Abstract
3-aminomethylalizarn-N-N diacetic acid chromogenic dye (AMADA) was used for the first time in uranium and thorium spectrophotometric estimation with accurate and sensitive result, their complexes having a maximum absorbance at 575 nm for U and at 510 nm for Th. This system allows uranium and thorium content to be calculated with a detection limit (LOD) of 0.16 µg L−1 and 0.14 µg L−1, respectively. The first-derivative spectra and EDTA were investigated to eliminate interference and provide selective estimation of uranium and thorium in presence of each other. This new spectrophotometric method was applied for the determination of U(VI) and Th(IV) in the standard reference materials (granite and Syenite) and some cataclastic rock samples with accurate results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since World War II, numerous research teams have put a lot of work into creating an economic method to recover thorium, uranium, and rare earth elements (REEs) from their sources. Accuracy, speed and efficiency are the three main factors which contribute to the selection of analytical techniques. The techniques of optical emission (ICP-ES and ICP-MS) and X-ray fluorescence are highly sensitive for U and Th analyses, primarily at trace and low concentration levels [1,2,3,4,5,6,7,8,9,10,11]. The disadvantages of the above mentioned techniques are due to high costs, special installation and complicated maintenance for the purpose of regular daily monitoring of the process (in the quality control/quality assurance programmes).
The popularity of spectrophotometry in determining U and Th arises, in addition to its economy, from the modest requirements of its apparatus, the short time needed for any analyst to get to know its use, an adequate level of sensitivity for many present-day purposes and accuracy often as good as, if not better than, any other method within its range [12].
The following survey is restricted by reagents suitable for thorium analyses; These include; 2-(4-sulphophenylazo)chromotropic acid (SPADNS) [13], Arsenazo I and III [14,15,16], n-phenylbenzohydroxamic acid [17], Arsenazo B [18], Dibromo-o-nitro-arsenazo [19], bromo cresol orange (BCO) [20], pyrimidine azo dyes [21], Chromo Azural S [22, 23], 3,5-Dimethoxy-4-hydroxybenzaldehyde isonicotinoyl hydrazone [24], Diacetyl Monoxime Isonicotinoyl Hydrazone [25], Eriochrome Cyanine R [26], Xipamide [27], 2-hydroxy-1-naphthaldehydep-hydroxybenzoichydrazone [28], 5-bromo salicylaldehyde isonicotinoyl hydrazone [29], Thorin [30], 5-Bromo-2-hydroxy-3-methoxy benzaldehyde-p-hydroxybenzoichydrazone [31], Alizarin Red S [32], ortho-ester tetraazophenylcalix [4] arene [33], Thoron I after separation by (E)-4-(2-hydroxyphenyl imino) pentane-2-one [34]. Specific organic dyes and reagents were used in complex materials for the spectrophotometric determination of UO +22 such as 4-(2-pyridylazo) resorcinol [35], thoronal [36], chrome azurol S [37], 1-(2-pyridylazo)-2-naphthol [38], guaiacol [39], 2-(5-bromo-2-pyridylazo)-5-(diethylamino)phenol [40], molybdophosphoric acid [41], N-m-tolyl-p-methoxybenzohydroxamic acid [42] 4-methyl-4-chlorodibenzoylmethane [43], 2-hydroxy-1-naphthaldehyde isonicotinoyl hydrazine [44], 2-(2-benzothiazolylazo)phenol(I), -4-methoxyphenol (II) [45], 2-hydroxynaphthaldehydebenzoylhydrazone (HNABH) [46], 2-(2- Thiazolylazo)-p-Cresol (TAC) [47], Eriochrome Black T [48], and arsenazo (III) [49]. However, most of these methods are tedious, requiring rigid control of pH, temperature, and reagent as well as requiring separation of U or Th from other elements (especially from each other) in advance. This is due to the fact that as for most chromogenic reagents, etc., the maximum absorption for uranium and thorium complexes is very close to each other, and is also time consuming. These methods are generally low in reproducibility and are not selective, either [50].
3-aminomethylalizarn-N-N diacetic acid has been applied earlier for spectrophotometric determination of F− and Cu2+ [51, 52].
Until now, the reagent studied here, 3-aminomethylalizarn-N-N diacetic acid, has not been reported as a ligand for the spectrophotometric determination of uranium or thorium.
Therefore, the present study was intended to propose a novel and very easy method for determining uranium and thorium by 3-aminomethylalizarn-N-N diacetic acid using spectrophotometer which is still frequently used due to its availability and simplicity.
Experimental
Materials
The primary chemicals used were of a category of analytical reagent. Uranyl nitrate hexahydrate (UO2(NO3)2·6H2O) and thorium nitrate tetrahydrate (Th(NO3)4.4H2O) were purchased from Merck, Germany, while Acetic acid (CH3COOH), Sulfuric acid (H2SO4), and 3-aminomethylalizarn-N-N diacetic acid chromogenic dye were purchased from BDH, England. On the other hand, the standard stock solutions Na+, K+, Ca2+, Mg2+, Fe3+, Zr4+, Hf4+, Cr6+, V5+, Cu2+, Co3+, La3+, Y3+, Ce4+, Ni2+, Co2+ Zn2+ and Cd2+, 1000 ppm, were analytical grade and purchased from Merck (Darmstadt, Germany).
Preparation of the working solutions
A stock solution (10−3 M) of U(VI) was prepared by dissolving 0.0251 g of uranyl nitrate hexahydrate (UO2(NO3)2·6H2O) in double-distilled water and completed up to volume in 50 mL standard flask. Thorium stock solution (10−3 M) has been obtained by solvating 0.0276 g of thorium nitrate tetrahydrate (Th(NO3)4·4H2O) in double-distilled water and completing it up to volume in 50 mL standard flask. Standard solutions of the required U(VI) and Th(IV) have been obtained by appropriate dilution.
A stock solution 10−3 M solution of 3-aminomethylalizarn-N-N diacetic acid chromogenic dye (AMADA) dye has been obtained by solvating 0.0963 g of the purified reagent in 50 mL of water containing 0.3 mL conc. ammonia solution. 0.25 mL glacial acetic acid was added and completed up to volume in 250 mL standard flask. More dilute solutions were obtained by appropriate dilution. The structure of AMADA is shown in scheme 1.
Instrumentations
-
UV-Double Beam Spectrophotometer (UNICAM) with 1 cm of cell was used. The optical system was automatically checked, and the instrument was calibrated periodically.
-
Used pH-meter (SCHOTT GERATE, Germany) for all the experiments in the present work. Before each experiment the pH-meter was calibrated using two consecutive buffer solutions (pH 4 and 7, pH 7 and 10).
Procedure
The decomposition procedure for reference and granite samples were done as follow: 0.2 g of sample was mixed with 20 ml HF, evaporates till dryness, then diluted with 15 ml HF (1:1), then filtration and treating the precipitate with 10 ml conc. HClO4 and 5 ml conc HNO3 and heat till dryness. 15 ml 1:1 HCl were added to the residue and completed to the required volume for analysis [32].
Uranium and thorium react sensitively with AMADA dye to form binary complex. These complexes were prepared by adding 0.5 mL of 10−3 M AMADA dye solution to 0.5 mL of 10−3 M of U(VI) or Th(IV) ions and diluted the volume with double distilled water in 10 mL standard measuring flask, finally scanned spectrophotometrically against a reagent blank in the visible region between 400 and 700 nm.
Statistical and accuracy evaluation
In the present work, statistical techniques are used to estimate the precision and accuracy of analytical data. A common practice in literature on analytical chemistry is to quote the mean (\(\bar{X}\)) as the standard consideration for precession estimation (degree of reproducibility or random error). A measurement method accuracy (Δ) is defined as measuring the closeness of results to reference (well known) or true results. Consequently, [53,54,55] applies the following equations:
where Xi = is an individual measurements, n = is the number of measurements.
where d represents the difference between the repeated measurements.
Results and discussion
Absorption spectrum of 3-aminomethylalizarn-N-N diacetic acid (AMADA) and their U(VI) and Th(IV) complexes
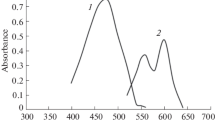
Spectrum of 10−3 M AMADA dye dissolved in water is shown in Fig. 1. The maximum absorption wavelength AMADA dye in aqueous phase is observed at λmax 425 nm. Uranium and thorium react sensitively with AMADA dye to form binary complex. These complexes were prepared by adding 0.5 mL of 10−3 M AMADA dye solution to 0.5 mL of 10−3 M of U(VI) or Th(IV) ions and diluted the volume with double distilled water in 10 mL standard measuring flask, finally scanned spectrophotometrically in the visible region between 400 and 700 nm. Th- AMADA dye complex has rose colour and makes shift to a longer wavelength at λmax 510 nm, while U- AMADA dye complex has purple colour at λmax 575 nm as shown in Fig. 1.
Optimization of the relevant factors
Effect of acidity
The effect of sulfuric acid, hydrochloric acid, nitric acid and perchloric acid with different concentrations (pH) on complex formation were studied. To determine the optimum concentration of acidic medium for optimum formation of U/Th—AMADA complex, a constant volume of 0.5 ml from each of the metal stock solution (10−3 M), and AMADA dye (10−3 M) were taken, while several aliquots from different acids (0.1 mL to 5 mL) from 10−2 M and 10−4 M were added to a series of 10 ml volumetric flasks. The absorbance of each acid concentration was measured in the range from 450 to 600 nm. The obtained data illustrated in Table 1 illustrated the effect of the studied acids on U/Th—AMADA complex formation. The maximum absorbance value of the complex was found at withdrawing of 1 mL from 10−4 M sulfuric acid (pH ≈ 5) at λmax = 575 for U and λmax = 510 for Th. Sulfuric acid was selected for optimum complex formation. For all subsequent measurements 1 mL of 10−4 M sulfuric acid (pH ≈ 5) was added.
Selection of suitable buffer pH solution
The selected optimum pH was 5 (1 mL of 10−4 M H2SO4) for optimal metal- AMADA complex formation, so two pH values were studied; 5 and 5.5. Different amounts of the studied buffer solution (0.2 M acetic acid and 0.2 M sodium acetate) have been applied to various solutions containing 0.5 mL of 10−3 M U(VI) or Th(IV) ions, 1 mL of 10−4 M sulfuric acid and 0.5 mL of 10−3 M AMADA dye where the final volumes of these solutions have been completed to 10 mL. By Fig. 2 it was recognized that the maximum absorbance value was observed with addition of 0.5 mL from buffer pH 5.
Effect of AMADA dye concentration on the absorbance of U and Th-dye complex
The concentration of AMADA dye should be optimized, since an amount which is less than enough or very higher than necessary would cause deviation from Beer’s law in the calibration curve. The optimization of the dye concentration beside pH value would prevent, to a certain extent, the dye from the formation of complexes with other ions that might be present in the aqueous solution.
To study the concentration effect, a series of experiments were performed. In these experiments, different volumes of 10−3 M AMADA dye were added to a series of measuring flasks (10 mL) containing fixed volumes of 0.5 mL of 10–3 M U or Th, 1 mL from 10−4 M sulfuric acid and 0.5 mL from buffer pH 5. Absorbance of each solution was then measured spectrophotometrically at λmax = 575 for U and λmax = 510 for Th. As shown from the obtained data (Fig. 3), the maximum absorbance of U or Th binary complexes occurs at a concentration of 5 × 10−5 M AMADA dye (0.5 mL from 10−3 M) under the above mentioned experimental conditions. Hence, 5 × 10−5 M of the reagent is maintained for all further studies.
Stoichiometry of the “metal - AMADA” complex
The molar ratio between metal (U or Th) and the dye was estimated using different spectrophotometric methods, namely the continuous variation method and molar ratio method.
Continuous variation method (Jobʼs method)
In this method [56], the molar ratio between the dye and the metal is continuously varied by changing their molarities while maintaining the total moles in both constituents constant.
For the determination of the molar ratio, the working solutions for each of metal and dye were prepared (10−3 M). In this experiment, the total number of moles of metal and dye is always kept constant through keeping the final volume constant 1 mL. From the obtained result shown in Fig. 4, it is clearly evident that metal (U or Th) associates with AMADA dye in a 1:1 molar ratio.
Molar ratio method (Yoe-Jones method)
The second method for estimating the molar ratio between metal (U or Th) and dye is molar ratio method [57]. In this method, the content of the metal ions was maintained constant (5 × 10−5 M) and the content of the ligands ranged from 0.5 × 10−5 to 7 × 10−5 M. Therefore, a sequence of aqueous metal–ligand solutions with various [L]/[M] proportions were prepared. The detection of all these solutions utilizing Spectrophotometer was assessed at λmax = 575 for U and λmax = 510 for Th as shown in Fig. 5.
The absorbance was perceived to increase linearly as the amount of ligand (AMADA) increased, as the complex was formed until the solution meets the specific molar ratio of the complex being examined. At this point, the excess quantity of the added ligand created an inflection in the straight line, since the ligand had an absorption value that varied from that of the complex at the complex’s λmax. The molar ratio method showed that the ratio between AMADA dye [L] and metal [M] at high absorbance [L]/[M] = 1/1 as shown in Fig. 5. This is identical with that obtained by the continuous variation method.
Effect of time on the stability of the metal binary complex
After optimizing the complex formation of metal (U or Th)- AMADA binary complex, the duration of the stability of complex was studied. The metal (U or Th)- AMADA binary complex using the optimum concentrations shown above was left for a long period, where the absorbance was measured periodically. From the recorded data in Fig. 6, it was observed that the complex was formed instantly and a maximum absorbance was remained strictly constant for more than two days (Fig. 6); no longer time was studied.
Construction of U(VI) and Th(IV) with AMADA calibration graph (Beer’s law and sensitivity)
After the comprehensive analysis of important factors influencing the AMADA spectrophotometric estimation of U(VI) and Th(IV), the concentration range was found to be sufficient above which no further U/Th complex could be formed and determined. On the other hand below this range (detection limit), U/Th could not be determined by using the studied optimized conditions. The building of the spectrometric calibrating curve will define all these limits for U/Th—AMADA complex. This spectrophotometric determination of both metals U/Th must be done within the concentration range in which the rule of Beer’s law is valued.
For this purpose, a series of solutions containing fixed concentrations of AMADA dye (5 × 10−5 M) and variable concentration of either U or Th were prepared. Each of the prepared solutions was adjusted to pH 5 (acetate buffer) and was measured at recommended wavelength and against blank solution. Figure 7 shows the obtained results. Form the obtained data; it is clearly evident that the maximum concentration limit attained is 15 µg mL−1 for U(VI) and 25 µg mL−1 for Th(IV). From this straight line, the average molar absorptivity (Ɛ) of the uranium and thorium complexes were calculated as 0.56 × 104 M−1 cm−1 and 0.688 × 104 M−1 cm−1, respectively. Beer’s law was obeyed in the range from 0.16 to 15 µg mL−1 U(VI) and from 0.14 to 25 µg mL−1 Th(IV).
Table 2 summarizes the calculated data for the molar absorptivity (Ɛ), the specific absorptivity (a), Sandell index (S), and limit of detection as well as the obeyance range of Beerʼs law for binary complex of uranium and thorium.
Studying of the possible interferences with uranium and thorium during its spectrophotometric determination using AMADA
Many elements which can be correlated with uranium and thorium in natural rocks had to be tested for interference effects. Generally, some of the common cations which are expected to be associated with uranium and thorium are; Na+, K+, Ca2+, Mg2+, Fe3+, Zr4+, Hf4+, Cr6+, V5+, Cu2+, Co3+, La3+, Y3+, Ce4+, Ni2+, Co2+ Zn2+ and Cd2+. These cations may interfere in the U/Th spectrophotometric estimation, thus they have been chosen to study their interference effects. From their working solutions, different volumes were added to a constant U/Th concentration (1 μg mL−1) and the spectrophotometric procedure under investigation was applied. The absorbances of these mixtures were measured at the optimized conditions. Then, it would be possible to identify the maximum permitted concentration of the examined cation, above which interference begins to appear. The data in Table 3 indicate the critical concentration of the examined cations, above which the estimation of the uranium and thorium complexes is affected by these examined cations. Masking with potassium cyanide (1 × 10−3 M) avoided significant interference from the cations mentioned above [58,59,60]. The interference effect of U and Th had to be tested one on the other. For this purpose, a mixture of uranium and thorium (10 μg mL−1) was mixed with 1 mL of 10−4 M sulfuric acid, 0.5 mL from buffer pH 5, and 0.5 mL of 10−3 M AMADA dye then completed up to volume in 10 mL volumetric flask. Figure 8 demonstrates spectral U and Th interference on each other. Therefore, EDTA was used as a Th masking agent [61, 62] for uranium determination in the presence of thorium. EDTA-2Na (0.1 M) was applied to the aforementioned mixture in various amounts and uranium was measured in each aqueous solution. The results were obtained from Fig. 9 showed that 0.3 mL in 10 mL of measuring solution was adequate to mask the interfering thorium. Using this study on uranium determination in the presence of thorium, it was found that uranium was determined without thorium interference, as shown in Fig. 10.
On the other hand, for determination of Th in presence of U, derivative spectrophotometric technique was used. Derivatisation of spectra is the easiest approach for increasing selectivity; this process allows for the overcoming of spectral interferences and, as a result, increases assay selectivity. Digital data derivatisation is a well-known method for extracting useful signals from noised data [63] as spectrophotometer integrated computers and accompanying software were built to generate derivative spectra of various orders. Hence, in the presence of U, it will test for determining Th. So, in order to eliminate interferences of U, the first-derivative spectra were investigated. Adjust the first-derivative spectrum in the range between 470 nm and 525 nm against a blank as a reference using 1.0 cm cells, band pass = 1.0 nm, Δλ = 1.8 nm, scanning speed = 20 nm. It was found from the first-derivative spectrum (Fig. 11) that there is significant peak for Th only at 497 nm. The first-order derivative was therefore the most suitable for selective estimation of Th in the presence of the U using AMADA dye method, which indicates that it is possible to determine Th individually in the presence of U.
A comparison of the reagent possibility (AMADA) under consideration for the spectrophotometric estimation of uranium (VI) and thorium (IV) with the other methods (Tables 4, 5) shows that the proposed reagent (AMADA) is one of the most powerful determinant reagents for the estimation of uranium (VI) and thorium (IV). The previously reported spectrophotometric methods suffer from interference from many metal ions, such as Fe, Cr, Cu, Co, Zn, Al, Ni, V, Zr, Cu, Hg, Mn, Ce, and Mo, which found in ore materials. These methods require prior separation and are tedious and time consuming. Also, the stability of complexes in the above methods is low. Whereas the present reported method is simple, nonextractive, and reasonably accurate methods. Further, the derivative method is found to be more selective than large number of the reported methods.
Application of proposed method on standard geological and granitic samples
The proposed method has been used for spectrophotometric estimation of U(VI) and Th(IV) in various samples including certified reference materials and cataclastic rocks from various locations in the Abu Rusheid region, South Eastern Desert, Egypt to verify applications and validations thereof. To overcome the high concentration of major oxides in the measured solutions, the samples were first decomposed by the method of Afifi et al., 2012 and Fouad et al. 2015 [32, 69]. Using this method for removing most of interfering ions and also it gives the maximum leaching efficiency of uranium and thorium. In the latter method, 0.2 g of sample was mixed with 20 ml HF, evaporate till dryness, then diluted with 15 ml HF (1:1), then filtration and treating the precipitate with 10 ml conc. HClO4 and 5 ml conc HNO3 and heat till dryness. 15 ml 1:1 HCl were added to the residue and completed to the required volume. Uranium was spectrophotometrically analyzed in solutions as follows: an aliquot portion of each solution was mixed with1 ml of 10−4 M sulfuric acid, 0.5 ml from buffer pH 5, 1 mL potassium cyanide (1 × 10−3 M), 0.3 ml EDTA, and 0.5 mL of 10−3 M AMADA dye then completed up to volume in 10 mL volumetric flask. The absorbance of these solutions has been calculated with λmax 575 UV spectrophotometer. On the other hand, for estimation of Th in presence of U the first-derivative spectra were investigated at λmax = 497 nm. Tables 6 and 7 detail the effects of the expected and obtained values. The agreement between the obtained and expected values showed that the method mentioned was accurate in the complex matrices for analysis of U(VI) and Th(IV). This means that both the reference and observed sample error percentage values using this spectrophotometric technique are small and similar to the approved values obtained by the ICP-OES and XRF techniques. This states that the findings obtained are highly accurate and precision. For the evaluation of the existing process, certain statistical parameters (Tables 6, 7) such as standard deviation (S.D), percentage error (% E), standard error (S.E), and accuracy (Δ) were measured. It has been found that appropriate statistical data have been obtained where the percentage errors (% E) are less than 0.75%, while the accuracy (Δ) is less than 0.8. Thus, these findings suggested that the proposed approach is reliable and effective in cataclastic rock samples to analyze uranium and thorium.
Conclusions
3-aminomethylalizarn-N-N diacetic acid chromogenic dye (AMADA) was found to be accurate and sensitive for the spectrophotometric estimation of U(VI) and Th(IV). The molar absorptivity at 575 nm is computed to be 0.56 × 104 L M−1 cm−1 for U-AMADA solution at buffer pH 5, while it is 0.688 × 104 L M−1 for Th-AMADA solution at 510 nm. Beer’s law was obeyed in the range from 0.16 to 15 µg mL−1 U(VI) and from 0.14 to 25 µg mL−1 Th(IV). The first-derivative spectra and EDTA were investigated to eliminate interference and selective estimation of uranium and thorium in presence of each other. The analytical features (limit of detection, precision and accuracy) achieved have demonstrated the feasibility of the new proposed method for uranium and thorium determination in standard reference and natural rocks.
References
Daneshvar G, Jabbari A, Yamini Y, Paki D (2009) Determination of uranium and thorium in natural waters by ICP-OES after on-line solid phase extraction and preconcentration in the presence of 2,3-dihydro-9,10-dihydroxy-1,4-antracenedion. J Anal Chem 64:602–608. https://doi.org/10.1134/S1061934809060112
Zoriy P, Ostapczuk P, Dederichs H, Hobig J, Lennartz R, Zoriy M (2010) Biomonitoring of environmental pollution by thorium and uranium in selected regions of the Republic of Kazakhstan. J Environ Radioact 101:414–420. https://doi.org/10.1016/j.jenvrad.2010.02.014
Abdul-Hadi A, Al-Qadhi W, El-Zeen E (2011) Determination of uranium, thorium and potassium contents of rock samples in Yemen. J Radioanal Nucl Chem 290:261–266. https://doi.org/10.1007/s10967-011-1214-8
Avivar J, Ferrer L, Casas M, Cerda V (2012) Fully automated lab-on-valve-multisyringe flow injection analysis-ICP-MS system: an effective tool for fast, sensitive and selective determination of thorium and uranium at environmental levels exploiting solid phase extraction. J Anal At Spectrom 27:327–334. https://doi.org/10.1039/C2JA10304D
Krejcova A, Cernohorsky T, Pouzar M (2012) O-TOF-ICP-MS analysis of rare earth elements, noble elements, uranium and thorium in river-relating species. Int J Environ Anal Chem 92:620–635. https://doi.org/10.1080/03067310903582382
Souza S, Ávila D, Monteiro A, Garcia A, Alves J, Maranhão T, Araujo R (2016) Simultaneous determination of thorium and uranium in mineral fertilizers by inductively coupled plasma optical emission spectrometry. J Braz Chem Soc 27:799–806. https://doi.org/10.5935/0103-5053.20150324
Rezaee M, Khalilian F (2016) A novel method for the determination of trace thorium by dispersive liquid-liquid microextraction based on solidification of floating organic drop. Quim Nova 39(2):167–171. https://doi.org/10.5935/0100-4042.20160012
Sathyapria R, Rao D, Prabhath R (2017) Choosing an appropriate method for measurement of 232Th in environmental samples. Radiat Prot Environ 40:90–94
Lim S, Park R, Choi E, Han S, Park J, Lee C (2017) Improvement of bulk analysis technique for uranium in environmental samples with high thorium contents. J Radioanal Nucl Chem 314:2047–2055. https://doi.org/10.1007/s10967-017-5550-1
Sergani F, Abdelbagi A, Shokali A (2017) Evaluation of uranium, thorium and some metal elements in rocks by HR-ICP-MS and LIBS. J Nucl Energy Sci Power Gener Technol 6:1. https://doi.org/10.4172/2325-9809.1000168
Ito S, Takaku Y, Ikeda M, Kishimoto Y (2017) Determination of trace levels of uranium and thorium in high purity gadolinium sulfate using the ICP-MS with solid-phase chromatographic extraction resin. Prog Theor Exp Phys 11H01:1. https://doi.org/10.1093/ptep/ptx145
Ali M, Issa Y, Abdel-Hamied M, Rezk A (1998) Extraction-spectrophotometric microdetermination of thorium using acridinic basic dyes. Egypt J Anal Chem 5:36
Banerjee G (1957) Direct spectrophotometric determination of zirconium with spadns. Anal Chim Acta 16:62–66. https://doi.org/10.1016/S0003-2670(00)89889-X
Marczenko Z (1986) Spectrophotometric determination of elements. Wiley, New York
Khan M, Ali A, Khan N (2001) Spectrophotometric determination of thorium with disodium salt of arsenazo-III in perchloric acid. J Radioanal Nucl Chem 250:353–357. https://doi.org/10.1023/A:1017968217578
Niazi A, Ghasemi N, Goodarzi M, Ebadi A (2007) Simultaneous Spectrophotometric determination of uranium and thorium using arsenazo III by H-point standard addition method and partial least squares regression. J Chin Chem Soc 54:411–418. https://doi.org/10.1002/jccs.200700058
Agrawal Y, Dayal U (1985) Spectrophotometric determination of trace level thorium with n-phenylbenzohydroxamic acid. J Radioanal Nucl Chem 90:303–308. https://doi.org/10.1007/BF02060785
Martinez-Calatayud J, Pascual-Marti M, Palau-Contell M, Martinez-Cervera J, Martinez-Palmer F (1987) Spectrophotometric determination of lanthanum(III) and thorium(IV) with arsenazo B. Batch and flow-injection procedures. Quim-Anal 6:333–342
Yang Y, Hsu C, Pan J (1993) Microdetermination of thorium by ion-exchanger phase spectrophotometry with dibromo-o-nitro-arsenazo. Microchem J 84(2):178–183. https://doi.org/10.1006/mchj.1993.1088
Khalifa M, Hafez M (1998) Spectrophotometric and complexometric methods for the determination of thorium and fluoride using bromocresol orange reagent. Talanta 47:547–559. https://doi.org/10.1016/S0039-9140(98)00078-2
Amin A, Mohammed T (2001) Simultaneous spectrophotometric determination of thorium and rare earth metals with pyrimidine azo dyes and cetylpyridinium chloride. Talanta 54:611–620. https://doi.org/10.1016/S0039-9140(00)00679-2
Upase A, Zade A (2005) Microspectrophotometric determination of thorium (IV) and uranium (VI) with methylthymol blue. J Indian Chem Soc 82:853
Upase A, Zade A, Kalbende P (2011) Spectrophotometric microdetermination of thorium(IV) and uranium(VI) with chrome azurol-s in presence of cationic surfactant. E-J Chem 8(3):1132–1141
Vallinath G, Chandrasekhar K, Devanna N (2010) Determination of thorium (IV) by derivative spectrophotometric technique. Int J Pharm Qual Assur 2(1):67–72
Reddy G, Devanna V, Chandrasekhar K (2011) Sensitive spectrophotometric determination of thorium (IV) using diacetyl monoxime isonicotinoyl hydrazone (DMIH). Int J Appl Biol Pharm Technol 2(2):133–139
Shiri S, Delpisheh A, Haeri A, Poornajaf A, Khezeli T, Badkiu N (2011) Floatation-spectrophotometric determination of thorium using complex formation with eriochrome cyanine R. Anal Chem Insights 6:1–6. https://doi.org/10.4137/ACI.S5949
Al-Kady A (2012) Optimized and validated spectrophotometric methods for the determination of trace amounts of uranium and thorium using 4-chloro-N-(2,6-dimethylphenyl)-2-hydroxy-5-sulfamoylbenzamide. Sens Actuators B 166–167:485–491. https://doi.org/10.1016/j.snb.2012.02.091
Devi A, Reddy V (2013) 2-Hydroxy-1-naphthaldehyde-P-hydroxybenzoichydrazone: a New chromogenic reagent for the determination of thorium(IV) and uranium(VI). J Chem 2013:1–10. https://doi.org/10.1155/2013/697379
Swetha M, Reddy R (2014) Sensitive spectrophotometric determinaton of thorium(IV) using 5-bromo salicylaldehyde isonicotinoyl hydrazone(5-BRSAINH). Exp J 19(2):1330
Khan M, Hafeez M, Bukhari S, Ali A (2014) Spectrophotometric determination of microamounts of thorium with thorin in the presence of cetylpyridinium chloride as surfactant in perchloric acid. J Radioanal Nucl Chem 301:703–709. https://doi.org/10.1007/s10967-014-3201-3
Reddy V, Saritha B, Giri A, Reddy T (2014) Direct spectrophotometric determination of Th(IV) in monazite sand using 5-Bromo-2-hydroxy -3-methoxybenzaldehyde-4-hydroxy benzoichydrazone. IJAPBC 3(3):667–672
Fouad H, Abu Elenein S, Elrakaiby R, Abdulmoteleb S (2015) A developed spectrophotometric method for thorium determination using alizarin red s dye in different types of its bearing rocks. Int J Sci Res 4:1611–1615
Tran Q, Le V, Nguyen V (2016) Solvent extraction of thorium using 5,11,17,23-Tetra[(2-ethylacetoethoxyphenyl)(azo)phenyl]calix[4]arene. J Chem 2016:1–6. https://doi.org/10.1155/2016/5078462
Cheira M, Orabi A, Hassanin M, Hassan S (2018) Solvent extraction of thorium (IV) from chloride solution using Schiff base and its application for spectrophotometric determination. Chem Collect 13–14:84–103. https://doi.org/10.1016/j.cdc.2018.01.003
Chen J, Khalili F, Mohammed A, Choppin G (1994) Extraction and spectrophotometric determination of Nd(III), Th(IV), and U(VI) in synthetic brine using chlorophosphonazo III. Anal Chim Acta 284:593–597. https://doi.org/10.1016/0003-2670(94)85064-X
Shamsipur M, Ghiasvand A, Yadollah Y (1999) Solid-phase extraction of ultratrace uranium(VI) in natural waters using octadecyl silica membrane disks modified by tri-n-octylphosphine oxide and its spectrophotometric determination with dibenzoylmethane. Anal Chem 71:4892–4895. https://doi.org/10.1021/ac981229o
Nakashima T, Yoshimura K, Taketutsu T (1992) Determination of uranium(VI) in seawater by ion-exchanger phase absorptiometry with arsenazo III. Talanta 39:523–527. https://doi.org/10.1016/0039-9140(92)80175-D
Narasimba B, Jagannath Y, Yadav R, Ramamurty C, Syamsundar S (1997) Spectrophotometric determination of uranium in process streams of a uranium extraction plant. Talanta 44:283–295. https://doi.org/10.1016/S0039-9140(96)02046-2
Kuroda R, Kurosaki M, Hayashibe Y, Ishimaru S (1990) Simultaneous determination of uranium and thorium with arsenazo III by second-derivative spectrophotometry. Talanta 37:619–624. https://doi.org/10.1016/0039-9140(90)80205-T
Mohapatra P, Manchanda V (1998) Ion-pair extraction of uranyl ion from aqueous medium using crown ethers. Talanta 47:1271–1278. https://doi.org/10.1016/S0039-9140(98)00215-X
Sasaki T, Meguro Y, Yoshida Z (1998) Spectrophotometric measurement of uranium(VI)-tributylphosphate complex in supercritical carbon dioxide. Talanta 46:689–695. https://doi.org/10.1016/S0039-9140(97)00331-7
Ru Y, Yan L, Guilan S, Tao W, Jiaomai P (1995) Spectrophotometric determination of uranium in natural water with the new chromogenic reagent p-carboxychlorophosphonazo. Anal Chim Acta 314:95–99. https://doi.org/10.1016/0003-2670(95)00258-2
Ramesh A, Liyanage J, Taylor D, Williams D (1995) Spectrophotometric analysis of uranyl as its 4-sulfonmic calix[6]arene complex. Anal Proc 32(6):217. https://doi.org/10.1039/ai9953200217
Krishnamacharyulu J (1992) Simultaneous determination of uranium and thorium by second derivative spectrophotometry. Analyst 117:1037–1039. https://doi.org/10.1039/AN9921701037
Amin A, El-Mossalamy E (2003) Simple spectrophotometric method for the quantitative determination of uranium. J Trace Microprobe Tech 21(4):637–648. https://doi.org/10.1081/TMA-120025815
Afrin A, Manirul M, Jamaluddin M (2019) A simple and selective spectrophotometric method for the determination of uranium at nano-trace levels in some real, environmental, biological food and soil samples using 2-hydroxynaphthaldehydebenzoylhydrazone. Int J Curr Res 11(9):7281–7291. https://doi.org/10.24941/ijcr.36413.09.2019
Teixeira L, Costa A, Ferreira S, Freitas M, Carvalho M (1999) Spectrophotometric determination of uranium using 2-(2-Thiazolylazo)-p-Cresol (TAC) in the presence of surfactants. J Braz Chem Soc 10(6):519–522
Gürkan R, İbrahim H, Akçay M (2011) Surfactant enhanced-spectrophotometric determination of uranium (VI) at trace levels by using eriochrome black t as a chelating agent. Eclética Química Journal 36(3):37–46. https://doi.org/10.26850/1678-4618eqj.v36.3.2011.p37-46
Golmohammadi H, Rashidi A, Safdari S (2012) Simple and rapid spectrophotometric method for determination of uranium (VI) in low grade uranium ores using arsenazo (III). Chem Chem Technol 6(3):245–249
Florence T, Farrar Y (1963) Spectrophotometric determination of Thorium(IV) and uranium (VI) in different samples. Anal Chem 35:1613–1616. https://doi.org/10.1021/ac60204a020
Dias F, Bonsucesso J, Oliveira L, Santos W (2012) Preconcentration and determination of copper in tobacco leaves samples by using a minicolumn of sisal fiber (Agavesisalana) loaded with Alizarin fluorine blue by FAAS. Talanta 89:276–279
Chikuma M, Okabayashi Y, Nakagawa T, Inoueb A, Tanakab H (1987) Separation and determination of fluoride ion by using ion exchange resin loaded with alizarin fluorine blue. Chem Pharm Bull 35(9):3734–3739
Davis J (1986) Handbook of statistics and analysis in geology, 2nd edn. Wiley, New York, p 646
Christian G (1994) Handbook of analytical chemistry, vol 2, 5th edn. Wiley, New York, pp 22–26
Leenheer A, Ruyter M, Steyaert H (1976) A method for the statistical evaluation of results in external quality control surveys. Clin Chim Acta 71:229–238. https://doi.org/10.1016/0009-8981(76)90535-0
Job P (1928) Anal Chem 9:113–203
Yoe J, Jons A (1944) Ind Eng Chem Anal Ed 16:111–115
Bale M, Sawant A (2011) Solvent extraction and spectrophotometric determination of uranium (VI) with pyridine- 2-carboxaldehyde 2-hydroxybenzoylhydrazone. J Radioanal Nucl Chem 247:531–534. https://doi.org/10.1023/a:1010626409358
Orabi A, Elenein S, Abdulmoteleb Sh (2019) Amberlite XAD-2010 impregnated with chrome azurol s for separation and spectrophotometric determination of uranium and thorium. Chem Africa 2:673–688. https://doi.org/10.1007/s42250-019-00072-z
Fouad H, Elenein S, Orabi A, Abdulmoteleb Sh (2019) A new extractant impregnated resin for separation of traces of uranium and thorium followed by their spectrophotometric determination in some geological samples. SN Appl Sci 1:309. https://doi.org/10.1007/s42452-019-0325-7
Fouad H, Elrakaiby R, Orabi A (2016) Direct spectrophotometric determination of uranium in granite after its selective leaching using lixiviant reagents without its prior separation. Int J Adv Res 4(4):1134–1144. https://doi.org/10.21474/IJAR01/263
Orabi A (2013) Determination of uranium after separation using solvent extraction from slightly nitric acid solution and spectrophotometric detection. J Radiat Res Appl Sci 6:1–12. https://doi.org/10.1016/j.jrras.2013.09.001
Abd El-Rehim S, Mohammed A, El-Wakil A, Abd Al Aziz R (2017) Int J Adv Res 5:1632–1643. https://doi.org/10.21474/ijar01/4292
Anusuya D, Parashuram L, Govinda C (2016) Direct and derivative spectrophotometric determination of thorium (IV) with 2-hydroxy-3-methoxybenzaldehyde isonicotinoyl hydrazone. Der Pharm Lett 8(9):307–314
Fouad H, Abu Elenein S, Elrakaiby R, Abdelmoteleb Sh (2015) Developed spectrophotometric method for thorium determination in different Rosetta monazite concentrates using thorin dye. Int J Adv Res 3(7):326–336
Nashine N, Mishra R (1995) Spectrophotometric determination of uranium (IV) with thorin and N-hydroxy-N, N0-diphenylbenzamidine. Analyst 120:197–199. https://doi.org/10.1039/AN9952000197
Ueda K, Yoshimura O, Okayama T, Michigami Y (1995) Complexing properties of uranium and lanthanoids with 4-(2-thiazolylazo)-6-chlororesorcinol. Anal Lett 28:157–168
Abu-Eid M, Zater N, Al-Nuri M, Khamis M, Khalaf S (1992) Spectrophotometric determination of uranium in ores using di-2-pyridyl ketone hydrazone derivatives. Spect Lett 24:585–592. https://doi.org/10.1080/00387019208021533
Afifi S, Mustafa M, El Sheikh E, Gado M (2012) Extraction and determination of thorium and its application on geologic samples using trioctyl phosphine oxide. Arab J Nucl Sci Appl 45(3):1–16
Acknowledgements
The author gratefully acknowledges to my institution (Nuclear Materials Authority) for its support and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orabi, A.H., Falila, N.I., Ismaiel, D.A. et al. An innovative spectrophotometric method for determination of uranium and thorium using 3-aminomethylalizarn-N-N diacetic acid in some geological samples. J Radioanal Nucl Chem 327, 239–250 (2021). https://doi.org/10.1007/s10967-020-07496-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07496-9