Abstract
A novel simple, sensitive, rapid, direct, and spectrophotometry-based procedure was investigated to determine Th(IV) at trace amounts. The new method is based on Th(IV) chelation with 3,6-dichloro-2,5-dihydroxy-l,4-benzoquinone (DDBQ). The reagent reacts with Th(IV) in 0.1 M HCl to form an orange 1:2 complex. The stability constant value is 6.62 × 104 for the Th(IV) complex. The Th(IV)-DDBQ obtained shows one peak with a maximum at about 346 nm. The chelate forms immediately and the absorbance remains stable for over 24 h. Beer’s law was obeyed in the concentration range 0–10 µg mL−1. The molar absorptivity and Sandell’s sensitivity were 4.4 × 104 L mol−1 cm−1 and 0.0053 μg cm−2, respectively. Different analytical parameters were tested in detail. Interfering ion (cations and anions) effects were tested. Methods for Th(IV) determination by second and third-derivative spectrophotometry were also introduced at about 344 and 341 nm, respectively. These two derivative orders offer the feature of sensitivity without the necessity for solvent extraction, heating, or pre-concentration steps. Finally, the methods were successfully utilized for Th(IV) determination in monazite, environmental water, and wastewater samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Thorium (Th) is a very significant metal [1]. It is widely utilized in medicine, catalysis, the fuel industry, ceramics, lenses, refractory materials, and electronic components [1, 2]. The haphazard release of Th(IV) species from different industries increases its content in the surrounding ecosystem. Increased concentrations of this metal increase the incidence of lung diseases, and pancreatic and bone cancers [1,2,3]. Thus, the analytical quantification of Th(IV) is helpful in ecological science, nuclear fuel chemistry, and geochemistry [4, 5].
Numerous procedures for Th(IV) ion determination have been elaborated. These methods include gravimetry, titrimetry, thin-layer chromatography, reverse-phase liquid chromatography, spectrofluorescence, X-ray fluorescence, capillary zone electrophoresis, inductively coupled plasma (ICP), electroanalytical techniques, activation analysis, and alpha spectrometry [6]. These reported procedures are precise but costly and require pre-treatment steps and complicated instrumentation. Furthermore, the use of these techniques is restricted because of their higher working cost in addition to the equipment cost [2, 4,5,6,7,8,9].
Spectrophotometry is predominantly a trace analysis technique and is one of the most widely available apparatuses in chemical analysis. It has gained much attention owing to its simplicity, accuracy, precision, and easy automation. It is a multi-use technique because of its wide availability, simple implementation, and economic feasibility in comparison with others [10]. The spectrophotometric estimation of Th(IV) ions using appropriate chromogenic reagents is one of the simplest, most precise, and most accurate tolls. There is therefore a need to propose new, simple-to-utilize methods for Th(IV) ion determination in different samples [7, 11]. Th(IV) determination at the microgram level using spectrophotometry remains more favorable than other analytical techniques [11]. Normal procedures for spectrophotometric Th(IV) determination are based on the reaction of Th(IV) ions with a chromogenic or complexing reagent [6, 10].
A search of the literatures shows that only a few chromogenic reagents are useful for spectrophotometric Th(IV) determination [7, 9,10,11]. Various chromogenic reagents for spectrophotometric Th(IV) determination have been recognized. These include methylthymol blue, xylenol orange, galleon, 5,8-dihydroxy-1,4-naphthoquinone, nitrosulfonazo III, 1-amino-4-hydroxyanthraquinone, 4-(2-triazolylazo)-resatophenone, 1-(2-thiazolylazo)-2-naphthol, arsenazo III, and thoron [6, 7, 10]. Among these chromogenic reagents, thoron, arsenazo III, and xylenol orange are particularly sensitive for Th(IV) determination [9, 10]. Few other analytical complexing reagents are helpful for direct Th(IV) ion determination [10]. The majority of the above procedures requires a preliminary step to separate closely associated ions and hence lack selectivity [6, 10].
Derivative spectrophotometry has the advantages of increased sensitivity and selectivity compared to zero-order (ordinary) spectrophotometry. The selectivity increase in the new derivative mode originates from the fact that peaks that are superimposed in the zero-order spectrum are separated in the new derivative mode; thus, derivative mode can reduce the interference effect. Derivatization can enhance sensitivity by reducing noise and amplification of the derivative signals [12, 13] and has been utilized successfully for the estimation of many ions [9, 12, 14]. In our laboratory, we have used various organic reagents for the determination of Nb(V), Zr(IV), U(VI), Th(IV), Cr(III), Cr(VI), V(V), Fe(III), and Mo(VI) in different environmental samples and alloys with derivative methods [5, 8, 15,16,17,18,19].
In the present investigation, a direct, non-extractive, simple, sensitive, and derivative method for spectrophotometric Th(IV) ion determination using a new reagent, 3,6-dichloro-2,5-dihydroxy-l,4-benzoquinone, is reported.
2 Experimental
2.1 Reagents
Most of the chemicals applied in our work were of analytical grade (AR) and were used as is. Deionized distilled water (DDW) was used for preparing aqueous solutions. The glassware used was immersed in 10% (v/v) HNO3 for 24 h and rinsed many times with DDW.
A stock solution of 1000 ppm of thorium was obtained by dissolving the appropriate amount of Th(NO3)4 (Loba) in 100 mL of DDW. Solutions with lower Th(IV) concentrations were obtained by dilution of the 1000 ppm Th(IV) stock solution. The thorium solution was standardized spectrophotometrically [10]. A chromogenic solution (0.1% (w/v) DDBQ, Aldrich) was obtained by dissolving 0.250 g DDBQ in 250 mL of DDW. In the interference study, solutions containing various ions were obtained by dissolving the calculated amount of each compound to give solutions containing 1000 ppm of each ion. All solutions were stored in bottles (made from polyethylene). Different concentrations of hydrochloric acid (Aldrich) were tested as the acidic complexation medium.
Environmental samples from different sources were collected and filtered using Whatman filter paper to retain the suspended particles. The filtered samples were then treated with 0.1 M HCl to prevent possible hydrolytic precipitation of some ions.
2.2 Equipment
Spectra of the thorium complex were measured using a Shimadzu UV–visible spectrophotometer-160A from Japan. This spectrophotometer (double beam) operates in the range of 200 to 1100 nm. The built-in software includes a program for obtaining different order derivative spectra and the automatic computation of derivative values. In all measurements, two quartz cells with a path length of 10 mm were used.
2.3 Analytical procedures
A thorium sample containing up to 100 µg of thorium was placed in a 10-mL standard flask, after which their acidity was adjusted to 0.1 M HCl and 1 mL of DDBQ solution [0.1% (w/v)] was added. The volume of the solution in the measuring flask was increased to 10 mL and mixed. The absorbance of the thorium complex [Th(IV)-DDBQ] was measured at 346 nm after 2 min versus blank containing the reagent but no thorium ions under similar conditions.
The second-derivative spectrophotometric determination procedure was carried out for thorium concentrations as low as 0.5 µg mL−1. The second-derivative spectrum was obtained at Δλ = 8 nm and a 1500 nm min−1 scan speed in the range of 320 to 400 nm. The analytical response was obtained by computing the vertical distance (H1) from the base line to the trough value at approximately 344 nm.
The third-derivative spectrophotometric procedure was carried out when the Th(IV) ion concentration was smaller than 0.5 µg mL−1 (0.25 to 8.0 µg mL−1). The third-derivative spectrum was obtained at Δλ = 8 nm and a 1500 nm min−1 scan speed in the range of 320 to 400 nm. The analytical response was obtained by computing the vertical distance (H2) from the zero line to the peak value at 341 nm.
2.4 Procedure for the continuous variation method
Thorium (IV) solutions of 4.3 × 10−4 M (0.1, 0.2, 0.3,… 1 mL) were pipette out and placed into ten flasks (10 mL), and aliquots (0.9, 0.8, 0.7,…, 0 mL) of 4.3 × 10−4 M DDBQ reagent were placed in the flasks, respectively, in such a way that the mole fraction of the solution remained fixed. The absorbances were recorded at 346 nm after 2 min versus a reagent blank that contained no thorium ions under similar conditions.
3 Results and discussion
DDBQ was applied as a chromogenic reagent for spectrophotometric Th(IV) estimation in an acidic medium to form an orange complex. Studies were carried out spectrophotometrically to determine the most effective conditions for the complexation of Th(IV) with DDBQ to achieve maximum color development during the quantitative measurement of the thorium ions.
3.1 Spectral characteristics
The reagent DDBQ reacted immediately with the thorium ions in the HCl medium (0.001 to 3.0 M) to form an orange complex at normal temperature. Normal (zero-order) absorption spectra of DDBQ and its Th(IV) complex obtained using the optimum conditions at 320–400 nm are given in Fig. 1. The spectrum of the Th(IV)-DDBQ complex versus the blank showed the greatest absorbance intensity at 346 nm (λmax), and the absorption reading was taken at this wavelength. The absorption was linear and obeyed Beer’s law for thorium ion concentrations up to 10 µg mL−1.
The uses of derivative spectrophotometric modes have become increasingly widespread, since they are more selective and sensitive than classical spectrophotometry. This tool has opened the possibility to increase the detection limit and selectivity of measurement without additional cost [15,16,17,18,19,20]. The properties of the investigated method (sensitivity and selectivity) can also be increased by utilizing derivative spectroscopy. Hence, different derivative orders were studied.
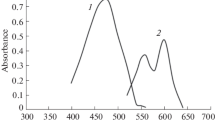
Studies of the first-, second-, third-, and fourth-derivative spectra of the Th(IV)-DDBQ complex under the optimum conditions were explored, and the second-order and third-order derivative spectra of the Th(IV)-DDBQ complex were found to be optimal (offering better detection limit and selectivity compared to those of other derivative profiles) at 1500 nm min−1 (scan speed) and the range of 320 to 400 nm at a wavelength interval Δλ(N) of 8. The second-order and third-order derivative spectra of the Th(IV)-DDBQ complex under the optimum conditions are shown in Figs. 2 and 3, respectively.
Second-derivative spectrum of the Th(IV)-DDBQ complex against the reagent blank under the optimum conditions used in Fig. 1
Third-derivative spectrum of the Th(IV)-DDBQ complex against the reagent blank under the optimum conditions used in Fig. 1
The second-order spectrum of Th(IV)-DDBQ shows one peak and two troughs in Fig. 2. The third-order derivative spectrum of Th(IV)-DDBQ also shows one peak and two troughs in Fig. 3.
In the second-order method, the analytical response was obtained by measuring the peak value of the valley at 344 nm. In the third-derivative method, the analytical response was obtained by calculating the peak value at 341 nm.
3.2 Effect of acidity
Hydrochloric acid was used as an acidic environment for thorium complexation [to form a colored complex between DDBQ and Th(IV) ions]. The absorbance behavior of Th(IV)-DDBQ as a function of HCl concentration (0.001–3.0 M) was studied; the data are shown in Fig. 4. The impact of the hydrochloric acid molarity on the peak intensity of the colored Th(IV) complex was investigated at a concentration of 2 μg mL−1 Th(IV) and a constant concentration of DDBQ.
This figure clearly shows that the absorbance of the Th(IV)-DDBQ complex decreases with an increase in hydrochloric acid concentration. Since lower acidities enhance the hydrolysis of thorium and increase the error caused by interference from other ions, 0.1 M HCl was applied throughout the rest of the work as the acidic complexing media to diminish or prevent interference.
3.3 Effect of the reagent concentration
It is very critical to optimize the concentration of reagent, which reacts quantitatively with the thorium ion to form a stable Th(IV)-DDBQ complex. The effect of the DDBQ concentration on absorbance intensity of Th(IV)-DDBQ at 0.5 and 2 μg mL−1 Th(IV) in 0.1 M HCl was investigated by measuring the absorbance of Th(IV)-DDBQ in solutions containing a constant Th(IV) concentration and varying volumes of 0.1% DDBQ.
The experiments revealed that the optimal signal of the thorium complex was obtained at about 1 mL of 0.1% DDBQ solution, and the signal remained almost constant up to 3.0 mL of the DDBQ reagent as shown in Fig. 5. However, 1 mL of the 0.1% DDBQ reagent solution was employed for all further studies.
3.4 Order of addition
The absorbance intensity of the thorium complex depended slightly on the addition order of the reactants, the best addition order being the thorium sample, hydrochloric acid (0.1 M), and DDBQ (1 mL), and then adding deionized distilled water to fill the 10-mL standard measuring flask. Other orders resulted in the same λmax, but with a reduction in absorbance. Hence, the first order of reactant addition was chosen as the optimal one.
3.5 Reaction rate and the effect of the reaction time and temperature on the complex stability
The effect of the reaction time on the formation of the thorium complex was also tested under the optimal conditions described earlier. The results indicated that the rate of color formation did not depend on the reaction time and that the Th(IV)-DDBQ complex was formed instantaneously, and reached its maximum concentration and remained constant after about 1 min. Thus, a 2-min reaction time was selected for the rest of the study. The thorium complex developed as mentioned above showed no significant changes in its absorbance value after being left at a normal temperature for about 24 h, which suggested that there was no time effect on the formation of Th(IV)-DDBQ.
Similarly, variation in the temperature (20 to 40 °C) did not result in any remarkable alteration in the color strength (absorbance) of the thorium complex. This was confirmation that a stable thorium complex was promptly created by the combination of Th(IV) ions and DDBQ (28–30 °C), and that no alteration in the absorption spectra was observed with an increase in temperature.
3.6 Optimization of parameters
The parameters that required optimization in order to achieve perfect selectivity in the thorium complexation and superior sensitivity in the measurement step were studied. The data obtained are listed in Table 1.
3.7 Analytical calibration curves and sensitivities
A standard graph for the estimation of thorium in multiple solutions was constructed according to the analytical procedure. The absorption was found to obey Beer’s law for thorium ion concentrations of 0.0–10 µg mL−1. The best range for thorium concentration determination was 1.0–10 µg mL−1. The data led to the following equation:
where A denotes the Th(IV) complex absorbance at 346 nm and [Th(IV)] is the thorium ion concentration in units of µg mL−1. The molar absorptivity (ε) and Sandell’s sensitivity (ζ) values were determined and were found to be 4.4 × 104 L mol−1 cm−1 and 0.0053 µg cm−2, respectively. The low Sandell’s sensitivity and high value of molar absorptivity indicated the high sensitivity of the new investigated method for the determination of Th(IV).
In the case of the second-derivative procedure, the derivative amplitude was computed by a peak-zero method at 344 nm versus the Th(IV) ion concentration and was linear for thorium concentrations of 0.5 to 10 µg mL−1 Th(IV). The data were fitted by the equation:
In the third-derivative procedure, the amplitude of the derivative, which was computed by a peak-zero method at 341 nm versus the Th(IV) ion concentration, was linear for thorium concentrations from 0.25 to 8.0 µg mL−1. The data were fitted by the equation:
where d2A/dλ2 and d3A/dλ3 are the second- and third-order derivative amplitude values at 344 and 341 nm, respectively.
3.8 Sensitivity of the different determination modes
Our obtained results indicated that the detection limit (sensitivity) of thorium in the second- and third-derivative procedures at 344 nm and 341 nm were two and four times smaller than that of the zero-order (normal mode) procedure, respectively, demonstrating the higher sensitivity of the second- and third-derivative modes.
3.9 Recovery and precision
Accuracy was calculated by analysis of synthetic Th(IV) samples. Thorium ions were added to DDW samples, and Th(VI) concentrations were measured using our investigated methods. The recoveries of Th(IV) ions obtained using the normal mode and second-order and third-order derivative modes were found to range from 100.26 to 106.33, 99.60 to 102, and 100.13 to 100.4%, respectively (Table 2).
The precision of the various modes was also determined as the RSD (relative standard deviation) computed by measuring a peak at 346 nm, and the second-order and third-order derivative amplitudes (H1 and H2) of five replicate thorium samples containing 2 µg mL−1 Th(IV) ions. These gave RSDs of 1.12, 0.64, and 0.72, respectively. These results demonstrate the small RSD and good percentage recoveries (values close to 100%). Based on the improved sensitivity and reproducibility, the estimation of the derivative amplitudes at H1 and H2 is recommended for Th(IV) in different samples.
3.10 Stability of DDBQ
Calibration plot obtained using the optimum conditions described above (in the general procedure) and DDBQ that had been stored at about 29 °C for 7 days matched well with the standard curve obtained using fresh DDBQ. A fresh DDBQ should be prepared after 7 days; alternatively, the DDBQ solution was stable for 14 days when kept at cooler temperatures.
3.11 Structure and stability constant of the thorium complex
We used Job’s method (method of continuous variation) [21] to determine the composition (stoichiometry) of the obtained Th(IV)-DDBQ complex. In this test, we prepared various solutions in which the concentrations of Th(IV) and DDBQ were varied, but the sum of their concentrations was kept fixed. Using these thorium and reagent solutions, the absorption values of Th(IV)-DDBQ were computed and plotted versus the mole fraction. The resulting plot displayed a maximum at the mole fraction identical to their thorium complex (Fig. 6).
From this curve, it was noted that the maximum value was located at about 0.4 mol fraction, which suggests a 1:2 Th(IV):DDBQ stoichiometry for this complex [22]. The stoichiometric ratio of the thorium complex was also examined using the molar ratio method [1]. The complex formation (or stability) constant was computed using Job’s method as follows [21, 22]:
where A1 is the absorbance at the break point, A2 is the actual absorbance, CTh is the concentration of thorium, and CDDBQ is the concentration of DDBQ. The stability constant was computed from the Job’s method results. The stability constant of Th(IV)-DDBQ was found to be equal to 6.62 × 104. Knowledge of this value is required to quantitatively determine the amount of uncomplexed Th(IV), DDBQ, and the complex species formed at different pH values. Thus, based on the data obtained above, the following mechanism is suggested:

In order to provide a useful understanding of the thermodynamics of the reaction (complexation) between the thorium ion and DDBQ, it is useful to compute the contributions of free energy, entropy, and enthalpy for this complexation reaction. The standard free energy change of thorium complex formation was determined using the formation constant (KDDBQ) as in the following equation [23, 24]:
where ΔG° is the change in free energy of the formation of the Th(IV)-DDBQ complex with units of kJ mol−1, R is the gas constant, which is equal to 8.314 J mol−1 K−1, T is the absolute temperature (273 + 30 K), and KDDBQ is the formation constant of Th(IV)-DDBQ at 30 °C.
The obtained ΔG° value of − 12.14 reveals that the Th(IV)-DDBQ formation process is spontaneous. The value of ΔG° becomes more negative as the stability constant of Th(IV)-DDBQ increases. As the binding of Th(IV) and DDBQ becomes stronger, and thus, the Th(IV) and DDBQ undergo additional physical strain or loss of freedom, ΔG° takes on more negative values.
3.12 Impacts of interfering ions
Derivatization increases not only the sensitivity of the investigated method, but also its selectivity. An estimation of the selectivity of the investigated new methods for Th(IV) determination using DDBQ was carried out by adding known concentrations of different ions to a constant concentration of Th(IV) ions (8 µg mL−1) and determining the thorium content using the above methods. The limits of tolerance corresponding to a ± 3% variation in absorbance are shown in Table 3.
The results indicated that many species did not interfere in the Th(IV) determination. The results in Table 3 clearly demonstrate that a variety of ions can tolerate in considerable concentrations in the classical and derivative modes. Similar selectivity was noted when the reagent thorin was applied for Th(IV) ion estimation [4]. The tolerance limit values for many ions were greater in the derivative mode. It was noted that the second-derivative method was more selective than the third-derivative method.
3.13 Analytical applications to real samples
3.13.1 Thorium determination in environmental and wastewater samples and monazite
To determine the analytical applicability and the accuracy of the investigated methods, the investigated procedures were applied to real matrices. The amount of thorium (IV) in samples collected from environment, including river water, tap water, ground water, and seawater, was detected using the new methods. Various spiked samples were synthesized by mixing Th(IV) ions into real water matrices, as shown in Table 4.
Wastewater and monazite samples were also analyzed. The effects of these matrices were tested. The obtained data are shown in Table 5. 1.0 g of monazite was mixed with concentrated H2SO4 (5 mL) and heated at 250 °C for about 4 h to digest the monazite. The obtained material (viscous) was dissolved using distilled water. After that, the solution was filtered and the Th(IV) ions in the filtrate were precipitated as the hydroxide by adding NH4OH. The precipitated thorium was filtered and dissolved in the minimum volume of HCl. After that, the solution was made up to a known volume with DDW [9, 25,26,27]. The specific volume was analyzed using the investigated method. The values obtained were compared by analyzing the sample using ICP, as shown in Table 5.
The concentrations of Th(IV) that were obtained using the new investigated method agreed well with the certified amounts and reference method, which demonstrated the good accuracy of the Th(IV) estimation in different matrices.
3.14 Comparison with other methods
The new method investigated in this paper is very simple, sensitive, and selective, does not require heating or extraction tools, and compares favorably to many methods in precision and accuracy. The limits of tolerance for a variety of ions were good. Moreover, the use of derivative spectrophotometry could accurately estimate thorium from various matrices. A comparison of the new method with some published ones for the direct determination of Th(IV) is given in Table 6.
In the case of our new methods, which utilize DDBQ as an analytical reagent, not only was the selectivity preferable, but the sensitivity was also better than that of the most used analytical methods.
4 Conclusion
The investigated normal and derivative spectrophotometric methods were simple, accurate, and highly sensitive, and had good precision. They also have the advantage of performing the analysis at a minimum cost without reducing its accuracy. The developed methods are based on common reagent and instrument, and could be used for routine measurements. The sufficiently high molar absorptivity of the Th(IV) complex makes it possible to use this complex for the determination of trace amounts of thorium in solutions and in various materials. The comparison of the data obtained in this paper and those from other investigations (Table 6) demonstrates that the new method is superior, as well as having good linear range sensitivity and a short standing time. It was demonstrated that the method could be effectively applied using a simple instrument (spectrophotometer), which would allow a large number of samples to be quantified daily for environmental or industrial applications.
References
S. Lutfullah, S. Sharma, N. Rahman et al., Determination of thorium (IV) with rifampicin in synthetic mixture and soil samples by spectrophotometry. Arab. J. Chem. 9, S1163–S1169 (2016). https://doi.org/10.1016/j.arabjc.2011.12.001
R. Selvakumar, S.K. Ashok Kumar, K. Vijayakrishna et al., Development of highly selective chemosensor for thorium estimation. Sens. Actuators, B 255, 1391–1400 (2018). https://doi.org/10.1016/j.snb.2017.08.131
T.S. Anirudhan, S.R. Rejeena, Thorium(IV) removal and recovery from aqueous solutions using tannin-modified poly(glycidylmethacrylate)-grafted zirconium oxide densified cellulose. Ind. Eng. Chem. Res. 50, 13288–13298 (2011). https://doi.org/10.1021/ie2015679
S. Rastegarzadeh, N. Pourreza, I. Saeedi, An optical chemical sensor for thorium (IV) determination based on thorin. J. Hazard. Mater. 173, 110–114 (2010). https://doi.org/10.1016/j.jhazmat.2009.08.055
M. Gök, Ş. Sert, G. Özevci et al., Efficient adsorption of Th(IV) from aqueous solution by modified SBA-15 mesoporous silica. Nucl. Sci. Tech. 29, 95 (2018). https://doi.org/10.1007/s41365-018-0432-y
D.E. Ryan, R.R. Brooks, H.F. Zhang, The analytical chemistry of thorium. Rev. Anal. Chem. 5(3–4), 281–318 (2011). https://doi.org/10.1515/revac.1981.5.3-4.281
M.H. Khan, M. Hafeez, S.M.H. Bukhari et al., Spectrophotometric determination of micro amounts of thorium with thorin in the presence of cetylpyridinium chloride as surfactant in perchloric acid. J. Radioanal. Nucl. Chem. 301, 703–709 (2014). https://doi.org/10.1007/s10967-014-3201-3
X. Zhou, Z. Liu, C. Chen et al., Real-time wide-range neutron flux monitor for thorium-based molten salt reactor. Nucl. Sci. Tech. 29, 107 (2018). https://doi.org/10.1007/s41365-018-0450-9
V.S.A. Devi, V.K. Reddy, 2-Hydroxy-1-naphthaldehyde-P-hydroxybenzoichydraz-one: a new chromogenic reagent for the determination of thorium(IV) and uranium(VI). J. Chem. 2013, 697379 (2013). https://doi.org/10.1155/2013/697379
Z. Marczenko, M. Balcerzak, Separation, preconcentration, and spectrophotometry in inorganic analysis (Elsevier, Amsterdam, 2001)
V.S.A. Devi, P. Lakshminarayana, P.G. Chowdary, Direct and derivative spectrophotometric determination of Th(IV)with 2-hydroxy-3-methoxybenzaldehyde isonicotinoylhydrazone (HMBAINH). Pharm. Lett. 8, 307–314 (2016)
H.M. Lotfy, D. Mohamed, M.S. Elshahed, Different mathematical processing of absorption, ratio and derivative spectra for quantification of mixtures containing minor component: an application to the analysis of the recently co-formulated antidiabetic drugs; canagliflozin and metformin. Spectrochim. Acta A 189, 100–109 (2018). https://doi.org/10.1016/j.saa.2017.08.015
G. Talsky, Derivative spectrophotometry of first and higher orders (VCH, New York, 1994)
C.B. Ojeda, F.S. Rojas, Recent applications in derivative ultraviolet/visible absorption spectrophotometry: 2009–2011 a review. Microchem. J. 106, 1–16 (2013). https://doi.org/10.1016/j.microc.2012.05.012
M.M. Hamed, R.F. Aglan, S.A. El-Reefy, Normal and second derivative spectrophotometric determination of niobium using solid phase extraction technique. J. Anal. Chem. 70, 1103–1110 (2015). https://doi.org/10.1134/s1061934815090075
A.A. El-Sayed, M.M. Hamed, S.A. El-Reefy, Determination of micro-amounts of zirconium in mixed aqueous organic medium by normal and first-derivative spectrophotometry. J. Anal. Chem. 65, 1113–1117 (2010). https://doi.org/10.1134/S1061934810110043
A.A. El-Sayed, M.M. Hamed, N.S. Awwad, S.A. El-Reefy, Novel reaction for rapid, simple, and sensitive determination of molybdenum in various samples. Anal. Lett. 41, 871–889 (2008). https://doi.org/10.1080/00032710801935095
A.A. El-Sayed, M.M. Hamed, H.A. Hamad, S.A. El-Reefy, Collection/concentration of trace uranium for spectrophotometric detection using activated carbon and first-derivative spectrophotometry. Radiochim. Acta 95, 43–48 (2007). https://doi.org/10.1524/ract.2007.95.1.43
A.A. El-Sayed, M.M. Hamed, Direct determination of U(VI) in TBP-kerosene extracts using first derivative ultraviolet spectroscopy. J. Radioanal. Nucl. Chem. 270, 629–635 (2006). https://doi.org/10.1007/s10967-006-0472-3
A.A.Y. El-Sayed, M.A.A. Rahem, A.A. Omran, Zero- and first-order derivative spectrophotometry for individual and simultaneous determinations of copper(II) and palladium(II) using an oxazolidine derivative as a new reagent. Anal. Sci. 14, 577–583 (1998). https://doi.org/10.2116/analsci.14.577
D. Kara, C. Karadas, A simple spectrophotometric method for the determination of trace levels of molybdenum using N,N-bis (2-hydroxy-5-bromo-benzyl) 1, 2 diaminopropane. Spectrochim. Acta A 147, 158–162 (2015). https://doi.org/10.1016/j.saa.2015.03.081
A. Beiraghi, S. Babaee, Spectrophotometric determination of trace amounts of beryllium using 1,8-dihydroxyanthrone as a new chromogenic reagent. J. Iran. Chem. Soc. 4, 459–466 (2007). https://doi.org/10.1007/BF03247233
A. Lakkadi, N. Baindla, P. Tigulla, Synthesis, spectroscopic and computational studies of charge-transfer complexation between 4-aminoaniline and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. J. Solution Chem. 46, 2171–2190 (2017)
M. Pandeeswaran, K.P. Elango, Solvent effect on the charge transfer complex of oxatomide with 2,3-dichloro-5,6-dicyanobenzoquinone. Spectrochim. Acta A 65, 1148–1153 (2006). https://doi.org/10.1016/j.saa.2005.12.037
A.R. Khan, M. Rafique, A. Jabbar et al., Radionuclide concentrations in sand samples from riverbanks of Muzaffarabad, Azad Kashmir. Nucl. Sci. Tech. 29, 93 (2018). https://doi.org/10.1007/s41365-018-0442-9
E.H. Borai, M.S. Abd El-Ghany, I.M. Ahmed, M.M. Hamed et al., Modified acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Int. J. Miner. Process. 149, 34–41 (2016). https://doi.org/10.1016/j.minpro.2016.02.003
E.H. Borai, M.M. Hamed, A.M. Shahr El-Din, A new method for processing of low-grade monazite concentrates. J. Geol. Soc. India 89, 600–604 (2017). https://doi.org/10.1007/s12594-017-0649-0
Y.K. Agarawal, U. Dayal, Spectrophotometric determination of trace level thorium with n-phenylbenzohydroxamic acid. J. Radioanal. Nucl. Chem. 90, 303–308 (1985). https://doi.org/10.1007/BF02060785
M.E. Khalifa, M.A.H. Hafez, Spectrophotometric and complexometric methods for the determination of thorium and fluoride using bromocresol orange reagent. Talanta 47, 547–559 (1998). https://doi.org/10.1016/S0039-9140(98)00078-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamed, M.M., Aglan, R.F. Simple and sensitive method for the determination of trace amounts of thorium using a benzoquinone derivative. NUCL SCI TECH 30, 73 (2019). https://doi.org/10.1007/s41365-019-0606-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-019-0606-2