dominated by agglutinated forms with complex internal structures or planktic forms. Exton and Grandstein (1984) confirmed that the Type-B1 of Gordon is restricted to the Tethys and the Type-A had a wide distribution during the Lower Jurassic. Later, Nikitenko (2008) added two subdivisions for the Boreal Realm: Boreal Artic, and Boreal Atlantic Realms. Finally, Copestake and Johnson (1984) argued that the Boreal Atlantic foraminiferal assemblages are present in the north and south of the Tethys. The foraminiferal assemblages studied in the Talveila and the Murtinheria sections are characteristic of the Jurassic carbonate platforms of the Boreal Atlantic Realm. Their composition is variable throughout the studied interval but, in both sections, the LOs of typical Lower Jurassic species and the FOs of typical Middle Jurassic species were recognized. The preservation of the specimens in both sections is relatively good, being slightly better in Murtinheira. As it was noticed, the taphonomic processes seem to have no influence in the studied assemblages, therefore any variation in the assemblages’ composition, abundance and diversity must be related to paleoenvironmental factors.
Regarding the composition of the assemblages, the Suborder Lagenina, whose percentages always exceed 38% and ranges up to 97% in the Talveila section and varies between 56 and 92% in the Murtinheira section, dominates all of them. The most abundant genus in almost all the assemblages from Talveila and in all the assemblages from Murtinheira is Lenticulina. According to Haynes (1981), the genus Lenticulina is, in general, the dominant taxon in the assemblages during the Jurassic and the most characteristic genus in the Middle and Upper Jurassic. Murray (1989) indicated that foraminiferal assemblages of the Middle Jurassic shallow carbonate sediments are dominated by Lenticulina, Citharina and Nodosaria. Along the stratigraphic interval, at Talveila and Murtinheira sections, Nodosaria and Citharina are not very important constituents of the assemblages; when present they can be considered as minor components. However, in both sections, the most abundant genera are Lenticulina, Astacolus and Spirillina.
At the specific rank, Lenticulina helios shows the highest relative abundance values in most of the assemblages from Talveila, a fact that is also verified in other areas, such as Hontoria del Pinar (Canales et al. 2013), Moyuela (Canales and Herrero 2000), Muro de Aguas and Fuentelsaz (Canales 2001) in the Iberian Range, some sections in the Basque-Cantabrian Basin (Canales 2001) and several sections of the Lusitanian Basin, like São Gião (Henriques and Canales 2013) and Serra da Boa Viagem II sections (Silva et al. 2014, 2015b). In contrast, Lenticulina muensteri shows the highest relative abundance values in most of the assemblages obtained in the Murtinheira section. This prevalence over other species has been verified in other European areas for the same stratigraphic interval, such as the Cotswolds, in Great Britain (Morris 1982), the Lusitanian Basin, in Portugal (Stam 1985; Carapito and Henriques 1999), the Iberian Range (Canales and Herrero 2000) and the Basque-Cantabrian Basin (Canales 2001), in Spain. According to Canales et al. (2014) the species Lenticulina muensteri is typical of distal platform environments. This may explain the relatively high abundance of this species in Murtinheira when compared to the relative low abundance values obtained in the Talveila section throughout the studied stratigraphic interval. During the Middle Jurassic, the Talveila section corresponded to a proximal and shallow position in the context of the Iberian Range (Ureta and Goy 1986; García-Frank 2006), while Murtinheira was located in a distal ramp in the context of the Lusitanian Basin (Duarte 1997; Azerêdo et al. 2003; Canales and Henriques 2008).
According to Johnson (1976) the Suborder Lagenina attains its maximum diversity in platforms during the Jurassic. Lenticulina could live in a variety of substrates, because it is one of the less specialized genera (Haynes 1981; Olóriz et al. 2003a; Reolid 2008; Reolid et al. 2008, 2013). This genus displays a large variability of lifestyles as deep infaunal, epifaunal and partially shallow infaunal (Tyszka 1994). As this genus can resist to major environmental changes, that could explain the fact that their representatives are a constant and predominant component in the studied assemblages throughout a relatively expanded time span.
Other important components in the assemblages of both sections are the representatives of the suborders Spirillinina (more abundant in the Murtinheira section) and Textulariina (more abundant in the Talveila section). According to Murray (1989) the presence of Spirillinina would indicate normal marine conditions, relatively shallow and well-oxygenated. This author also indicates, about representatives of the Suborder Textulariina, that the assemblages dominated by small agglutinated genera would indicate brackish water and probably a certain oxygen deficit, while assemblages where large agglutinated forms prevail would be characteristic of deep areas. All the specimens of Textulariina obtained from both Talveila and Murtinheira sections correspond to small agglutinated genera. García-Frank et al. (2012) indicated that in the Talveila section an alternation of periods of good oxygenated conditions with other with restriction or changes in the redox boundary within the substrate in the upper part of the Aalenian can be inferred. Tyszka (2001) indicated that Spirillina usually dominates assemblages from inner to middle neritic zones, but this genus can occur in a wide variety of paleoenvironments at nearly all depths. However, Canales (2001) and Canales et al. (2014) considered that the representatives of this genus (namely Spirillina numismalis) are typical of distal platforms environments. This is verified in both sections that record low percentages of Spirillina at Talveila and high percentages in Murtinheira when compared to other genus. Reolid and Martínez-Ruiz (2012) indicate that Spirillina can be affected by the oxygen disponibility, independently of the food availability. Bouhamdi et al. (2001) indicate that the prevalence of the Spirillinina representatives over those of Textulariina, as well as the presence of agglutinated shells with elongated morphologies, would be indicative of assemblages developed in a platform environment. They also indicate that the presence of Spirillinina would be related whith trophic inputs, while trochospiral or planispiral siliceous test indicate poor trophic sources. When compared to Lagenina in the Talveila and Murtinheira assemblages, the percentages of Spirillinina and Textulariina are variable, but never dominant.
The representatives of the suborders Miliolina, Robertinina and Rotaliina can be considered minor components of the studied assemblages in both sections. According to Murray (1989), the presence of Ophthalmidium indicates normal, relatively shallow and well-oxygenated marine conditions. Reolid and Martínez-Ruiz (2012) indicate that this genus is tolerant to low oxygen levels but is less tolerant to variations in paleoproductivity. Murray (1989) indicates that, in general, the representatives of Robertinina are characteristic of normal marine conditions, except for those assemblages clearly dominated by the representatives of the genus Reinholdella, an opportunistic genus (Reolid and Martínez-Ruiz, 2012).
In most of the studied assemblages from the Talveila section, a large number of adherent foraminifers (foraminifers fixed to hard substrates or other shells of vagile mode of life) were recovered, whose representatives in some cases were not possible to determine at the specific level. In addition to these indeterminable specimens, the species Ammovertella liassica, Tolypammina flagella, Vinelloidea rosacea, Bullopora globulata and Bullopora rostrata were recognized. The presence of these forms is remarkable, because the high percentage of foraminifers with this mode of life has not been recognized in any of the other coeval Iberian Plate sections. In the case of the Talveila section, the percentage of adherent foraminifers reaches 6.5% over the total number of specimens. This fact can be related, following Olóriz et al. (2003b), Reolid et al. (2005) and Reolid and Gaillard (2007) with a decreasing sedimentation rate or with the presence of appropriate substrates for attaching or encrusting, such as macroinvertebrate remains. In the Talveila section, most of the adherent foraminifera are attached to fragments of bivalve shells. Barnard (1950) described the occurrence of Tolypammina and Ammovertella representatives associated to Ostraea fragments spp. and Gryphaea spp., thus indicating the preference for shallow marine environments. García-Frank (2006) and García-Frank et al. (2012) reported the occurrence of adherent foraminifers in the microfacies study of thin sections from the same stratigraphical interval in the NW Iberian Range; the author refers to them as “encrusters” together with serpulids and bryozoans, and indicates that across the Aalenian–Bajocian transition an increase in condensation takes place, allowing the occurrence of iron-coat particles, which included adherent foraminifera.
From a biostratigraphical point of view, the species of benthic Jurassic foraminifera show wide distribution ranges, when compared with other groups such as ammonites, which base the zonal scales for marine sediments of this system. Hence, the zonal scale based on this group of microfossils show relative low stratigraphic resolution when compared with zonal scales based in other groups (Canales and Henriques 2008). For the studied interval (lower Aalenian–lower Bajocian) biostratigraphical studies based on foraminifera are scarce and, according to Morris and Coleman (1989) and Canales and Henriques (2008), more detailed studies should be carried out to develop the knowledge of this issue.
For the studied stratigraphic interval, some zonal scales have already been proposed (Fig. 8). The Lenticulina d’orbignyi Biozone, characteristic of the Late Toarcian and lower Aalenian (Wernli and Septfontaine 1971; Gradstein 1977, 1978; Exton and Gradstein 1984; Copestake and Johnson 1984; Boutakiout 1990; Tyszka 1999) was first proposed by Bartenstein and Brand (1937). In the Iberian Plate, Canales (2001) proposed the Dorbignyi Biozone for the Late Toarcian–Middle Aalenian interval. Astacolus dorbignyi shows a continuous record in both Murtinheira and Talveila sections, ranging from the Comptum Subzone to the upper part of the Concavum Subzone. Barbieri (1964) and Boutakiout (1990) characterized the Aalenian–Bajocian boundary based on the FO of Lenticulina quenstedti, and Tyszka (1999) defined the Lenticulina quenstedti Biozone in the Bajocian (from the upper part of the Sauzei Biozone). Canales (2001) established in the Basque-Cantabrian Basin the Quenstedti Biozone for the interval between the lower part of the Bradfordensis Subzone and the Laeviuscula Biozone (Lower Bajocian). In the Talveila section, the FO of this species was recorded at the Bradfordensis Biozone. At Murtinheira, the FO of this species was recorded at the top of the Gigantea Subzone (also Bradfordensis Biozone). Canales and Henriques (2013) characterized the lower Bajocian by the FO of Ramulina spandeli at Murtinheira, a species which was also recognized at the Serra da Boa Viagem II section (Silva et al. 2014, 2015b) in the Lusitanian Basin and in the Hontoria del Pinar section (Canales et al. 2013) in the Iberian Range, but not recognized in the Talveila section, until now.
To complement these biozones based in foraminifers, some bioevents that may have local or regional range were identified (Fig. 9). In both Murtinheira and Talveila sections four different regional bioevents throughout the lower Aalenian–lower Bajocian stratigraphic interval were recognized, some of them also recorded in other sections located around the Iberian Plate and other European basins. Firstly, the progressive disappearance of typical Lower Jurassic species and the FOs of characteristic Middle Jurassic species are recorded. This progressive renewal in the taxonomical composition of the foraminiferal assemblages was also observed in other Lower–Middle Jurassic sections such as Muro de Aguas, Fuentelsaz and several sections in the Basque-Cantabrian Basin (Canales 2001); Moyuela (Canales and Herrero 2000); Maria Pares (Figueiredo and Guterres 2012); Zambujal de Alcaria (Figueiredo et al. 2010, 2014) and Hontoria del Pinar (Canales et al. 2013). Morris (1982) and Morris and Coleman (1989) also recognized this renewal in Great Britain. In both studied sections, as well as in the Basque-Cantabrian Basin (Canales 2001), the FO of the species Lenticulina quenstedti is recorded in the middle Aalenian (Bradfordensis Biozone). The assemblages recorded in the upper Aalenian show an increase in the relative abundance of the representatives of the Suborder Spirillinina. Finally, at the Aalenian–Bajocian boundary interval a decrease in the foraminiferal abundance and diversity is also identified in several sections located in the Basque-Cantabrian Basin (Canales 2001), in Hontoria del Pinar section (Iberian Range) (Canales et al. 2013), in the Serra da Boa Viagem II section (Lusitanian Basin) (Silva et al. 2014, 2015b) and in the Agua Larga section, located in the Betic Range (Silva et al. 2015a, 2017).
The application of several diversity indexes allow some paleoecological considerations. According to Murray (1991), foraminiferal assemblages display low, irregular and variable Fisher’s α index values when they developed in marginal marine biotopes, where paleoecological factors as water temperature, oxygen content, salinity, etc., may experience variations. In normal marine environments, paleoecological factors can remain more stable and, consequently, higher and stable α values are recorded. In the Talveila section, from the Comptum Subzone to the middle part of the Murchisonae Biozone, heterogeneous Fisher’s α values were obtained. In both Bradfordensis and Concavum biozones, the values are relatively high and homogeneous. Finally, at the Aalenian–Bajocian boundary, the recorded α values are again irregular. Taking into account Murray’s (1991) interpretations, the obtained values indicate that different environmental conditions took place along the studied stratigraphic interval. From the Comptum Subzone to the Murchisonae Biozone, the conditions would have been unstable but have allowed the development of relatively abundant and diverse foraminiferal assemblages. In the Bradfordensis and Concavum biozones, stable paleoenvironmental conditions can be inferred, allowing the good development of the foraminiferal assemblages. Finally, in both Discites and Laeviuscula biozones, variable values indicate again relatively unstable paleoenvironmental conditions, but allowing the development of less abundant and diverse foraminiferal assemblages. For the Iberian Range, García-Frank et al. (2008) defined tectonic pulses which lead to important changes in the sedimentary architecture and, therefore in the environments, both in Opalinum-Murchisonae and Concavum-Discites biozones. In the Murtinheira section, from the Comptum Subzone to the lower part of the Limitatum Subzone, the Fisher’s α values obtained indicate stable paleoenvironmental conditions, appropriate for the good development of the foraminiferal assemblages, with the exception of M-269 (α = 0) which is associated with a sedimentary discontinuity (Henriques 1992; Canales and Henriques 2008). From the lower part of the Limitatum Subzone to the Aalenian–Bajocian transition, the values tend to slightly decrease, but continues homogeneous, so the paleoenvironmental conditions would have stayed stable but with a slight tendency to difficult the normal development of the foraminiferal assemblages, since they are composed of relatively small number of specimens and taxa. At Murtinheira, this diversity decrease is not noticed in the nektonic and planktic organisms, such as ammonoids and calcareous nannoplankton (Henriques et al. 1994). However, paleoenvironmental changes seem to have affected benthic organisms such as foraminifers and brachiopods (Andrade 2004). In the lower Bajocian, the α values tend to increase and indicate stable paleoenvironmental conditions, allowing the development of abundant and diverse foraminiferal assemblages.
Considering the Margalef’s richness index results, the values show the same trends in both sections. In the Comptum Subzone and the Murchisonae Biozone the values are irregular, as result of unstable paleoenvironmental conditions, allowing the development of more or less abundant and diverse foraminiferal assemblages. In the Bradfordensis and Concavum biozones, the homogeneous values indicate stable paleoenvironmental conditions allowing the development of the foraminiferal assemblages. Finally, in the Aalenian–Bajocian transition the values become irregular again, which are indicative of unstable paleoenvironmental conditions but with relatively good development of the recorded foraminiferal assemblages.
Regarding the Simpson’s index, the values obtained are relatively high in both sections, which indicate that there is no clear dominance of any species in any assemblage, the most abundant species being Lenticulina helios in Talveila and Lenticulina muensteri in Murtinheira. The Shannon–Wiener index values in the Talveila and Murtinheira sections are relatively high (always above 1) and they allow the recognition of the same intervals established using the above mentioned indexes. In the Comptum Subzone and Murchisonae Biozone, the values obtained are irregular mainly in the Talveila section where, probably, local changes took place. As it can be observed in the stratigraphical column, a noticeable change in facies takes place from the Comptum Subzone, where marly levels are abundant, to the Murchisonae Biozone, where limestones are predominant. However, in the Murtinheira section paleoenvironmental conditions seem to have been more stable. In the Bradfordensis and Concavum biozones values are relatively homogeneous, but it is necessary to take into account the discontinuity located in the Bradfordernsis-Concavum biozones boundary in the Murtinheira section. In the Aalenian–Bajocian boundary, the values become variable again, showing a decrease related to a regional empoverishment of the foraminiferal assemblages, affecting both basins. Beerbower and Jordan (1969) consider that diversity is low when H′< 0.6, moderate when 0.6 < H′> 1 and high when H′> 1. Therefore, the values of H’ obtained in the Talveila and Murtinheira assemblages indicate high diversity. Murray (1991) considers that the values of diversity are related to different paleoenvironmental conditions, being 0 < H′> 1.8 brackish wetlands; 0 < H′> 2.1 brackish lagoons; 0.7 < H′> 2.7 marine platform with normal salinity; 0.8 < H′> 4 bathyal zones and 2.6 < H′> 3.1: abyssal zones. Thus, the values of H′ obtained in the Talveila and Murtinheira assemblages, together with the sedimentological features observed in both sections, indicate an environment of marine platform with normal salinity.
Pielou’s equitability values are high and relatively constant in both sections. Observed trends in this index are comparable with the previous ones. Again, it can be observed a sharp decrease in this index around the boundary between the Comptum Subzone-Murchisonae Biozone in the Talveila section, probably related to a sedimentological change from marly to predominantly calcareous. Haynes (1981) considers that low values indicate conditions of high environmental stress. The dominance of any species and therefore stressful environmental conditions, have not been observed in the studied sections.
The values of all the indexes throughout the studied stratigraphic interval enable the differentiation of three intervals in each section, that represent different paleoenvironmental conditions in terms of stability or instability which have controlled the abundance and diversity of the foraminiferal assemblages. At Talveila, the first recognized interval extends from the Comptum Subzone to the Murchisonae Biozone with unstable paleoenvironmental conditions; even so, the paleoenvironmental conditions allowed the development of foraminiferal assemblages. The second interval includes the Bradfordensis and Concavum Biozones, where values are homogeneous and relatively high, indicating that the paleoenvironmental conditions must have been stable and favorable for the development of the foraminiferal assemblages. The last interval corresponds to the Bajocian assemblages, when the paleoenvironmental conditions were also unstable, but not as much as during the first differentiated interval.These differentiated intervals again coincide with the tectonic pulses recognized by García-Frank et al. (2008), which lead to important changes in the sedimentary architecture and in the environments in the Iberian Range across the Opalinum-Murchisonae and Concavum-Discites intervals. The values of the diversity indexes indicate that these conditions have also enabled the development of the foraminiferal assemblages. In the Murtinheira section, the first recognized interval extends in the Comptum Subzone and displays relatively unstable and homogeneous values, indicating that no major paleoenvironmental changes occurred. It is important to emphasize that a major stratigraphic discontinuity, representing a hiatus affecting the Murchisonae Biozone, was previously recognized in the Lusitanian Basin (Henriques 1992). However, the foraminiferal assemblages and the indexes do not reflect major changes across this discontinuity (Canales and Henriques 2008). The second interval comprises the Bradfordensis Biozone and the Concavum Biozone where the values obtained are homogeneous and relatively high, indicating that the paleoenvironmental conditions must have been stable and favourable for the development of the foraminiferal assemblages. The last interval corresponds to the Bajocian assemblages, where the indexes show a clear upward tendency but also show irregular values; therefore in this interval the paleoenvironmental conditions must have also been unstable.
6 Conclusions
The foraminiferal assemblages of the lower Aalenian–lower Bajocian of the Talveila located in a proximal setting in the Iberian Range (NE Spain) and Murtinheira, located in a distal position of the platform of the Lusitanian Basin (Western Portugal) are composed of a relatively high number of taxa. A lower relative abundance of foraminifera characterizes the assemblages from the Talveila section and from the Murtinheira section, located in proximal and distal positions in their respectives basins, when compared with other Iberian sections located in the middle part of the platform. From a taphonomic point of view, no evidence of any alteration of the original composition of the assemblages can be inferred. The composition of the foraminiferal assemblages in both sections are typical of the Jurassic carbonate platforms of the Boreal Atlantic Realm, so they are comparable with coeval assemblages recorded in other European basins.
From a biostratigraphical point of view, some of the identified taxa show wide distribution ranges, which together with their high abundance assign a homogeneous character to the assemblages. Moreover, the main foraminiferal-based zones, established by different authors for the studied stratigraphic interval, are recognizable at Talveila and at Murtinheira. The presence of Astacolus dorbignyi throughout the lower Aalenian, and Lenticulina quenstedti from the Bradfordensis Biozone (middle Aalenian) upwards, allowed the recognition of the Astacolus dorbignyi and Lenticulina quenstedti biozones in both sections. Also the presence of Ramulina spandeli at Murtinheira in the lower Bajocian enabled the recognition of the Ramulina spandeli Biozone. Some bioevents that complement these biostratigraphic units were also recognized, including the gradual replacement of typical Lower Jurassic taxa by characteristic species of the Middle Jurassic, as well as changes in the relative abundance and diversity in the assemblages.
The paleoecological analyses show that the studied foraminiferal assemblages of the two sections were developed in a well-oxygenated and normal salinity shelf basin. The most abundant suborder is Lagenina and the most abundant genera under these conditions are Lenticulina, Astacolus and Spirillina. However, the increase of simple agglutinated forms in the upper part of the studied stratigraphical interval in the Talveila section can be related with changes in the oxygen content. The application of diversity indexes indicates that the paleoenvironmental conditions did not remain constant throughout the studied stratigraphic interval, with observed changes similar and coeval in both sections. The first interval (Comptum Subzone and Murchisonae Biozone) reflects unstable conditions, more noticeable in the Talveila section than in the Murtinheira section, but they were in general suitable for the development of foraminiferal assemblages. On the contrary, in the second interval (Bradfordensis and Concavum biozones) the paleoenvironmental conditions were stable and favourable for the normal development of the foraminiferal assemblages. In the third and last interval (Discites and Laeviuscula biozones) the paleoenvironmental conditions became unstable again, being more noticeable in Murtinheira than in Talveila.
In conclusion, the taxonomic composition of the foraminiferal assemblages of the Talveila and Murtinheira sections was controlled by the paleoegeographical position occupied by each studied section within the respective basin. However, regarding abundance and diversity, the analysed assemblages from both sections during the Early Aalenian–Early Bajocian display the same trends. Therefore, the development of the foraminiferal assemblages seems to have been conditioned by environmental changes of regional scale, affecting both the Iberian and the Lusitanian basins.
References
Andrade, J. B. (2004). Los braquiópodos del tránsito Jurásico Inferior-Jurásico Medio de la Cuenca Lusitánica (Portugal). Ph.D. thesis. Madrid: Universidad Complutense de Madrid.
Andrade, J. B. (2006). Los braquiópodos del tránsito Jurásico Inferior-Jurásico Medio de la Cuenca Lusitánica (Portugal). Coloquios de Paleontología, 56, 5–194.
Azerêdo, A. C., Duarte, L. V., Henriques, M. H., & Manuppella, G. (2003). Da dinâmica continental no Triásico aos mares do Jurássico Inferior e Médio. Cadernos de Geología de Portugal Instituto Geológico e Mineiro, 1, 6–43.
Barbieri, F. (1964). Micropaleontologia del Lias e Dogger del pozzo Ragusa 1 (Sicilia). Rivista Italiana di Paleontologia e Stratigrafia, 70, 709–830.
Barnard, T. (1950). The uses of foraminifera in Lower Jurassic stratigraphy. In Reports of the 18th session of the international geological congress (Vol. 1, pp. 34–41.
Bartenstein, H., & Brand, E. (1937). Mikro-paläontologische Untersuchungen zur Stratigraphie des nordwest–deutschen Lias und Doggers. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 439, 1–224.
Beerbower, J. R., & Jordan, D. (1969). Application of information theory to paleontologic problems: taxonomic diversity. Journal of Paleontology, 43, 1184–1198.
Bouhamdi, A., Gaillard, C., & Ruget, C. (2001). Spirillines versus agglutinants: impact du flux organique et intérêt paléoenvironnemental (Oxfordien moyen du Sud-Est de la France). Geobios, 34, 267–277.
Boutakiout, M. (1990). Les foraminifères du Jurassique des Rides sudrifaines et des régions voisines (Maroc). Documents des Laboratoires de Géologie de Lyon, 112, 1–247.
Buzas, M. A. (1979). The measurement of species diversity. Society of Economic Paleontologists and Mineralogists: Short Course, 6, 3–10.
Canales, M. L. (2001). Los foraminíferos del Aaleniense (Jurásico Medio) en la Cuenca Vasco Cantábrica (N de España). Revista Española de Micropaleontología, 33, 253–438.
Canales, M. L., & Herrero, C. (2000). Asociaciones de foraminíferos del Toarciense superior y Aaleniense en la sección de Moyuela (Zaragoza, España). Revista Española de Micropaleontología, 32, 301–317.
Canales, M. L., & Henriques, M. H. (2007). Análisis Cuantitativo de los Foraminíferos del Aaleniense (Jurásico Médio) de la Sección de Murtinheira (Cabo Mondego, W de Portugal): Consideraciones Paleoecológicas. In Carvalho, I. S., Cassab. R. C. T., Schwanke, C., Carvalho, M. A., Fernandes, A. C. S., Rodrigues, M. A. C., Carvalho, M. S. S., Arai, M., & Oliveira, M. E. Q. (Eds.), Paleontologia: Cenários de Vida 2 (pp. 382–393). Editoral Interciência.
Canales, M. L., & Henriques, M. H. (2008). Foraminifera from the Aalenian and the Bajocian GSSP (Middle Jurassic) of Murtinheira section (Cabo Mondego, west Portugal): Biostratigraphy and paleoenvironmental implications. Marine Micropaleontology, 67, 155–179.
Canales, M. L., & Henriques, M. H. (2013). Foraminiferal assemblages from the Bajocian Global Stratotype Section and Point (GSSP) at Cape Mondego (Portugal). Journal of Foraminiferal Research, 43(2), 182–206.
Canales, M. L., Henriques, M. H. (2015). Paleoenvironmental significance of the foraminiferal assemblages from the Bajocian GSSP (Cape Mondego, Portugal). In Abstracts of the AAPG European and Regional conference and exhibition 2015: Tethys–Atlantic interaction along the European Iberian–African plate boundaries, Lisbon (pp. 82–83).
Canales, M. L., Henriques, M. H., and Ureta, S. (2000). Análisis de las asociaciones de foraminíferos del Aaleniense en los márgenes oriental y noroccidental de la Placa Ibérica: implicaciones biogeográficas y bioestratigráficas. In Actas do I Congreso Ibérico de Paleontología/XVI Jornadas de la Sociedad Española de Paleontología, Évora, Portugal (pp. 8–9).
Canales, M. L., Ureta, M. S., Hernández, L., & García-Frank, A. (2013). Bioestratigrafía y bioeventos (ammonoideos y foraminíferos) del tránsito Aaleniense/Bajociense (Jurásico Medio) en Hontoria del Pinar (Noroeste de la Cordillera Ibérica). In Álvarez-Vázquez, C., & López Rodríguez, I. (Eds.), Libro de Resúmenes de las XXIX Jornadas de la Sociedad Española de Paleontología y Simposio del Proyecto PICG 596, Córdoba (pp. 139–140).
Canales, M. L., García-Baquero, G., Henriques, M. H., & Figueiredo, V. L. (2014). Palaeoecological distribution pattern of Early-Middle Jurassic benthic foraminifera in the Lusitanian Basin (Portugal) based on multivariate analysis. Palaeogeography, Palaeoclimatology, Palaeoecology, 410, 14–26.
Carapito, M. C., Henriques, M. H. (1999). Foraminiferal biostratigraphy and paleoecology of the Aalenian–Bajocian boundary at the Cabo Mondego area. In Proceedings of the European palaeontological association workshop, Lisbon (pp. 35–38).
Copestake, P., & Johnson, B. (1984). Lower Jurassic (Hettangian– Toarcian) Foraminifera from the Mochras Borehole, North Wales (UK) and their application to a worldwide biozonation. In Oertli, H. J. (Ed.), Second international symposium on benthic foraminifera elf aquitaine, esso REP and total CFP (pp. 183–184).
de Kaenel, E., & Bergen, J. A. (1993). New Early and Middle Jurassic coccolith taxa and biostratigraphy from the eastern Proto-Atlantic (Morocco, Portugal and DSDP site 547B). Eclogae Geologicae Helveticae, 86(3), 861–907.
de Kaenel, E., Bergen, J. A., & von Salis Perch-Nielsen, K. (1996). Jurassic calcareous nannofossil biostratigraphy of western Europe. Compilation of recent studies and calibration of bioevents. Bulletin Societé géologique France, 167(1), 15–28.
Duarte, L. V. (1997). Facies analysis and sequential evolution of the Toarcian–Lower Aalenian series in the Lusitanian Basin (Portugal). Comunicações do Instituto Geológico e Mineiro, 83, 65–94.
Ellis, B. F., & Messina, A. (1940–1990). Catalogue of Foraminifera. New York: Museum of natural history.
Exton, J., & Gradstein, F. M. (1984). Early Jurassic stratigraphy and micropaleontology of the Grand Banks and Portugal. In Westermann, G.E.G. (Ed.), Jurassic–Cretaceous biochronology and paleogeography of North America: Geological Association of Canada, Special Paper, 27 (pp. 13–30).
Fernández López, S., Henriques, M. H. P., Mouterde, R., Rocha, R., & Sadki, D. (1988). Le Bajocien inférieur du Cap Mondego (Portugal). Essai de biozonation. In Rocha. R. B., and Soares, A. F. (Eds.), 2nd international symposium on Jurassic stratigraphy, Lisbon (Vol. 1, pp. 301–313).
Figueiredo, V. L. (2009). Foraminíferos da passagem Jurássico Inferior–Médio do Sector Central da Bacia Lusitânica: o perfil de Zambujal de Alcaria. Unpublished M.Sc. thesis (pp. 88). Coimbra: Faculdade de Ciências e Tecologia, Universidade de Coimbra.
Figueiredo, V. L., & Guterres, H. C. (2012). Análise Quantitativa das Associações de Foraminíferos da Passagem Jurássico Inferior—Médio do Perfil de Maria Pares (Setor Norte da Bacia Lusitânica, Portugal)—Implicações Paleontológicas. In Lopes, F. C., Andrade, A. I., Henriques, M. H., Quinta-Ferreira, M., Barata, M. T., & Pena dos Reis, R. (Eds.), Para Conhecer a Terra—Memórias e Notícias de Geociêcias no Espaço Lusófono: Imprensa da Universidade de Coimbra (pp. 151–159).
Figueiredo, V. L., Henriques, M. H., & Canales, M. L. (2010). Foraminíferos da Passagem Jurássico Inferior—Médio do Sector Central da Bacia Lusitânica: o Perfil de Zambujal de Alcaria. Boletim de Geociências da Petrobras, 19(1), 207–234.
Figueiredo, V. L., Canales, M. L., & Henriques, M. H. (2014). Foraminifera of the Toarcian–Aalenian boundary from the Lusitanian Basin (Portugal): a paleoecological analysis. Journal of Iberian Geology, 40(3), 409–450.
García-Frank, A. (2006). Evolución biosedimentaria y secuencial del Jurásico Medio inferior en la Cuenca Ibérica (Sector NO). Ph.D. thesis. Madrid: Universidad Complutense de Madrid.
García-Frank, A., Ureta, S., & Mas, R. (2008). Aalenian pulses of tectonic activity in the Iberian Basin, Spain. Sedimentary Geology, 209, 15–35.
García-Frank, A., Ureta, S., & Mas, R. (2012). Iron-coated particles from condensed Aalenian–Bajocian deposits: Evolutionary model (Iberian Basin, Spain). Journal of Sedimentary Research, 82(12), 953–968.
Giraud, F., Mattioli, E., López-Otálvaro, G. E., Lécuyer, C., Suchéras-Marx, B., Almerás, Y., et al. (2016). Deciphring processes controlling mid-Jurassic coccolith turnover. Marine Microapleontology, 125, 36–50.
Gómez, J. J., Comas-Rengifo, M. J., & Goy, A. (2003). Las unidades litoestratigráficas del Jurásico Inferior de las Cordilleras Ibérica y Costero Catalana. Revista de la Sociedad Geológica de España, 16, 227–237.
Gómez, J. J., & Fernández-López, S. R. (2004). Las unidades litoestratigráficas del Jurásico Medio de la Cordillera Ibérica. Geogaceta, 35, 94–97.
Gordon, W. A. (1970). Biogeography of Jurassic foraminifera. Bulletin of the Geological Society of America, 81, 1689–1704.
Gradstein, F. M. (1977). Biostratigraphy and biogeography of Jurassic Grand Banks foraminifera. In Schafer, G. H. T., & Bernard, R. P. (Eds.), First international symposium on Benthonic Foraminifera of continental margins. Part B. paleoecology and biostratigraphy: maritime sediments special publication (Vol. 1, pp. 567–583).
Gradstein, F. M. (1978). Jurassic Grand Banks foraminifera. Journal of Foraminiferal Research, 8, 97–109.
Hammer, Ø., & Harper, D. A. T. (2006). Paleontological Data Analysis. Oxford: Blackwell Publishing.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontología Electronica, 4, 9.
Haynes, J. R. (1981). Foraminifera (p. 433). London: Macmillan Publishers Limited.
Henriques, M. H., (1992). Biostratigrafia e Paleontología (Ammonoidea) do Aaleniano em Portugal (Sector Setentrional da Bacia Lusitaniana). Ph.D. thesis. Coimbra: Universidade de Coimbra.
Henriques, M. H. (1995). Les faunes d’ammonites de l’Aalenien portugais: composition et implications paleobiogéographiques. Geobios, Memoire Special, 18, 229–235.
Henriques, M. H., & Canales, M. L. (2013). Ammonite-benthic foraminifera turnovers across the Lower-Middle Jurassic transition in the Lusitanian Basin (Portugal). Geobios, 46, 395–408.
Henriques, M. H., Gardin, S., Gomes, C. R., Soares, A. F., Rocha, R. B., Marques, J. F., Lapa, M. R., & Montenegro, J. D. (1994). The Aalenian–Bajocian boundary at Cabo Mondego (Portugal). In: Cresta, S. G., & Pavia, G. (Eds.), Proceedings of the 3rd International Meeting on Aalenian and Bajocian Stratigraphy. Miscellanea del Servizio Geologico Nazionale, (Vol 5, pp. 63–77).
Henriques, M. H., Canales, M. L., & Magno, C. (2008). Paragem 2A—Fácies Distais de Rampa Carbonatada (Sag do 1 Rifte): Jurássico Médio. In Pena dos Reis, R., Pimentel, N., & Bueno, G. (Eds.), Roteiro: III Curso de Campo na Bacia Lusitânica (Portugal), Coimbra (pp. 33–42).
Henriques, M. H., Canales, M. L., Neto, K., & Antunes, R. L. (2010). Significant bioevents across the Bajocian GSSP (Cape Mondego, Portugal). In Pena dos Reis, R., & Pimentel, N. (Eds.), II Central and North Atlantic conjugate margins conference, Lisbon 2010, Field-Trip Guidebook, Lusitanian Basin (Portugal) (pp. 33–42).
Henriques, M. H., Canales, M. L., Silva, S. C., & Figueiredo, V. (2016). Integrated biostratigraphy (Ammonoidea, Foraminiferida) of the Aalenian of the Lusitanian Basin (Portugal: A synthesis). Episodes, 39(3), 482–490.
Hernández, L. (2015). Foraminíferos da passagem Aaleniano-Bajociano no perfil de Talveila (Cordilheira Ibérica, Espanha), Unpublished MSc thesis (p. 115). Coimbra: Faculdade de Ciências e Tecologia, Universidade de Coimbra.
Herrero, C., & Canales, M. L. (1997). Diversidad en los foraminíferos del tránsito Toarciense/Aaleniense en la sección de Fuentelsaz (Cordillera Ibérica). Revista Española de Paleontología, 12, 233–242.
Herrero, C., & Canales, M. L. (2002). Taphonomic processes in selected Lower and Middle Jurassic foraminifera from the Iberian Range and Basque-Cantabrian Basin (Spain). Journal of Foraminiferal Research, 32(1), 22–42.
Johnson, B. (1976). Ecological ranges of selected Toarcian and Domerian (Jurassic) foraminiferal species from Wales. In Schafer, G. H. T., & Bernard, R. P. (Eds.), 1st intrantional symposium on Benthonic Foraminifera of continental margins. Part B: paleoecology and biostratigraphy (Halifax, 1975): marine sediments, special publication, (Vol. 1, pp. 545–556).
Loeblich, A. R., Jr., & Tappan, H. (1987). Foraminiferal genera and their classification (Vol. 2, p. 1182). New York: Van Nostrand Reinhold Company.
López-Otálvaro, G. E., Suchéras-Marx, B., Giraud, F., Mattioli, E., & Lecuyer, C. (2012). ). Discorhabdus as a key coccolith genus for paleoenvironmental reconstructions (Middle Jurassic, Lusitanian Basin): biometry and taxonomic status. Marine Micropaleontology, 94(95), 45–57.
Magno, C., Henriques, M. H., & Canales, M. L. (2008). Foraminíferos do Aaleniano (Jurássico Médio) da Ibéria: bacias Lusitânica (Portugal), Basco-Cantábrica (Espanha) e Cordilheira Ibérica (Espanha). Memórias e Notícias, 3(Nova Série), 115–122.
Magurran, A. E. (1988). Ecological diversity and its measurement. London: Chapman and Hall.
Morris, P. H. (1982). Distribution and palaeoecology of Middle Jurassic Foraminifera from the Lower Inferior Oolithe of the Cotswolds. Palaeogeography, Palaeoclimatology, Palaeoecology, 37, 319–347.
Morris, P. H., & Coleman, B. (1989). The Aalenian to Callovian (Middle Jurassic). In D. G. Jenkins & J. W. Murray (Eds.), Stratigraphical atlas of fossil foraminifera: British Micropaleontological Society Series (2nd ed., pp. 189–236). Chichester: Ellis Horwood Limited.
Murray, J. W. (1989). An outline of faunal changes through the Phanerozoic. In D. G. Jenkins & J. W. Murray (Eds.), Stratigraphical atlas of fossil foraminifera (2nd ed., pp. 570–573). Chichester: Ellis Horwood Limited.
Murray, J. W. (1991). Ecology and distribution of benthic foraminifera. In J. J. Lee & O. R. Andreson (Eds.), Biology of foraminifera (pp. 221–253). UK: Academic Press Limited.
Neto, K. (2010). Nanofósseis calcários da passagem Aalenian–Bajociano do perfil do Cabo Mondego—Portugal (GSSP do Bajociano). Unpublished MSc thesis. Coimbra: Faculdade de Ciências e Tecologia, Universidade de Coimbra.
Nikitenko, B. L. (2008). The Early Jurassic to Aalenian paleobiogeography of the Arctic Realm: Implications of microbenthos (foraminifers and ostracods). Stratigraphy and Geological Correlation, 16, 59–80.
Olóriz, F., Reolid, M., & Rodríguez-Tovar, F. J. (2003a). Palaeogeographic and stratigraphic distribution of mid-late Oxfordian foraminiferal assemblages in the Prebetic Zone (Betic Cordillera, Southern Spain). Geobios, 36(6), 733–747.
Olóriz, F., Reolid, M., & Rodríguez-Tovar, F. J. (2003b). A Late Jurassic carbonate ramp colonized by sponges and benthic microbial communities (external prebetic, Southern Spain). Palaios, 18(6), 528–545.
Pavia, G., & Enay, R. (1997). Definition of the Aalenian–Bajocian stage boundary. Episodes, 20, 16–22.
Perilli, N., Henriques, M. H., & Giannetti, M. (2002a). Aalenian calcareous nannofossil changes and Lotharingius/Watznaueria turnover: evidence from the Lusitanian Basin (Portugal). Journal of Nannoplankton Research, 24(2), 145.
Perilli, N., Henriques, M. H., & Ureta, M. S. (2002b). Aalenian calcareous nannofossils biohorizons of some sections from Lusitanian Basin and Basque-Cantabrian Area. Publicaciones del Seminario de Paleontología de Zaragoza, 5, 162–166.
Reolid, M. (2008). Taphonomic features of Lenticulina as a tool for paleoenvironmental interpretation of midshelf deposits of the Upper Jurassic (Prebetic Zone, Southern Spain). Palaios, 23, 482–494.
Reolid, M., & Gaillard, C. (2007). Microtaphonomy of bioclasts and paleoecology of microencrusters from upper Jurassic spongiolithic limestones (External Prebetic, southern Spain). Facies, 53(1), 97–112.
Reolid, M., & Martínez-Ruiz, F. (2012). Comparison of benthic foraminifera and geochemical proxies in shelf deposits from the Upper Jurassic of the Prebetic (Southern Spain). Journal of Iberian Geology, 38(2), 449–465.
Reolid, M., Gaillard, C., Olóriz, F., & Rodríguez-Tovar, F. J. (2005). Microbial encrustations from the Middle Oxfordian–earliest Kimmeridgian litofacies in the Prebetic Zone (Betic Cordillera, southern Spain): characterization, distribution and controlling factors. Facies, 50, 529–543.
Reolid, M., Rodríguez-Tovar, F. J., Nagy, J., & Olóriz, F. (2008). Benthic foraminiferal morphogroups of mid to outer shelf environments of the Late Jurassic (Prebetic Zone, southern Spain): charactirzation of biofacies and environmental significance. Palaeogeopgraphy, Palaeoclimatology, Palaeoecology, 261, 280–299.
Reolid, M., Chakiri, S., & Bejjaji, Z. (2013). Adaptative strategies of the Toarcian benthic foraminiferal assemblages from the Middle Atlas (Morocco): paleoecological implications. Journal of African Earth Sciences, 84, 1–12.
Sánchez-Moya, Y., & Sopeña, A. (2004). El rift Mesozoico Ibérico. In J. A. Vera (Ed.), Geología de España (1ª ed., p. 484). Madrid: Madrid Sociedad Geológica de España, Instituto Geológico y Minero de España.
Silva, S.C. (2013). Foraminíferos da passagem Aaleniano–Bajociano: O perfil da Serra da Boa Viagem II. M.Sc. thesis. Coimbra: Universidade de Coimbra.
Silva, S., Henriques, M. H., & Canales, M. L. (2014). Paleoecological analysis based on foraminifera of the Aalenian–Bajocian (Middle Jurassic) boundary at the Serra da Boa Viagem II section. Comunicações Geológicas, Especial I, Laboratório Nacional de Energia e Geologia, Lisboa, 101, 573–576.
Silva, S., Canales, M. L, Sandoval, J., & Henriques, M. H. (2015a). Análisis paleoecológico de las asociaciones de foraminíferos bentónicos del tránsito Aaleniense- Bajociense en la sección Barranco de Agual Larga (Dominio Subbético, España). In Reolid, M. (Ed.), Actas de las XXXI Jornadas de Paleontología, Sociedad Española de Paleontología (pp. 296–298).
Silva, S., Henriques, M. H., & Canales, M. L. (2015b). High resolution ammonite-benthic foraminiferal biostratigraphy across the Aalenian–Bajocian boundary in the Lusitanian Basin (Portugal). Geological Journal, 50(4), 477–496.
Silva, S., Canales, M. L., Sandoval, J., & Henriques, M. H. (2016). Benthic foraminiferal biostratigraphy of the Aalenian–Bajocian (Middle Jurassic) boundary in the Barranco de Agua Larga section (Betic Cordillera, Southern Spain). In Abstract EGU 2016-15878.
Silva, S. C., Canales, M. L., Sandoval, J., & Henriques, M. H. (2017). Paleoecological analysis based on benthic foraminifera of the Aalenian–Bajocian boundary in Barranco de Agua Larga section (Betic Cordillera, SE Spain). Journal of Iberian Geology, 43(1), 1–22.
Stam, B. (1985). Quantitative analysis of Middle and Late Jurassic foraminifera from Portugal and its implications for the Grand Banks of Newfoundland. Utrecht Micropaleontological Bulletins, 34, 1–168.
Tyszka, J. (1994). Response of Middle Jurassic benthic foraminiferal morphogroups to dysoxic/anoxic conditions in the Pieniny Klippen Basin, Polish Carpathians. Palaeogeography, Palaeoclimatology, Palaeoecology, 110, 55–81.
Tyszka, J. (1999). Foraminiferal biozonation of the Early and Middle Jurassic in the Pieniny Klippen Belt (Carpathians). Bulletin of the Polish Academy of Sciences, Earth Sciences, 47, 27–46.
Tyszka, J. (2001). Microfossil assemblages as bathymetric indicators of the Toarcian/Aalenian “Fleckenmergel”-facies in the Carpathian Pieniny Klippen Belt. Geologica Carpathica, 52, 147–158.
Ureta, S., & Goy, A. (1986). El Aaleniense en el Área de Talveila (Soria)—Bioestratigrafía y Evolución Sedimentaria. Estudios Geológicos, 42, 331–339.
Wernli, R., & Septfontaine, M. (1971). Micropaléontologie comparée du Dogger du Jura méridional (France) et des Préalpes Médianes Plastiques romandes (Suisse). Eclogae Geologicae Helvetiae, 64, 437–458.
Acknowledgements
The authors are grateful to Dr. Alejandra García-Frank and an anonymous reviewer for the careful revision of the manuscript and hopeful suggestions and comments which have contributed for the improvement of its final version. Also to Dr. Kaminski for the critical review of the manuscript. This work was supported by FCT—Fundação para a Ciência e a Tecnologia, I.P., through Portuguese funds, in the research project UID/Multi/00073/2013 of the GeosciencesCenter of the University of Coimbra (Portugal). The study is a contribution for the Projects CGL2011-23947, CGL2011-25894 and CGL2015-66604-R (Ministerio de Ciencia e Innovación, Spain) and for the Grupo de Investigación UCM 910431 (Complutense University, Madrid, Spain). The laboratory work was supported by the Petrobras-Galp-Partex Consortium of Portugal. The authors are grateful to the Centro Nacional de Microscopía Electrónica (Complutense University of Madrid, Spain) for the SEM photographs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernández, L., Canales, M.L. & Henriques, M.H. Response of benthic foraminiferal assemblages to contrasting environments during the Aalenian–Bajocian in the Iberia: a case study from the Talveila section (Iberian Range) and Murtinheira section (Lusitanian Basin). J Iber Geol 44, 447–478 (2018). https://doi.org/10.1007/s41513-018-0067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41513-018-0067-1