Abstract
This study described the effectiveness of Bacillus cereus on potato bacterial wilt caused by Ralstonia solanacearum under greenhouse and field conditions, as well as the correlation between the disease-suppression impact of B. cereus and resistance-related enzymes produced in potatoes during infection. Based on the outcomes of the in vitro screening, the bacterial isolate exhibited inhibitory effects against the pathogen R. solanacearum. This manifested as an inhibition zone measuring 16 mm. The bacterial identity was subsequently confirmed through molecular analysis using 16S rRNA gene partial sequencing, which positively identified the isolate as Bacillus cereus. The findings of greenhouse trials demonstrated that the potato plants cv. Berema treated with Bacillus cereus exhibited a significant reduction in the disease severity of bacterial wilt by 80.99% compared to that in the pathogen-inoculated and untreated control. In addition, a significant decrease in disease severity was recorded under field conditions (41.67 and 25.67% in the two seasons). B. cereus treatment significantly increased tuber yield by 70% and 203% in both seasons. Application of Bacillus cereus increased the total phenolic and salicylic acid contents of potato leaves. In addition, the treatment enhanced the activities of defense-related enzymes, including peroxidase, polyphenol oxidase, phenylalanine ammonia-layse, and lipoxygenase, and decreased the catalase activity. The results showed that the disease-suppressive effect of B. cereus on bacterial wilt was significantly positively correlated with the activities of peroxidase, polyphenoloxidase, phenylalanine ammonialayse and lipoxygenase, whereas it was negatively correlated with catalase. These findings suggest that Bacillus cereus has potential for use as a biological control agent against R. solanacearum in potato plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato wilt caused by Ralstonia solanacearum (Yabuuchi et al. 1995), also known as bacterial wilt or brown rot, is a significant challenge in Africa (Charkowski et al. 2020). R. solanacearum is a soil-borne bacterium that can infect potato (Solanum tuberosum) plants through wounds in the roots, leading to wilt and eventually plant death. It is highly contagious and can spread easily through contaminated soil, water, tools, and plant material, making it difficult to control (Bereika et al. 2022).
In Egypt, potato production is an important agricultural activity, and the prevalence of R. solanacearum has become a major concern for potato growers. The bacterium can survive in the soil for long periods of time, making it difficult to eradicate once it becomes established in a field. Infected plants may show symptoms such as wilting, yellowing, and eventual death, which can result in significant yield losses and economic losses for potato farmers (Sallam et al. 2021).
Integrated pest management (IPM) strategies are typically employed to manage potato wilt caused by R. solanacearum. These strategies include crop rotation, planting resistant potato varieties, using certified disease-free seed potatoes, managing irrigation practices to avoid overwatering, and practicing good sanitation measures to prevent the spread of the bacterium (Altaf et al. 2023). In some cases, chemical treatments may also be used, although their usage has decreased due to concerns about environmental impact and resistance development (Okonya and Kroschel 2015). IPM for disease management also involves a combination of approaches, including the use of resistant varieties, clean seeds, and cultural practices such as crop rotation (Abd El-Wahed et al. 2023). These strategies aim to minimize the impact of potato wilt and reduce reliance on chemical pesticides (Barea and Jeffries 1995).
Endophytic microorganisms are important constituents of the plant microbiota and play a vital role in promoting plant growth. These microbes are not pathogenic to plants and live within their tissues for part of their life cycle (Ek-Ramos et al. 2019). Endophytic bacteria such as Bacillus cereus have been reported to promote plant growth through mechanisms such as siderophores, salicylic acid, indole-3-acetic acid, and hydrogen cyanide production (Rahman et al. 2023). Moreover, they can indirectly control plant diseases by acting as antagonists (Compant et al. 2005). Bacillus endophytes are among the most promising microorganisms used in the biological control of plant diseases, including potato wilt (Compant et al. 2010).
In this study, an evaluation was conducted on endophytic bacteria derived from potato plants cultivated in Assiut governorate, Egypt, with the aim of managing potato wilt disease. The evaluation was conducted in vitro, in a greenhouse, and under field conditions. We also investigated the production of siderophores, salicylic acid, indole-acetic acid, and hydrogen cyanide in plants as biochemical responses to defense enzymes that induce resistance against the causal pathogen of potato bacterial wilt. This study offers insight into the potential use of endophytic bacteria as a biological control agent for potato wilt disease in an integrated disease management strategy.
Materials and methods
Bacterial pathogen and growth conditions
Ralstonia solanacearum isolate PHYRS3 was obtained from a previous study (Bereika 2008). The bacterial pathogen isolate was grown on 2,3,5-triphenyl tetrazolium chloride (TZC) and stored at 4 °C (Abd-Alla and Bashandy 2007).
Isolation of the endophytic bacteria
Endophytic bacterial isolates were obtained from healthy potato plants growing in Assiut governorate, Egypt, during the winter of 2021. The surface of the potato stem segments (2 cm) was sterilized in 2 percent sodium hypochlorite for 3 min and then in 70% ethanol for 30 s. The segments were then washed three times with sterile distilled water before being homogenized in 10 ml of acetate buffer (pH 5.2). A loop was used to collect the homogenized plant tissue and streak it on the surface of Petri plates with nutritional agar media (NA) (Mohamed et al. 2020). The plates were then incubated for 48 h at 28 °C. Pure cultures were maintained on NA slants and stored at 4 °C until future use.
Assessment of antagonistic capability of the endophytic bacteria against R. solanacearum
Antagonists and pathogenic bacteria were grown separately in 250 ml Erlenmeyer flasks containing 100 ml of sucrose nutrient broth and incubated at 28 °C for 48 h at 150 rpm. After incubation, the bacterial growth was centrifuged at 10,000×g in sterile microcentrifuge tubes. The supernatant was discarded, and the bacterial cells were harvested. The bacterial cell density was adjusted to 108 CFU/ml using a spectrophotometer (at 600 nm). The antagonistic activity of ten endophytic bacterial isolates against R. solanacearum PHYRS3 was studied using the dual culture method based on a modified method described by Abo-Elyousr et al. (2012). Briefly, 100 μl of R. solanacearum PHYRS3 (108 CFU) was applied to the agar surface. After drying, 100 μl of each antagonist isolate (108 CFU) was individually pipetted into a 5 mm punch line from the same agar inoculated with the pathogen. Streptomycin was used as the positive control. After two days of incubation at 28 °C, the antibacterial effect of the strain was monitored by measuring the diameter of the inhibition zone (mm).
Identification of the potent antagonistic endophytic bacteria
The most effective antagonistic bacterial isolate was chosen for identification by 16 s rRNA sequencing, based on a previous in vitro screening test.
DNA isolation
Genomic DNA was extracted from the endophytic bacterial isolates using a genomic DNA Prep kit (SolGent, Daejeon, Korea) according to the manufacturer’s instructions (Weisburg et al. 1991). The isolated DNA served as a template for the PCR amplification of the 16S rRNA gene. Universal bacterial primers 27F (5′-GTT TGA TCC TGG CTC AG-3) and 1492R (5′-TAC CTT GTT ACG ACT T-3) were used to amplify the complete 16S rRNA gene (Lane 1991).
PCR amplification
PCR amplification was conducted in a reaction volume of 25 μl with 0.4 μM of each primer, 0.75 U of EF-Taq DNA polymerase from SolGent in Daejeon, Korea, 0.2 mM of each dNTP, 10–50 ng of the template DNA, and 1 × EF-Taq reaction buffer. The thermocycling conditions were as follows: initial denaturation at 95 °C for 15 min, followed by 30 cycles of 95 °C for 20 s, 50 °C for 40 s, and 72 °C for 1.5 min, with a final extension step at 72 °C for 5 min. The PCR product was then separated by 1.5% agarose gel electrophoresis containing ethidium bromide with 0.5 × Tris–acetate-EDTA (TAE) buffer and visualized using a UV illuminator (Saiki et al. 1988).
DNA sequencing
The PCR product was purified using a SolGent PCR purification kit (SolGent, Daejeon, Korea), according to the method of Sanger et al. (1977). The amplified 16S rRNA gene was sequenced using an ABI BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, Cal., USA) and an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, Cal., USA). A BLAST search (NCBI) was used to compare the incomplete 16S rRNA gene sequence with the full sequences available in the GenBank database to identify the bacterial strain. The sequences collected from the GenBank database, as well as the sequencing data, were deposited in GenBank under accession numbers. The phylogenetic analysis of the antagonistic bacterial endophyte was performed using the neighbor-joining method in BLAST pairwise alignments (Ulrich et al. 2005).
Effect of Bacillus cereus on diseases severity under greenhouse conditions
During the 2022 growing season, greenhouse trials were conducted in an open-air Experimental Greenhouse of Plant Pathology Department, Faculty of Agriculture, Assiut University, Assiut, Egypt. Potato seed tubers cv. Berema were surface-sterilized by soaking for 3 min in 2% sodium hypochlorite, rinsed completely with sterilized distilled water, and planted immediately in sterilized experimental pots (25 cm diameter) filled with 5 kg sterilized sandy-clay soil (3:1 w/w). Every 15 days, the plants were fertilized with 2 g of urea per pot and watered as needed. Four replicates were set up for each treatment. After 45 days of planting, sterilized knives were used to cut the roots of potato plants along two sides (4–5 cm deep), and 20 ml of R. solanacearum PHYRS3 suspension (108 CFU/ml) was added around the bases of each plant (Abd El-Wahed et al. 2023). Plants were inoculated with 20 ml sterile distilled water as a control. All potato plants were kept in a moist chamber at 25 °C for 2 days after inoculation. Two days after inoculation with the pathogen, 20 ml of B. cereus (108 CFU/ml) was added to the bases of the plants. Symptom development of bacterial wilt was observed after six weeks, and disease severity was recorded.
Disease assessment
Disease severity was recorded using the scale of Kempe and Siqueira (1983) follows: 0 = no symptoms, 1 = 1–25% of leaves wilted, 2 = 26–50% of leaves wilted, 3 = 51–75% of leaves wilted, 4 = more than 75%, less than 100% of leaves wilted, and 5 = all leaves wilted and died. The following equation was used to compute the percentage of disease severity, the disease severity percentage (DS%) was calculated according to the formula described by Kempe and Siqueira (1983).
where ds is the disease rating for each plant, dsmax is the maximum disease rating possible, and n is the total number of plants observed in each replicate.
The disease-suppression impact of Bacillus cereus under field conditions
The experiments were conducted at the experimental farm of the Faculty of Agriculture, Assiut University, Egypt. The treatment included four replicates, and the experiment was distributed in a completely randomized block design. The experimental plot area was 25 m2, consisting of five rows, each row was 4.5 m in length and separated by 0.5 m. Potato seed tubers cv. Berema were sowed 0.4 m apart in the center of the ridge. Thirty days after planting, 20 ml of B. cereus was drenched individually around each plant 48 h before inoculation with R. solanacearum PHYRS3 (108 cell/ml), as described in the greenhouse experiment. The plants were treated with 20 ml of distilled water as the infected control; in the healthy control, plants were not infected with the pathogen (Abo-Elyousr et al. 2017). Six weeks after inoculation, the disease severity was determined according to Kurabachew and Wydra (2013). At harvest time (approximately 110 days after planting), ten plants from each replicate were randomly selected to measure the total tuber yield (kg) per plant.
Biochemical analyses
The impact of B. cereus treatment on biochemical changes in potato plants infected with R. solanacearum PHYRS3 was examined. Total phenol content, salicylic acid, and enzyme activities were determined in potato plant leaves samples obtained at zero time, 2, 4, 6, and 8 days after inoculation.
Enzymes activities
To determine the activity of peroxidase (PO), polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL), catalase (CAT), lipoxygenase (LO), One-gram fresh weight of potato plant leaves were treated with liquid nitrogen and homogenized with 10 ml of 0.1 M Na-acetate buffer (pH 5.2). The mixture was centrifuged at 1000×g for 30 min at 4 °C, and enzyme activities were determined in the supernatants (Rapp and Ziegler 1973). Four replicates were used for each treatment.
Peroxidase activity (PO)
The activity of peroxidase was measured spectrophotometrically according to Putter (1974), using guaiacol as a substrate. The reaction mixture consisted of 0.2 ml supernatant, 1 ml of 0.1 M Na-acetate-buffer (pH 5.2), 0.2 ml of 1% guaiacol and 0.2 ml of 1% H2O2 after that incubated at 25 °C for 5 min, and then the absorbance was measured at 436 nm. The extraction buffer was used as blank. The change in absorbance was used to quantify enzyme activity, which was represented as enzyme per 1 mg protein.
Polyphenol oxidase (PPO) activity
Polyphenol oxidase was measured as proposed by Batra and Kuhn (1975) in a spectrophotometer (model 6405UV/VIS), recording changes in absorbance (410 nm) using 100 mM 4-methylcatechol as substrate and 0.2 M phosphate buffer pH 6.5 and represented as enzyme per 1 mg protein.
Phenylalanine ammonia-lyase (PAL) activity
Phenylalanine ammonia-lyase activity was determined by mixing 0.5 ml of the supernatant, 2 ml of 50 mM Na-borate/HCl buffer (pH 8.8), Mercapto ethanol, and 1 ml of 60 mM phenylalanine, followed by incubation at 37 °C for 2 h. The absorbance was measured spectrophotometrically at 290 nm using cinnamic acid as the standard (Silva et al. 2004).
Catalase activity
The catalase activity was determined according to the method described by Aebi (1984). The reaction mixture of 3 ml consisted of 0.05 ml extract, 1.5 ml phosphate buffer (100 mM buffer, pH 7.0), 0.5 ml H2O2, and 0.95 ml distilled water. Catalase activity was measured spectrophotometrically at 240 nm and expressed as μmol of H2O2 oxidized per minute per gram of FW.
Lipoxygenase activity
Lipoxygenase activity was determined by spectrophotometric measurement of the formation of conjugated dienes at 234 nm produced from linoleic acid (10 mM sodium linoleate; pH = 9) according to the method described by Axelrod et al. (1981). The LOX assay mixture consisted of 1 ml of 50 mM sodium phosphate buffer (pH 6), 20 µL substrate, and 10 µL plant extract. Absorbance of the reaction mixture was recorded every 30 s for 3 min. A mixture containing the substrate and buffer was used as the blank for each sample. The activity was calculated using an extinction coefficient of 25 mM−1 cm−1.
Investigation of total phenol and salicylic acid contents
Preparation of samples
One gram of potato leaves was mashed in liquid nitrogen and mixed with 10 ml of 80% methanol. The samples were centrifuged for 30 min at 4 °C and 1000×g. After the addition of ascorbic acid (0.1 g/5 ml), the pellet was discarded. The mixture was evaporated in a rotary evaporator for 5 min at 65 °C, and the procedure was repeated thrice. The residues were dissolved in 5 ml 80% methanol (Rapp and Zeigler 1973).
Total phenols content
Phenol content was measured using the Folin-Ciocalteu reagent, as described by Rapp and Ziegler (1973). The reaction mixture made of 0.02 ml methanol extract, 0.5 ml Folin reagent, 0.75 ml of 20% Na2CO3 solution and 8 ml water then incubated for one hour at 37 °C in water bath. The absorbance of the solution was measured spectrophotometrically at 767 nm, and the results were expressed as mg/g plant fresh weight using gallic acid as the standard. A blank sample containing methanol and the reagents was used as the negative control.
Salicylic acid content
Salicylic acid content was measured using a spectrophotometer at 254 nm and expressed as µg salicylic acid/g plant material, according to the modified method described by Scott and Yamamoto (1994). A 500 µl homogenate sample was mixed with 250 µl of 10 N HCl and 1000 µl methanol, and then incubated in a water bath at 80 °C for 2 h. Next, the sample was neutralized with 4–5 drops of 1 M NaHCO3 and 1000 µL methanol was added to the mixture.
Statistical analysis
All statistical analyses were carried out using the statistical package SPSS software version 27 (SPSS Inc., Chicago, IL, USA) and subjected to mean separation by the least significant difference (LSD) test (P ≤ 0.05). Correlations between the disease-suppression effect of Bacillus cereus on bacterial wilt and biochemical changes in potato plants were performed using bivariate Pearson’s test at P ≤ 0.05 (Gomez and Gomez 1984).
Results
Antibacterial activity of some entophytic bacteria against the pathogen
Ten unknown isolates of bacterial endophytes were evaluated against R. solanacearum PHYRS3 using the dual culture method, and the results showed that only one isolate had an inhibitory effect against the pathogen, causing an inhibition zone of 16 mm compared with streptomycin (1.0 mg/ml), which caused an inhibition zone of 20 mm (Table 1).
Identification of the potent bacterial endophyte
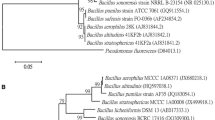
According to the in vitro screening, the effective isolate was molecularly identified using 16S rRNA gene partial sequencing. The obtained sequence was submitted to GenBank under accession number OR484917. A phylogenetic analysis was performed using the maximum likelihood method in BLAST pairwise alignments. The isolate was identified as Bacillus cereus with 100% identity and 99% query coverage (Fig. 1).
Disease suppression capacity of Bacillus cereus on Ralstonia solanacearum-inoculated potato plants
Greenhouse trial results showed that potato bacterial wilt severity, recorded 42 days post-inoculation, varied significantly depending on treatment with Bacillus cereus. As shown in Fig. 2, a significant decrease in disease severity by 80.99% over pathogen-inoculated and untreated controls was observed in potato plants cv. Berema infected with R. solanacearum and treated with B. cereus. In addition, a significant decrease in disease severity was observed in potato plants treated with B. cereus under field conditions. Decreases in bacterial wilt severity, of 41.67 and 25.67% were noted in potato plants treated with B. cereus in the 2020/2021 and 2021/2022 seasons, respectively (Fig. 3).
Effect of Bacillus cereus on potato bacterial wilt after 87 days post-sowing in Ralstonia solanacearum-inoculated potato cv. Berema compared with untreated controls under open-air greenhouse conditions. UC: Uninoculated and untreated controls; IC: Ralstonia solanacearum-inoculated and untreated controls. Results are presented as mean ± SE (n = 10, P ≤ 0.05). Bars sharing the same letter are not significantly different according to the least significant difference (LSD) test (P ≤ 0.05)
Effect of Bacillus cereus on potato bacterial wilt after 87 days post-sowing in Ralstonia solanacearum-inoculated potato cv. Berema, compared to untreated controls under field conditions. UC: Uninoculated and untreated control; IC: Ralstonia solanacearum inoculated and untreated control. Results are presented as mean ± SE (n = 10, P ≤ 0.05). Bars sharing the same letter are not significantly different according to least significant difference (LSD) test (at P ≤ 0.05)
Effect of Bacillus cereus treatment on tuber yield of potato under field conditions
As presented in Fig. 4, treatment with Bacillus cereus resulted in a significant increase in tuber yield by 70% and 203% in both seasons, respectively (40.75 and 18.58 ton ha−1, respectively), in pathogen-inoculated and untreated controls.
Effect of Bacillus cereus on tube yield of potato cv. Berema, under field conditions. UC: Uninoculated and untreated control; IC: Ralstonia solanacearum-inoculated and untreated control. Results are presented as mean ± SE (P ≤ 0.05). Bars sharing the same letter are not significantly different according to least significant difference (LSD) test (at P ≤ 0.05)
The influence of Bacillus cereus on production of activate defense-related metabolites in potato plant
Salicylic acid
Figure 5 shows a significant increase in the salicylic acid content of potato plants compared with uninoculated and untreated controls, and Ralstonia solanacearum-inoculated and untreated controls. SA accumulation increased 2 days after inoculation of plants with the bioagents until the 8th day, and SA content in plants (4.033, 3.7, 3.74 and 3.682 µg/g, respectively).
Effect of Bacillus cereus application on salicylic acid content in potato plants after inoculation with Ralstonia solanacearum PHYRS3. UC: Uninoculated and untreated control; IC: Ralstonia solanacearum-inoculated and untreated control. Results are presented as mean ± SE (P ≤ 0.05). Columns with the same letter are not significantly different (P ≤ 0.05)
Total phenol content
Figure 6 displays that the total phenol content of potato plants treated with the endophytic bacteria was higher than that of uninoculated and untreated control and Ralstonia solanacearum-inoculated and untreated control plants after two days (4.2, 4.61, 5.1 and 5.01 mg/g, respectively).
Effect of Bacillus cereus application on total phenol contents (TPC) in potato plants after inoculation with Ralstonia solanacearum PHYRS3. UC: Uninoculated and untreated control; IC: Ralstonia solanacearum-inoculated and untreated control. Results are presented as means ± SE (P ≤ 0.05). Columns with the same letter are not significantly different (P ≤ 0.05)
Enzymatic activities
Application of Bacillus cereus led to a significant increase (P ≤ 0.05) in peroxidase, polyphenol oxidase, phenylalanine ammonia-layer, and lipoxygenase in potato plant leaves compared with the control treatments (Table 2). The leaves of potato plants treated with B. cereus had peroxidase 2.65 and 3.95 (unit/mg protein) 2 and 4 days after application, respectively. Also, the level of polyphenol oxidase was 0.67 (unit/mg protein) after 2 and 4 days from application. In addition, the levels of phenylalanine ammonia-layse were 122.6 and 85 (unit/mg protein) 2 and 4 days after application, respectively. Moreover, the leaves of the same treatment group had lipoxygenase 3.1, 3.2, 1.5 and 1.9 (unit/ mg protein) after 2, 4, 6, and 8 days. In addition, the same treatment resulted in a significant decrease (P ≤ 0.05) in catalase by 2.585 (unit/mg protein) after 4 days of application.
Correlation between the disease-suppression impact of Bacillus cereus and resistance-related enzymes in potato
The correlation coefficient between the disease-suppressive effect of B. cereus on bacterial wilt and resistance-related enzymes in potato plants was analyzed (Table 3). The results indicated that the disease-suppressive effect of B. cereus on bacterial wilt was significantly positively correlated with the activities of peroxidase, polyphenoloxidase, phenylalanine ammonia-lyse, and lipoxygenase (P ≤ 0.01), whereas it was negatively correlated with catalase (P ≤ 0.01).
Discussion
This study aimed to identify a bacterial endophyte that can control Ralstonia solanacearum in potato plants. Ten bacterial endophytes were isolated and evaluated using the dual culture method, and only one isolate showed an inhibitory effect against R. solanacearum. The isolate was identified as Bacillus cereus by partial sequencing of its 16S rRNA gene (Clarridge 2004). The inhibitory ability of B. subtilis was demonstrated to be significantly higher than that of other bacteria (Basha et al. 2017). Previous studies have shown that B. cereus produces antimicrobial compounds, such as antibiotics and antifungal metabolites, which inhibit the growth and development of R. solanacearum (Köberl et al. 2013). The srfA gene of B. subtilis was found to be related to the inhibition of R. solanacearum, with significant changes in its transcription (Li et al. 2022). Previous studies have suggested that B. subtilis can be used as a biocontrol agent to effectively inhibit the growth of R. solanacearum through a bacteriostatic mechanism (Prihatiningsih et al. 2015, 2020). Additionally, B. subtilis has been suggested to provide long-lasting protection against R. solanacearum in crops (Huang et al. 2016). The formulation of B. subtilis as a biocontrol agent should be considered in future field studies to suppress R. solanacearum wilt disease (Chandrasekaran et al. 2016). The results of this study are essential because R. solanacearum is a pathogen responsible for bacterial wilt in potato plants, which causes significant losses in yield and quality. Our greenhouse and field trials demonstrated that B. cereus significantly reduces the severity of bacterial wilt in potato plants infected with R. solanacearum. Additionally, the application of B. cereus led to a significant increase in tuber yield in both the seasons, with a 203% increase in the second season. These findings demonstrated that B. cereus has a remarkable ability to control bacterial wilt caused by R. solanacearum in potato plants. Previous studies have indicated that B. subtilis B315 can be used for biocontrol of bacterial wilt and promotion of potato growth (Prihatiningsih et al. 2015). The use of B. cereus as a biological control agent in agriculture has several advantages. First, it is environmentally friendly and does not involve the use of harmful chemicals. Bacillus cereus is a naturally occurring bacterium that is safe for human consumption, making it an attractive alternative to chemical pesticides. Lastly, B. cereus is known to have beneficial effects on plant growth and development, making it a suitable candidate for use in sustainable agriculture. Previous investigations reported that B. subtilis has been shown to be an effective biocontrol agent against R. solanacearum in crops, and further studies are needed to optimize its mode of application and formulation for agricultural use (Basha et al. 2017; Puspita Saridewi et al. 2020; Prihatiningsih et al. 2015; Prihatiningsih et al. 2020 and Prihatiningsih et al. 2021). This study also investigated the effect of B. cereus on the production of activated defense-related metabolites in potato plants. Lin et al. (2020) reported that Bacillus cereus enhances the production of defense-related reactive oxygen species and callose deposition in potato plants. Bacillus strains have also been shown to control plant diseases by producing secondary metabolites (Hassan et al. 2009 and Ramarathnam et al. 2007). The results of the current study showed that the application of B. cereus increased salicylic acid and total phenol contents in potato plants. Moreover, it led to a significant increase in the activities of peroxidase and polyphenol oxidase. Prior studies have suggested that B. cereus can regulate salicylic acid (SA) signaling pathways in plants, which could play a role in controlling bacterial wilt disease (Yang et al. 2023). The results of this study also indicate that the application of B. cereus to potato plants can enhance the production of activated defense-related metabolites, such as salicylic acid and total phenol, which are known to be involved in plant defense mechanisms. Furthermore, the significant increase in the activities of peroxidase and polyphenol oxidase observed in this study suggests that B. cereus can activate the plant’s defense system against R. solanacearum. This is in line with the findings of Dutta et al. (2013), who demonstrated that B. cereus strains have been shown to produce bioactive metabolites that enhance defense-related enzymes and metabolites in plants, thereby potentially controlling diseases. This is in agreement with previous studies on the role of small RNAs in plant immune responses (Niu et al. 2016). In conclusion, the findings of this study provide a promising strategy for controlling bacterial wilt caused by R. solanacearum in potato. The use of B. cereus as a biological control agent offers many advantages, and can lead to the development of sustainable agricultural practices. Further studies are needed to investigate the effectiveness of B. cereus in controlling other plant pathogens, and to determine its potential for commercial use in agriculture.
References
Abd El-Wahed MH, Bereika MF, Abo-Elyousr KA, Almasoudi NM (2023) Integration of Pseudomonas fluorescens and Rosemarinus officinalis for controlling of potato bacterial wilt. Egypt J Biol Pest Control. https://doi.org/10.1186/s41938-023-00677-0
Abd-Alla MH, Bashandy SR (2007) Bacterial wilt and spot of tomato caused by Xanthomonas Vesicatoria and Ralstonia solanacearum in Egypt. World J Microbiol Biotechnol 24(2):291–292. https://doi.org/10.1007/s11274-007-9385-8
Abo-Elyousr KA, Ibrahim YE, Balabel NM (2012) Induction of disease defensive enzymes in response to treatment with acibenzolar-S-methyl (ASM) and pseudomonas fluorescens PF2 and inoculation with Ralstonia solanacearum race 3, biovar 2 (phylotype II). J Phytopathol 160(7–8):382–389. https://doi.org/10.1111/j.1439-0434.2012.01915.x
Abo-Elyousr KAM, Seleim MEA, El-sharkawy RM, Khalil-Bagy HMM (2017) Effectiveness of Egyptian propolis on control of tomato bacterial wilt caused by Ralstonia solanacearum. J Plant Dis Prot 124(5):467–472. https://doi.org/10.1007/s41348-017-0120-x
Aebi H (1984) [13] catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Altaf M, Sharma N, Singh J, Samota MK, Sankhyan P, Singh B, Kumar R (2023) Mechanistic insights on melatonin-mediated plant growth regulation and hormonal cross-talk process in solanaceous vegetables. Sci Hortic 308:111570. https://doi.org/10.1016/j.scienta.2022.111570
Axelrod B, Cheesbrough TM, Laakso S (1981) [53] lipoxygenase from soybeans. Methods Enzymol 71:441–451. https://doi.org/10.1016/0076-6879(81)71055-3
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil-plant systems. Mycorrhiza. https://doi.org/10.1007/978-3-662-08897-5_23
Basha CRJ, Manjula CP, Kumar MKP (2017) Management of bacterial wilt of tomato caused by Ralstonia solanacearum by bacterial antagonists and botanicals. Int J Plant Sci 12(2):114–119
Batra GK, Kuhn CW (1975) Polyphenoloxidase and peroxidase activities associated with acquired resistance and its inhibition by 2-thiouracil in virus-infected soybean. Physiol Plant Pathol 5(3):239–248. https://doi.org/10.1016/0048-4059(75)90090-9
Bereika MFF (2008) Studies on induction of resistance against potato brown rot caused by Ralstonia solanacearum. M.Sc. Thesis, Faculty of Agriculture, Assiut University, Egypt, p 142
Bereika MF, Moharam MH, Abo-Elyousr KA, Asran MR (2022) Investigation of virulence diversity in Ralstonia solanacearum isolates by a random amplified polymorphic DNA collected from Egyptian potato fields. Arch Phytopathol Plant Prot 55(10):1201–1218. https://doi.org/10.1080/03235408.2022.2081770
Chandrasekaran M, Subramanian D, Yoon E, Kwon T, Chun SC (2016) Meta-analysis reveals that the genus pseudomonas can be a better choice of biological control agent against bacterial wilt disease caused by Ralstonia solanacearum. Plant Pathol J 32(3):216–227. https://doi.org/10.5423/ppj.oa.11.2015.0235
Charkowski AO, Sharma K, Parker M, Secor GA, Elphinstone JG (2020) Bacterial diseases of potato. Potato Crop. https://doi.org/10.1007/978-3-030-28683-5_10
Clarridge JE (2004) Impact of 16S RNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17(4):840–862. https://doi.org/10.1128/cmr.17.4.840-862.2004
Compant S, Duffy B, Nowak JZ, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959. https://doi.org/10.1128/aem.71.9.4951-4959.2005
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Dutta S, Morang P, Nishanth Kumar S, Dileep Kumar BS (2013) Fusarial wilt control and growth promotion of pigeon pea through bioactive metabolites produced by two plant growth promoting rhizobacteria. World J Microbiol Biotechnol 30(3):1111–1121. https://doi.org/10.1007/s11274-013-1532-9
Ek-Ramos MJ, Gomez-Flores R, Orozco-Flores AA, Rodríguez-Padilla C, González-Ochoa G, Tamez-Guerra P (2019) Bioactive products from plant-endophytic gram-positive bacteria. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00463
Gomez K, Gomez A (1984) Statistical procedures for agricultural research, 2nd edn. Wiley, New York, p 680
Hassan MAE, Bereika MFF, Abo-Elnaga HIG, Sallam MAA (2009) Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. Plant Pathol J 25(4):352–360. https://doi.org/10.5423/ppj.2009.25.4.352
Huang CN, Lin CP, Hsieh FC, Lee SK, Cheng KC, Liu CT (2016) Characterization and evaluation of bacillus amyloliquefaciens strain WF02 regarding its biocontrol activities and genetic responses against bacterial wilt in two different resistant tomato cultivars. World J Microbiol Biotechnol 32:183. https://doi.org/10.1007/s11274-016-2143-z
Kempe J, Sequeira L (1983) Biological control of bacterial wilt of potatoes: attempts to induce resistance by treating tubers with bacteria. Plant Dis 67(5):499. https://doi.org/10.1094/pd-67-499
Köberl M, Ramadan EM, Adam M, Cardinale M, Hallmann J, Heuer H, Berg G (2013) Bacillus and Streptomyces were selected as broad-spectrum antagonists against soilborne pathogens from arid areas in Egypt. FEMS Microbiol Lett 342(2):168–178. https://doi.org/10.1111/1574-6968.12089
Kurabachew H, Wydra K (2013) Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacearum. Biol Control 67(1):75–83. https://doi.org/10.1016/j.biocontrol.2013.07.004
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, pp 115–175
Li Q, Ndayambaje JP, Qian X, Jin L, Jia Q, Liu M, Hu X, Chen J (2022) Transcriptome analysis of biocontrol strain bacillus subtilis pro-2 and its mutants. J Biobased Mater Bioenergy 16(2):191–197. https://doi.org/10.1166/jbmb.2022.2164
Lin CH, Lu CY, Tseng AT, Huang CJ, Lin YJ, Chen CY (2020) The ptsG gene encoding the major glucose transporter of Bacillus cereus C1L participates in root colonization and beneficial metabolite production to induce plant systemic disease resistance. Mol Plant Microbe Interact 33(2):256–271. https://doi.org/10.1094/mpmi-06-19-0165-r
Mohamed BFF, Sallam NM, Alamri SA, Abo-Elyousr KA, Mostafa YS, Hashem M (2020) Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt J Biol Pest Control 30:61. https://doi.org/10.1186/s41938-020-00262-9
Niu D, Xia J, Jiang C, Qi B, Ling X, Lin S, Zhang W, Guo J, Jin H, Zhao H (2016) Bacillus cereus AR156 primes induced systemic resistance by suppressing miR825/825* and activating defense-related genes in Arabidopsis. J Integr Plant Biol 58(4):426–439. https://doi.org/10.1111/jipb.12446
Okonya JS, Kroschel J (2015) A cross-sectional study of pesticide use and knowledge of smallholder potato farmers in Uganda. Biomed Res Int 2015:1–9. https://doi.org/10.1155/2015/759049
Prihatiningsih N, Arwiyanto T, Hadisutrisno B, Widada J (2015) Antibiosis mechanism of Bacillus subtilis B315 for controlling potato bacterial wilt disease. J Trop Plant Pests Dis 15:64–71. https://doi.org/10.23960/j.hptt.11564-71
Prihatiningsih N, Arwiyanto T, Hadisutrisno B, Widada J (2020) Characterization of Bacillus spp. from the rhizosphere of potato granola varieties as an antibacterial against Ralstonia solanacearum. Biodiversitas J Biol Divers 21(9):4199–4204. https://doi.org/10.13057/biodiv/d210934
Prihatiningsih N, Asnani ARI, Djatmiko HE (2021) Extracellular protease from bacillus subtilis B315 with antagonistic activity against bacterial wilt pathogen (Ralstonia solanacearum) of chili. Biodivers J Biol Divers 22(3):1291–1295. https://doi.org/10.13057/biodiv/d220327
Puspita Saridewi L, Prihatiningsih N, Adi Djatmiko H (2020) Characterization of eggplant endophyte bacteria and rhizobacteria as well as their antagonistic ability against Ralstonia solanacearum. J Trop Plant Pests Dis 20(2):150–156. https://doi.org/10.23960/jhptt.220150-156
Putter J (1974) Peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhan, pp 685–690
Rahman M, Borah SM, Borah PK, Bora P, Sarmah BK, Lal MK, Kumar R (2023) Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1141506
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuewen G, de Kievit T (2007) Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53(7):901–911. https://doi.org/10.1139/w07-049
Rapp A, Ziegler A (1973) Bestimmung der Phenolcarbonsaure in Rebblattern Weintraube und Wein mittels Polamyid-Dunnschicht Chromatographie. Vitis 12:226–236
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239(4839):487–491. https://doi.org/10.1126/science.2448875
Sallam NM, Ali EF, Abo-Elyousr KA, Bereika MF, Seleim MA (2021) Thyme oil treatment controls bacterial wilt disease symptoms by inducing antioxidant enzyme activity in solanum tuberosum. J Plant Pathol 103(2):563–572. https://doi.org/10.1007/s42161-021-00808-2
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 74(12):5463–5467. https://doi.org/10.1073/pnas.74.12.5463
Scott IM, Yamamoto H (1994) Mass spectrometric quantification of salicylic acid in plant tissues. Phytochemistry 37(2):335–336. https://doi.org/10.1016/0031-9422(94)85056-9
Silva HS, Romeiro R, Macagnan D, Halfeld-Vieira B, Pereira MC, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: non-specific protection and increase in enzyme activities. Biol Control 29(2):288–295. https://doi.org/10.1016/s1049-9644(03)00163-4
Ulrich LE, Zhang F, Zhulin IB (2005) One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 13(2):52–56. https://doi.org/10.1016/j.tim.2004.12.006
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16s ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia genus nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Douderoff 1973) comb.nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol 39:897–904. https://doi.org/10.1111/j.1348-0421.1995.tb03275.x
Yang B, Zheng M, Dong W, Xu P, Zheng Y, Yang W, Luo Y, Guo J, Niu D, Yu Y, Jiang C (2023) Plant disease resistance-related pathways recruit beneficial bacteria by remodeling root exudates upon Bacillus cereus AR156 treatment. Microbiol Spectr 11(2):e03611-e3622. https://doi.org/10.1128/spectrum.03611-22
Acknowledgements
This research work was Funded by Institutional Fund Project under grant no “IFPIP: 36-155-1443.” The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Funding
This research work was Funded by Institutional Fund Project under grant no “IFPIP: 36-155-1443” by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent statement
Not applicable.
Institutional review board statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seleim, M.A.A., Bereika, M.F.F., Ibrahim, O.H.M. et al. Effectiveness of Bacillus cereus in controlling potato bacterial wilt caused by Ralstonia solanacearum: greenhouse and field studies with insights into resistance-related enzymes in potatoes. J Plant Dis Prot 131, 65–75 (2024). https://doi.org/10.1007/s41348-023-00810-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00810-z