Abstract
This paper involves the use of state-of-the-art optimization techniques to conduct the wastewater treatment process in the most beneficial manner possible. Rigorous single- and multi-objective optimal control tasks are performed on the reduced BSM1 (benchmark simulation model 1) for the wastewater treatment process problem. The multiobjective optimization does not involve weight functions or additional constraints. The state-of-the-art global optimization solver BARON is used. The concentrations of the Nitrite/Nitrate, ammonium and biodegradeable organic contaminants are minimized. While the single and multiobjective optimal control profiles for the Nitrite/Nitrate and the biodegradable substance concentrations were qualitatively similar, there was considerable difference between the two profiles when the ammonium concentration profile was minimized. However, when the time for the multiobjective optimal control was increased, resulting profiles for the ammonium concentrations became similar. This demonstrated that increasing the time enabled the multiobjective optimal control to be as effective as the single objective optimal control while controlling all the variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Purifying wastewater from the contaminants is very essential to safeguard public health and wastewater treatment plants are needed to purify the water from contaminants.
The contaminants commonly occurring in the wastewater that are hazardous to human health include nitrate compounds, nitrite compounds, ammonium compounds, and biodegradable substances. The main aim of wastewater treatment plants is to reduce the concentration of these substances before discharge into the surroundings. While there can be a process to minimize biodegradable residue within the wastewater treatment itself, it is important to develop strategies to minimize this as much as possible. Wastewater treatment plants have to deal with continuous and unpredictable changes in the composition of the incoming untreated impure water. This paper demonstrates the use of a rigorous multiobjective optimal control strategy to ensure that the wastewater treatment plant is operated in a manner such that the amounts of all the problem-causing contaminants in the effluent water are minimized. This multiobjective optimal control strategy does not involve the use of arbitrary weight functions or additional constraints and the state-of-the-art global optimization solver BARON is used.
Background
The nonlinearity of the wastewater treatment process has led to a lot of modeling work, (Martin 2000; Meijer 2001; Singh et al. 2002; Hulsbeek, et al. 2002) and more recently by (Daughton 2018; Chen et al. 2019; Barceló 2020; Bijlsma et al. 2021; Adhikari and Halden 2022) The need to obtain optimal parameters in order to ensure the efficient operation of the process was investigated by Olsson et al. (2005) and Santin et al. (2016). The irregular variation of the influent along with the complex biochemical processes cause the optimization and control of the process to be challenging. Since the influent composition and the amount of contaminants changes irregularly, it is necessary to use a dynamic optimization to be able to ensure an effective continuous operation of the process. Amand et al. (2013) investigated in detail the effect of controlling the aeration in the wastewater treatment process. The use of advanced control strategies on the wastewater treatment plant process was also studied by (O’Brien et al; 2011; Amand et al. 2013; and Santin et al. 2016). The wastewater treatment is an aerobic process and since aeration demands a considerable amount of energy (McCarty et al. 2011; Daverey et al. 2019) the process must be operated in an effective manner. Real-time optimization and model predictive control of waste water treatment plants were studied by Vega et al. (2014); Santin et al. (2016); Piotrowski et al. 2008; Brdys et al. 2008; Tatjewski 2008 and Darby et al. 2011).
Two of the common process configurations for biological wastewater treatment process are the continuous-flow activated sludge process and the sequencing batch reactor process (Henze et al., 2008). Sun et al. compared the nitrogen and ammonia removal from waste water for both these processes and reported almost complete ammonia removal in both processes but observed higher total nitrogen removal in the continuous-flow activated sludge process than in the sequencing batch reactor process A complete ammonia removal in the sequencing batch reactor process was confirmed in a number of experimental studies (Yalmaz and Öztürk 2001; Andreottola et al. 2001). A comparison of the two processes for the treatment of industrial wastewater was performed by Papadimitriou et al. (2009), who found a higher removal of contaminants by the sequencing batch reactor process. Dionisi et al. 2016, and Dionisi 2017 developed a strategy to calculate the periodic steady state of sequencing batch reactors for biological wastewater treatment.
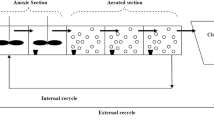
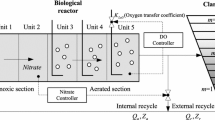
The reduced BSM1 (benchmark simulation model 1) is commonly used for the waste water treatment process (Julien et al. 1999; Gómez-Quintero and Spérandio 2004; Steffens et al. 1997; Zhao et al. 1994; Silvana et al. 2017). Details of this model can be found in all these articles. This model considers the anoxic and aerobic zones in the wastewater treatment plant. The process details of these zones are given in Silvana et al. 2017.
The values of the parameters, constants and the names of the variables for the equations in this model are shown in Tables 1, 2 and 3. The equations that constitute the model are
Optimal Control
In the multiobjective nonlinear optimal control (MOOC) strategy (Flores Tlacuahuaz et al. 2012; Sridhar 2019) used in this work the single objective optimal control problem is first solved for each of the objective functions. For a multiobjective optimal control problem
the single objective optimization problems are solved independently minimizing each \(\phi_{i}\) (i = 1, 2, 3…n) individually. This will lead to minimized values.
\(\phi_{i}^{*}\)(i = 1, 2, 3,..n). Then the problem that will be solved is
The optimization program, Pyomo (Hart et al. 2017), where the differential equations are automatically converted to a Nonlinear Program (NLP) using the orthogonal collocation method (Biegler 2007) is used for performing the dynamic optimization calculations. The Lagrange-Radau quadrature with three collocation points and ten finite elements are chosen. The resulting nonlinear optimization problem was solved using the solver BARON 19.3 (Tawarmalani and Sahinidis 2005), accessed through the Pyomo-GAMS27.2 (Bussieck and Meeraus 2004) interface. BARON implements a Branch-and-reduce strategy and provides a guaranteed global optimal solution. This procedure does not involve the use of weighting functions nor does it impose additional parameters or additional constraints on the problem unlike the weighted function or the epsilon correction method. (Miettinen 1999).
Results and Discussion
Three single objective and one multiobjective optimal control problems were solved. First, the biodegradable substrate was minimized (objective value = 1.4958E-5), subject to Eqs. 1 to 14 The second optimal control problem involves the minimization of ammonium concentration in the effluent (objective value = 13.1765), while the third optimal control problem deals with the minimization of the nitrite and nitrate compounds (objective value = 23.3579). In the multiobjective optimal control problem the function minimized is \(\sqrt {(\sum {S_{S2} - } 1.4958E - 5)^{2} + (\sum {S_{NH2} - } 13.1765)^{2} + (\sum {S_{NO2} - } 23.3579)^{2} }\). The multiobjective optimal control objective function value obtained is 0.5927. Table 4 shows the values of the objective functions.
Figure 1a–f shows the concentration profiles when the concentration of the biodegradable substance was minimized Fig. 2a–f shows the profiles when the concentration of the ammonium compounds was minimized while Fig. 3a–f is obtained when the nitrite/nitrate compositions are minimized. Figure 4a–f shows the profiles when the multiobjective optimal control was performed. Figure 5a shows the Pareto curve that gives the variation of \(S_{S2}\) with \(S_{O2}\).
a Multiobjective Optimal Control(Ss1 vs t), b Multiobjective Optimal Control(Ss2 vs t), c Multiobjective Optimal Control(Snh1 vs t), d-a Multiobjective Optimal Control(Snh2 vs t, t = 5), d-b Multiobjective Optimal Control (Snh2 vs t) with total time increase, e Multiobjective Optimal Control(Sno1 vs t), f Multiobjective Optimal Control(Sno2 vs t), g Multiobjective Optimal Control(So1 vs t), h Multiobjective Optimal Control(So2 vs t)
Two following important issues can be observed from these results:
-
1.
The multiobjective optimal control yields qualitatively similar results in the cases of \(S_{s2}\) (Figs. 1b and 4b) and \(S_{NO2}\) (Figs. 3f and 4f) while in the case of \(S_{NH2}\) (Figs. 2d and 4d–a) the multiobjective optimal control profile seems to be qualitatively different from the single optimal control where only \(S_{NH2}\) was minimized. However, this could be remedied by increasing the total operation time from 5 to 8 h when the multiobjective optimal control was performed (Figs. 2d and 4d–b). This demonstrates that increasing the time will cause the multiobjective optimal control to be as effective as the single objective optimal control while controlling all the variables.
-
2.
The Pareto curve 5a shows a spike. This indicates that the wastewater treatment problem is highly nonlinear and local optimal strategies may not be as effective as global optimization strategies in obtaining the most beneficial solution.
The objective of this work was to minimize Nitrite/Nitrate, ammonium, and biodegradable organic contaminants. It was seen that in the case of the Nitrite/Nitrate and the biodegradable contaminants the multiobjective and single objective profiles were qualitatively similar, but this was not true in the case of the ammonium compounds. However, increasing the time of the operation for the multiobjective optimal control caused the profiles for the ammonium contaminant to be qualitatively similar. This implies that by increasing the time of the operation the multiobjective optimal control could be made as effective as the single objective optimal control operation with the added advantage of being able to control all the variables. This is very beneficial to industry workers who can effectively purify the wastewater as much as possible before it is discharged into the environment.
Conclusions
Single and multiobjective optimal control using BARON which is a state of the art global optimization solver of the Wastewater treatment process is performed. The main aim of this optimal control was to minimize the Nitrite/Nitrate, ammonium and biodegradable organic contaminants. It is shown that by increasing the time of the operation the multiobjective optimal control could be made as effective as the single objective optimal control operation with the added advantage of being able to control all the variables. This provides an effective strategy for industries to obtain a more beneficial and to minimize the contaminants and ensure that the wastewater discharged into the surroundings is not harmful to the environment.
References
Adhikari S, Halden RU (2022) Opportunities and limits of wastewater-based epidemiology for tracking global health and attainment of UN sustainable development goals. Environ Int 163:107217. https://doi.org/10.1016/j.envint.2022.107217
Amand L, Olsson G, Carlsson B (2013) Aeration Control—a review. Water Sci Technol 67:2374–2397
Andreottola G, Foladori P, Ragazzi M (2001) On-line control of a SBR system for nitrogen removal from industrial wastewater. Water Sci Technol 43(3):93–100
Barceló D (2020) Wastewater-Based Epidemiology to monitor COVID-19 outbreak: present and future diagnostic methods to be in your radar Case Stud. Chem Environ Eng 2:100042. https://doi.org/10.1016/j.cscee.2020.100042
Barrou O, Karama A, Lakhal EK, Bernard O, Pons M-N, Corriou J-P (2008) Estimation of a Reduced Model of the BSM1 Activated Sludge Wastewater Treatment Plant, Vol 6, Article A63, (2008)
Biegler LT (2007) An overview of simultaneous strategies for dynamic optimization. Chem Eng Process.: Process Intensif 46:1043–1105
Bijlsma L, Bade R, Been F, Celma A, Castiglioni S (2021) Perspectives and challenges associated with the determination of new psychoactive substances in urine and wastewater—a tutorial Anal. Chim Acta 1145:132–147. https://doi.org/10.1016/j.aca.2020.08.058
Brdys MA, Grochowski M, Gminski T, Konarczak K, Drewa M (2008) Hierarchical predictive control of integrated wastewater treatment systems. Control Eng Pract 16:751–767
Bussieck MR, Meeraus A (2004) General Algebraic Modeling System (GAMS). In Kallrath J. (eds). Modeling Languages in Mathematical Optimization. Applied Optimization, vol 88. Springer, Boston, MA
Chen J, Venkatesan AK, Halden RU (2019) Alcohol and nicotine consumption trends in three U.S. communities determined by wastewater-based epidemiology. Sci Total Environ pp. 174–183. doi:https://doi.org/10.1016/j.scitotenv.2018.11.350.656
Darby ML, Nikolau M, Jones J, Nicholson D (2011) RTO: An overview and assessment of current practice. J Process Control 21:874–884
Daughton CG (2018) Monitoring wastewater for assessing community health: Sewage Chemical-Information Mining (SCIM). Sci Total Environ 619–620:748–764. https://doi.org/10.1016/j.scitotenv.2017.11.102
Daverey A, Pandey D, Verma P, Verma S, Shah V, Dutta K, Arunachalam K (2019) Recent advances in energy efficient biological treatment of municipal wastewater. Bioresour Technol Rep 7:100252
Dionisi D (2017) Biological Wastewater Treatment Processes, 1st edn. CRC Press, Boca Raton, FL, US
Dionisi D, Rasheed AA, Majumder A (2016) A new method to calculate the periodic steady state of sequencing batch reactors for biological wastewater treatment: model development and applications. J Environ Chem Eng 4(3):3665–3680
Flores-Tlacuahuac A, Morales P, Riveral Toledo M (2012) Multiobjective Nonlinear model predictive control of a class of chemical reactors. I & EC Res 51:5891–5899
Gómez-Quintero C-S, Spérandio IQM (2004) A reduced linear model of an activated sludge process. IFAC Proc 37(3):219–224
Hart WE, Laird CD, Watson J-P, Woodruff DL, Hackebeil GA, Nicholson BL, Siirola JD (2017) Pyomo – Optimization Modeling in Python. Second Edition. Vol. 67. Springer
Henze M, Van Loosdrecht MCM, Ekama GA, Brdjanovic D (2008) Biological Wastewater Treatment: Principles. Iwa publishing, London, Modelling and Design
Hulsbeek JJW, Kruit J, Roeleveld PJ, van Loosdrecht MCM (2002) A practical protocol for dynamic modelling of activated sludge systems. Water Sci Technol 45(6):127–136
Julien S, Lessard P, Babary JP (1999) A reduced-order model for control of a single reactor activated sludge process. Math Comp Mod Dyn Syst 5(3):337–350
Martin AD (2000) Interpretation of residence time distribution data. Chem Eng Sci 55:5907–5917
McCarty PL, Bae J, Kim J (2011) (2011) Domestic wastewater treatment as a net en- ergy producer–can this be achieved? Environ Sci Technol 45:7100–7106
Meijer SCF, van Loosdrecht MCM, Heijnen JJ (2001) Metabolic modelling of full scale biological nitrogen and phosphorus removing wwtp. Water Res 35:2711–2723
Miettinen, KM (1999) Nonlinear Multiobjective Optimization; Kluwers international series
O’Brien M, Mack J, Lennox B, Lovett D, Wall A (2011) Model predictive control of an activated sludge process: a case study. Control Eng Pract 19:54–61
Olsson G, Nielsen M, Yuan Z, Lynggaard-Jensen A, Steyer J-P (2005) Instrumentation, Control and Automation in Wastewater Systems. IWA Publishing, London, UK
Papadimitriou CA, Samaras P, Sakellaropoulos GP (2009) Comparative study of phenol and cyanide containing wastewater in CSTR and SBR activated sludge reactors. Bioresour Technol 100(1):31–37
Piotrowski R, Brdys MA, Konarczak K, Duzinkiewicz K, Chotkowski W (2008) Hierarchical dissolved oxygen control for activated sludge processes. Control Eng Pract 16:114–131
Santín I, Pedret C, Vilanova R (2015a) Applying variable dissolved oxygen set point in a two level hierarchical control structure to a wastewater treatment process. J Process Control 28:40–55
Santín I, Pedret C, Vilanova R (2015b) Fuzzy control and model predictive control configurations for effluent violations removal in wastewater treatment plants. Ind Eng Chem Res 54:2763–2775
Santin I, Pedret C, Vilanova R (2016) Control and Decision Strategies in Wastewater Treatment Plants for Operation Improvement. Springer, Cham, Switzerland
Silvana R, Vega P, Vilanova R, Francisco M (2017) Optimal control of wastewater treatment plants using economic-oriented model predictive dynamic strategies. Appl Sci 7:813
Singh KS, Viraraghavan T (2002) Modelling of sludge blanket height and flow pattern in UASB reactors treating municipal Wastewater. Wastewater Treatment 1:1–8
Sridhar LN (2019) Multiobjective optimization and nonlinear model predictive control of the continuous fermentation process involving Saccharomyces Cerevisiae. Biofuels. https://doi.org/10.1080/17597269.2019.1674000 (ISSN:1759-7269(Print)1759-7277)
Steffens MA, Lant PA, Newell RB (1997) A Systematic Approach for Reducing Complex Biological Wastewater Treatment Models. Wat Res 31(3):590–606
Tatjewski P (2008) Advanced control and on-line process optimization in multilayer structures. Ann Rev Control 32:71–85
Tawarmalani M, Sahinidis NV (2005) A polyhedral branch-and-cut approach to global optimization. Math Program 103(2):225–249
Vega P, Revollar S, Francisco M, Martin JM (2014) Integration of set point optimization techniques into nonlinear MPC for improving the operation of WWTPs. Comput Chem Eng 68:78–95
Yalmaz G, Öztürk I (2001) Biological ammonia removal from anaerobically pre-treated landfill leachate in sequencing batch reactors (SBR). Water Sci Tech- Nol 43(3):307–314
Zeng J, Liu J (2015) Economic model predictive control of wastewater treatment processes. Ind Eng Chem Res 54:5710–5721
Zhao H, Isaacs SH, Soeberg H, Kümmel M (1994) A Novel control strategy for improved nitrogen removal in an alternating activated sludge process - part i wat. Res 28(3):521–534
Funding
There is no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. All the codes of ethical conduct have been adhered to.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sridhar, L.N. Single and Multiobjective Optimal Control of the Wastewater Treatment Process. Trans Indian Natl. Acad. Eng. 7, 1339–1346 (2022). https://doi.org/10.1007/s41403-022-00368-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-022-00368-6