Abstract
The bioactive metabolites produced by two plant growth promoting rhizobacteria strains, a Pseudomonas aeruginosa strain RRLJ 04 and a Bacillus cereus strain BS 03, which showed growth promotion and disease control in pigeon pea against Fusarium udum, were isolated and screened for their efficacy to control fusarial wilt of pigeon pea under gnotobiotic and nursery condition. Bioactive metabolites viz., BM 1 and BM 2 from RRLJ 04 and BM 3 from BS 03 also showed in vitro antibiosis against F. udum. Seeds treated with 50 μl seed−1 of BM 1, 30 μl seed−1 of BM 2 and 70 μl seed−1 of BM 3 and grown in pathogen infested soil showed suppression of wilt disease besides growth enhancement. Per cent disease control was 90 % with BM 2 application as compared to 87 and 83 %, respectively in BM 1 and BM 3 after 90 days of growth. BM 2 treated plants were more resistant to the pathogen as compared to the other fractions tested. Mycelial dry weight was found to be reduced on treatment with the bioactive metabolites. Formation of chlamydospore-like structures was observed in the pathogen mycelium treated with BM 3. The analytical studies confirmed that two of these metabolites are phenazine derivatives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Utilization of plant growth promoting rhizobacteria (PGPR) is an eco-friendly method adopted for crop improvement and disease control in modern agricultural practices. The strains belonging to the genus of Bacillus and Pseudomonas have been mostly utilized for biomass improvement, yield enhancement and management of diseases in many agriculturally important crop plants (Compant et al. 2005; Cook 2007; Dutta et al. 2008; Dutta and Podile 2010: Bhattacharyya and Jha 2012; Perez et al. 2012) Production of secondary metabolites with antibiotic activity in several strains of fluorescent pseudomonads has been recognized as a major factor in suppression of root pathogens (Dwivedi and Johri 2003). Similarly, certain compounds produced by bacilli exhibited inhibitory activities against several plant pathogens (GaoFen et al. 2004; Chaurasia et al. 2005). The role of siderophores and phenazine type antibiotics produced by the fluorescent Pseudomonas species in growth promotion and/or disease control been reported (Defago and Haas 1990; Sharma and Johri 2003). However, the utilization of the metabolites produced by the PGPR strains has not attained the momentum, as it deserves.
Vascular wilt of pigeon pea, caused by Fusarium udum, is an agriculturally important disease in many parts of the world, particularly in Asian and African continents (Kiprop et al. 2002). Efficient high-yielding, disease resistant varieties are still lacking to tackle this problem (Reddy et al. 1990). The pathogen is soil borne and chemical control is impractical in established cases. Several cultural practices are recommended but the disease still poses a serious threat to this important pulse crop.
In this study, we examined the efficacy of three metabolites produced by a Pseudomonas aeruginosa strain RRLJ 04 and a Bacillus cereus strain BS 03 on growth promotion and fusarial wilt control in pigeon pea.
Materials and methods
Soil
The experiments were conducted on sandy–loam soil with pH 6.10, total nitrogen 0.015 %, organic carbon 0.268 % and have no previous history of any pesticide or synthetic agrochemical application.
Organisms
Two bacterial strains, P. aeruginosa RRLJ 04 (MTCC 7277) and a B. cereus strain BS 03 (MTCC 7278), which showed plant growth promotion and suppression of fusarial wilt in pigeon pea on our earlier investigation (Dutta et al. 2005), was selected for this study. The confirmatory identification of the strains was done from the Microbial Type Culture Collection and Gene Bank (MTCC) Division of Institute of Microbial Technology (CSIR), Chandigarh, India. The fungal pathogen F. udum was procured from International Crop Research Institute for Semi-Arid Tropics (ICRISAT), Hyderabad, India.
Extraction of bioactive metabolites from pseudomonad strain RRLJ O4
The bioactive metabolites produced by strain RRLJ 04 were extracted according to Dileep Kumar and Bezbaruah (1997). For this, a lawn of RRLJ 04 was grown in King’s B media for 7 days at 28 ± 2 °C. Then the bluish-green bacterial grown medium was cut into small pieces (≈1 cm2) and extracted with 80 % aqueous acetone till the medium turned into colourless. The extract was then filtered through a double layer of cheese cloth and the acetone was removed under vaccum pressure through a rota evaporator. The resultant aqueous part was then treated with sodium chloride (50 g l−1) and centrifuged at 11,000 rev min−1 for 20 min. The supernatant was collected and further extracted with diethyl ether (3:1 v/v) in a separating funnel. The resultant aqueous fraction then treated with chloroform (3:1 v/v) and the chloroform fraction was collected. The yellowish diethyl ether (BM 1) and the bluish green chloroform fractions (BM 2) were concentrated to get dried extracts.
Extraction of bioactive metabolites from bacilli strain BS 03
The bioactive compounds from BS 03 were extracted according to Kajimura et al. (1995). The strain was grown in potato dextrose broth (PDB) for 48 h was centrifuged at 8,000 rev min−1 and the supernatant was collected and extracted twice with equal volumes of n-butanol. The n-butanol extracts were combined and concentrated to give an oily residue. The residue was added to 20 ml of silica gel and slurried with methanol. After evaporating the methanol, the resulting powder was applied on top of a silica gel column (1.5 × 25 cm) that had been pre-packed with chloroform–methanol (3:1 v/v). The column was eluted stepwise with mixtures of chloroform–methanol from 3:1 to 1:1. Elutes thus obtained were combined (BM 3) and concentrated to get crude powder.
Characterization of bioactive metabolites
Crude extract of BM 1, BM 2 and BM 3 were further purified by thin layer chromatography (TLC) and the purified band were subjected to IR, 1H NMR, 13C NMR and mass spectrum studies for characterization studies.
In vitro antibiosis studies with bioactive metabolites
The experiments were conducted on three different media viz. Potato dextrose agar (PDA), Nutrient agar (NA) and King’s B medium (KB). For this, an actively growing mycelial disc (≈6 mm2) of F. udum was placed on the center of a Petri plate containing respective media. Sterilized filter paper discs (5 mm diameter) were impregnated with 50 μl of different concentrations of metabolites (0, 10, 20 mg bioactive metabolite in 100 ml−1 methanol) and two such discs were placed on either side of the mycelial disc at equal distances. Sterilized filter paper disc dipped in only methanol served as control. The plates were then incubated at 28 ± 2 °C and inhibition zone was measured as distance (in cm) between the filter paper disc and mycelium of the fungus after 7 days of growth.
Growth of the pathogen in media amended with different concentrations of bioactive metabolites
Stock solutions of BM 1, BM 2 and BM 3 were prepared to give 5 % solution in sterile distilled water. PDA and PDB amended with different concentrations of respective bioactive metabolites (0, 5, 10, 20, 30, 40 and 100 μl stock solution in 100 ml−1 media) were introduced with a mycelial disc (≈6 mm2) of F. udum and the radial growth in PDA and dry weight of mycelium in PDB were recorded after 7 days of growth.
Effect of bioactive metabolites under gnotobiotic condition
The gnotobiotic system consisted of a glass tube of 26 cm long and 1.5 cm in diameter, connected with a 100 ml flask at the bottom. The tube was filled with sand up to 12 cm by securing it with a piece of muslin cloth at the bottom of the tube. The tube was then fixed tightly to a conical flask containing Hoffland’s Plant Nutrient Solution (PNS) consisting 5 mM Ca(NO3)2; 5 mM KNO3; 2 mM MgSO4.7H20; 1 mM KH2PO4 and micronutrient (g l−1) MnSO4—0.61; ZnSO4—0.50; H3BO3—1.27; Na2MoO4—0.40; CuSO4—0.20. The bottom portion of the tube was immersed in the nutrient solution to keep the sand column moist. The whole system was autoclaved at 120 °C for 15 min before the use.
Optimization of the concentration of the metabolites was done by treating the seeds with 10, 20, 30, 40 and 50 μl of bioactive metabolite stock solution (20 mg 100 ml−1) and placing in Petri dishes containing sterile filter paper soaked with PNS. The concentration at which the seeds were able to survive and showed emergence of shoot and root were taken as the optimum concentration. Seeds with optimum concentration of respective treatments (BM 1, BM 2 and BM 3) were aseptically transferred approximately 5 mm below the surface of the sand column. For disease control studies, 2.0 ml of the conidial suspension (containing 1.0 × 107 conidia ml−1) was given in the sand column. The treatments taken were as below:
-
1.
Control
-
2.
BM 1
-
3.
BM 2
-
4.
BM 3
-
5.
F. udum
-
6.
BM 1 + F. udum
-
7.
BM 2 + F. udum
-
8.
BM 3 + F. udum
Shoot height, root length, fresh and dry weight of shoot and root were recorded after 15 days of growth.
Effect of bioactive metabolites under nursery condition
Pigeon pea seeds treated with optimum concentration of bioactive metabolites were sown in earthen pots (size 21 × 15 mm) filled with a mixture of soil and Farm Yard Manure (FYM) in 3:1 ratio. Treatments were initially optimized by placing surface sterilized seeds treated with different concentrations of bioactive metabolites in earthen pots (Size 21 × 15 mm) with sand. The concentrations used were 0, 10, 20, 30, 40, 50, 60, 70, 80 and 100 μl of bioactive metabolite stock solutions (20 mg 100 ml−1). The concentration in which the plants showed better growth as compared to only PNS (control) treated was taken as the optimum concentration in all the cases. For disease suppression studies, seeds were treated with different concentrations of bioactive metabolites, as given above, and placed in pathogen infested soil (2 ml containing 1.0 × 107 conidia ml−1). The concentration which showed better growth in comparison to only pathogen treatment and control was taken for further studies for each bioactive fraction. The treatments taken under gnotobiotic condition were also repeated in nursery condition. Observation for growth promotion and disease symptom development were noted up to 90 days of growth. Plants were carefully uprooted after 90 days of growth and shoot height, root length, fresh and dry weight of plants were recorded. Per cent disease incidence (PDI) and per cent disease control (PDC) were also calculated on 90 days after sowing (DAS).
Effect of bioactive metabolites on mycelium morphology and dry weight
For this, 1 ml of respective stock solution of bioactive metabolites (BM 1, BM 2 and BM 3) was introduced to the PDB medium growing F. udum at 0, 12 and 24 h. Dry weight of the mycelium was measured after 48 h of pathogen growth. Any morphological change of the mycelium was observed through a Leitz photomicroscope for 0–96 h at an interval of 6 h.
Statistical analyses
All the data obtained were subjected Duncan’s Multiple Range Test (DMRT) using a software programme with the help of a computer.
Results
Bioactive compounds from RRLJ 04 and BS 03
The strain RRLJ 04 produced approximately 0.15 g l−1 of crude BM 1. TLC studies showed that BM 1 consisted of three bands of which a yellowish compound was stable at room temperature. The IR spectrum of this compound showed absorption peaks at νmax 3,070, 2,959.5, 2,927.2 and 2,857.1 cm−1. It also showed strong absorption peaks at νmax 1,727.9, 1,275 cm−1 indicating the presence of ester group. 1HNMR (300 MHz): d 0.897–0.999 (m, 7′–11′–15′ CH3, m, 24H), 1.25–1.32 (m, 2′, 3′, 4′, 5′, 6′, 8′, 9′, 10′, 12′, 13′, 14′, 44H), 1.68 (m, 7′–11′–15′ CH, 6H), 4.2–4.23 (M, 1′ CH2, 4H), 7.5–7.7 (M, 6H) (Supplementary Fig. 1). Mass: m/z 804.0. The 1H NMR and mass spectrum studies of this compound confirmed that it is a symmetric phenazine 1, 6-dicarboxylate derivative (Fig. 1a).
The strain RRLJ 04 also produced approximately 0.50 g l−1 of crude BM 2 fraction. From the TLC studies of BM 2, it was found to be a mixture of three prominent compounds—yellow, green and blue colored of which blue compound was found to be unstable. The blue compound eventually decomposes into the yellow and green ones even at room temperature or in presence of light. The IR spectrum of yellow compound showed characteristic bands of phenazine at νmax 3,063.8, 2,960, 2,925.8 and 2,854.2 cm−1. 1HNMR (300 MHz): d 0.799–0.866 (m, 3′–7′–1′1 CH3, 12H), 1.172–1.259 (M, 2′–4′–5′–6′–8′–9′–10′, CH2, 14H), 4,12–4.16(M, 1′, CH2, 2H), 7.14–8.20 (m, 7H). 13CNMR (75 MHz):d 10.91, 14.2, 14.09, 22.64, 22.95, 23.67, 28.86, 29.33, 29.66, 30.14, 30.29, 31.38, 38.66, 68.1, 108.83, 119.98, 128.74, 129.61, 131.798, 141.15, 143.74, 144.09, 151.64 (Supplementary Fig 2–3). Mass: m/z 405.1. Considering the 1H NMR, 13C NMR and mass spectrum of this compound, it was confirmed to be an ether derivative of 1-hydroxy phenazine (Fig. 1b).
The strain BS 03 produced approximately 0.35 g l−1 of crude BM 3. Characterization of this compound was unsuccessful.
In vitro antibiosis studies with bioactive metabolites
The data recorded confirmed that the bioactive fractions obtained from both RRLJ 04 and BS 03 was able to control the growth of the test fungal pathogen in all three media tested. Among the metabolite fractions tested, BM 2 gave a better zone of inhibition (Table 1). PDA showed the best inhibition followed by NA and KB. In all cases, inhibition zone increased with increase in concentration of the bioactive metabolites.
Growth of the pathogen in media amended with different concentrations of bioactive metabolites
A gradual decrease in the mycelial growth, in terms of diameter in PDA and dry weight in PDB was observed with increase in concentration of respective bioactive metabolites. BM 2 gave the best inhibition as compared to other fractions at lower concentrations (Table 2). It is evident from the data that for BM 1, minimum of 10 μl 100 ml−1 media was required to inhibit the growth of F. udum whereas, inhibition was recorded at 5 μl 100 ml−1 media of BM 2. For BM 3, concentration below 30 μl 100 ml−1 media did not have any effect on the growth of the pathogen.
Gnotobiotic studies with bioactive metabolites
The recorded data confirmed that high concentration of BM 1 and BM 2 were detrimental to the plant growth. Combination of both the fractions did not have significant effect on the growth. BM 1 (20 μl), BM 2 (10 μl) and BM 3 (30 μl) when applied to the seed showed emergence of shoot and root.
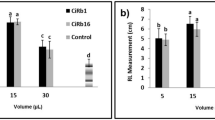
In soil without pathogen, highest shoot height and root length were given by BM 1 but it was statistically at par with BM 2 and BM 3 (Table 3; Fig. 2). Fresh weight was found to be statistically at par in BM 1 and BM 2 and dry weight was statistically higher in BM 1 than in BM 2.
In pathogen infested soil, it was found that BM 2 was better than the other two fractions in terms of growth (Table 3). Lowest shoot height, root length, fresh and dry weight of plants were recorded in pathogen alone treated plants.
Optimization of concentration required for nursery studies
It was recorded that 40 μl seed−1 of BM 1 and BM 2, respectively and 60 μl seed−1 of BM 3 fraction were optimum for growth promotion (Table 4). Concentrations above this were found detrimental to the plants whereas concentrations below this did not have any significant effect on growth improvement. Among all the concentrations tested from all the fractions, highest shoot height, fresh and dry weight of plant were recorded in 40 μl seed−1 of BM 1 followed by 40 μl seed−1 of BM 2. Root length was highest in 40 μl seed−1 of BM 2 followed by 40 μl seed−1 of BM 1.
In pathogen infested soil, plants in the treatment 50 μl seed−1 of BM 1 and 30 μl seed−1 BM 2 gave better results than the other concentrations. Higher concentrations than these suppressed the effect of the pathogen but growth of the plants were also retarded. 70 μl seed−1 of BM 3 was found to be optimum for growth in pathogen infested soil for further studies (Table 5). Beyond this concentration, seeds did not germinate. Shoot height, fresh and dry weight of plants were found to be highest in control followed by 30 μl seed−1 of BM 2. Root length was found to be maximized in control but it was statistically at par with 50 μl seed−1 of BM 1, 30 and 40 μl seed−1 of BM 2, 40, 50, 60 and 70 μl seed−1 of BM 3.
Effect of bioactive metabolites on growth under nursery condition
Under nursery condition, it was recorded that plants treated with BM 1 gave the highest increase in growth in terms of shoot height, root length, fresh and dry weight of plants. In pathogen infested soil, plants with BM 2 treatment showed a better enhancement in growth as compared to other treatments (Table 6). But all the treatments were found to be statistically significant over control. In terms of disease control, it was observed that at 90 DAS, per cent disease control was 90 % in BM 2 treated plants whereas it was 87 and 83 % in BM 1 and BM 3 treated plants, respectively (Table 7).
Effect of bioactive metabolites on mycelial morphology and dry weight of mycelium
Among the bioactive metabolites, lowest dry weight was recorded in F. udum + BM 2 (46.25) and highest dry weight in F. udum alone (126.79) which were found to be statistically at par with F. udum + BM 3 (Table 8).
It was recorded that the dry weight of the mycelium was significantly reduced on introduction of BM 1 and BM 2 to the fungal culture media. BM 3 gave a statistically significant reduction in dry weight of the mycelium, when simultaneously introduced together with the fungus (0 h). Introduction of BM 3 after 12 or 24 h of fungal growth did not have any significantly effect on the dry weight of mycelium.
When BM 3 was simultaneously introduced with the fungus (0 h), chlamydospore-like structures developed in mycelium after 18 h of growth. Introduction of BM 1 and BM 2 gave no such structures at any growth period (Fig. 3).
Discussion
Production of antibiotics in several strains of fluorescent pseudomonads has been recognized as a key factor in suppression of root pathogens (Lugtenberg and Kamilova, 2009; Saharan and Nehra 2011; Perez et al. 2012). Bakker et al. (2003) reported that antibiotics do have direct effects on plants and, therefore, might induce systemic resistance. The bioactive fractions BM 1 and BM 2 isolated from P. aeruginosa strain RRLJ 04 showed inhibition of the pathogen in antibiosis studies. Earlier, we have reported the plant growth promoting and disease control properties of an antibiotic and siderophore producing fluorescent Pseudomonas strain in some important crop plants (Dileep Kumar and Bezbaruah 1997). Seeds treated with respective fractions showed an enhancement in growth besides suppressing the wilt disease in pathogen infested soil. The growth improvement and disease suppression due to the presence of the bioactive fractions was confirmed by the gnotobiotic studies. Analytical studies of the bioactive compounds showed that these are phenazine derivatives.
Growth enhancement and disease suppression in seeds treated with bioactive fraction from BS 03 was also observed in pathogen infested soil. It has been reported that the addition of B. coagulans to culture of fungi resulted in inhibition of ergosterol biosynthesis in mycelium (Czaczyk et al. 2002). Some of the antibiotics produced by Bacillus (iturin and surfactin) are able to modify bacterial surface hydrophobicity and, consequently, microbial adhesion to surfaces of mycelium (Ahimou et al. 2000). Moyne et al. (2001) identified an antifungal peptide Bacillomycin D from a B. subtilis strain AU195. Three isomers of iturin A, a cyclic lipopeptide antibiotic produced by B. subtilis, was also found to be produced by a B. amyloliquefaciens strain B94 (Yu et al. 2002). The strain was used as a biocontrol agent to suppress R. solani and other fungal plant pathogens. GaoFen et al. (2004) reported the production of antifungal substance from B. cereus strain, which exhibited inhibitory activities against F. oxy. f. sp. cucumerinum, F. oxy. f. sp. niveum and F. oxy. f. sp. vasinfectum. Sadfi et al. (2002) reported that a B. cereus strain X16 produced more than one antifungal metabolite.
An enhancement in growth as compared to control was observed when respective bioactive fraction treated seeds were sown in soil without any pathogen. This improvement in growth may be attributed to successful colonization of beneficial microbes to the host roots. Phenazine plays an important role in microbial competition in the rhizosphere, including survival and competence (Mazzola et al. 1992). Fernando and Pierson (1999) reported three phenazine antibiotics to be responsible for the ability of a P. aureofaciens strain 30–84 to compete with the indigenous microflora in the rhizosphere.
Growth enhancement may also result due to increase in nodulation by rhizobia thereby increasing nitrogenase activity (Alagawadi and Gaur 1988; Zhang et al. 1997). Rhizobium strains face stiff competition from the rhizosphere microorganisms, like deleterious microbes, to colonize the roots. Phenazine and other antibiotics produced by pseudomonads and bacilli may help in suppressing these deleterious microbes facilitating successful nodulation by the rhizobial strains (Kloepper et al. 1991).
It has been observed that two bioactive fractions from RRLJ 04 affected the plant growth differently in presence and absence of pathogen. While BM 1 showed better growth in absence of pathogen, BM 2 showed a slightly better result in terms of growth and disease control in presence of the pathogen. Several abiotic factors such as oxygen, temperature, specific carbon and nitrogen sources and microelements have been identified to influence antibiotic production by bacterial biological control agents. Nutritional inadequacy has been found to be the main constraint in antibiotic production and activity. Among the biotic factors that may play a determinative role in antibiotic production are the plant host, the pathogen, the indigenous microflora and the cell density of the producing strain (Raaijmakers et al. 2002). Moreover, in many biocontrol systems, multiple factors have been shown to play a role in disease suppression. Kumar et al. (2005) reported the production of phenazine-1-carboxamide and IAA by a saprophytic P. aeruginosa strain PUPa3 which also produced siderophores, growth hormones, protease and phosphatase and has fungicidal properties. The strain RRLJ 04 is also found to produce siderophore. Disease suppression is a multifunctional attribute and hence it is possible that none of the mechanisms are mutually exclusive.
From the studies with bioactive metabolites on mycelial morphology, it was observed that BM 3 induced production of chlamydospore-like structures in the mycelium when grown together. Presence of BM 1 and BM 2 although did not form any such structures, but mycelial dry weight was drastically reduced. Chlamydospores are resting spores which are produced by the pathogen to tide over unfavourable condition. Thus, it can be concluded that presence of BM 3 in the medium made the environment unsuitable for the normal growth of the pathogen.
This result also supports the work of Harish et al. (1998) who reported that in vitro interaction of F. udum and B. subtilis strain AF 1 showed that the fungus formed chlamydospore-like structures and increased vacuolation, when both cultures are simultaneously inoculated into PDB. Vasudeva et al. (1958) reported that in the presence of an antibiotic from Bacillus, a characteristic bulb formation was observed in the spores and hyphae of test fungi. The active principle had been shown to be thermolabile and the antibiotic was named ‘bulbiformin’ in view of its distinctive property of forming bulbs on the test fungi. This bulb formation takes place only in fungi having chitin in their cell wall. The mycolytic activity of antagonists could be mainly due to the lytic enzymes β-1,3-glucanase and chitinase (Srivastava et al. 2001). Development of fusarial wilt symptoms was prevented in pigeon pea seeds treated with B. brevis which produced extracellular substances (Bapat and Shah 2000). The extracellular antagonistic substances which induced swelling of the pathogen’s hyphal tips and cells were bulbous and swollen with shrunken and granulated cytoplasm. Cell free culture filtrates of B. subtilis strain were active against macroconidium germination and hyphal growth of F. graminearum, depending on the initial macroconidium density. It induced formation of swollen hyphal cells in liquid cultures of this fungus grown from macroconidia (Chan et al. 2003).
It can be concluded from the results of this study that the bioactive metabolites produced by PGPR strains play an important role in plant growth promotion and disease control of plants provided they are produced in adequate amount which is dependent upon the biotic and abiotic factors. The bioactive compounds produced by RRLJ 04 and BS 03 can be further exploited for growth enhancement and disease control of other crops.
References
Ahimou F, Jacques P, Deleu M (2000) Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb Technol 27:749–751
Alagawadi AR, Gaur AC (1988) Asociative effect of Rhizobium and phosphate-solubilising bacteria on the yield and nutrient uptake of chickpea. Plant Soil 105:241–246
Bakker PAHM, Ran LX, Pieterse CMJ, Van Loon LC (2003) Understanding the involvement of rhizobactria mediated induction of systemic resistance in biocontrol of plant diseases. Can J Plant Pathol 25:5–9
Bapat S, Shah AK (2000) Biological control of fusarial wilt of pigeon pea by Bacillus brevis. Can J Microbiol 46:125–132
Bhattacharyya N, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Chan Y, McCormick WA, Seifert KA (2003) Characterization of an antifungal soil bacterium and its antagonistic activities against Fusarium species. Can J Microbiol 49:253–262
Chaurasia B, Pandey A, Palani LS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Compant S, Duffy B, Nowak J, Cle’ment C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principle, mechanisms of action and future prospects. Appl Environ Microbiol 71:4951–4959
Cook RJ (2007) Management of resident plant growth—promoting rhizobacteria with the cropping system: a review of experience in the US Pacific Northwest. Eur J Plant Pathol 119:255–264
Czaczyk K, Trojanowska K, Stachowiak B (2002) Inhibition of ergosterol biosynthesis in fungal plant pathogens by Bacillus species. Pol J Environ Stud 11:93–597
Defago G, Haas D (1990) Pseudomonas as antagonists of soil borne plant pathogens: modes of action and genetic analysis. In: Bollag J, Stotzky G (eds) Soil biochemistry, vol 6. Marcel Dekker, New York, pp 249–291
Dileep Kumar BS, Bezbaruah B (1997) Plant growth promotion and fungal pest control through an antibiotic and siderophore producing fluorescent Pseudomonas strain from tea (Camellia sinensis (L) O. Kuntze) plantations. Indian J Exp Biol 35:89–292
Dutta S, Podile AR (2010) Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit Rev Microbiol 36:232–244
Dutta S, Mishra AK, Dileep Kumar BS (2005) Efficacy of two rhizobacterial strains on growth promotion, nodulation and disease control of pigeon pea in presence of a rhizobial strain in soil infested with Fusarium udum. In: Chakraborty U, Chakraborty B (eds) Stress biology. Narosa Publishing House Pvt. Ltd., New Delhi, pp 247–251
Dutta S, Mishra AK, Dileep Kumar BS (2008) Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol Biochem 40:452–461
Dwivedi D, Johri BN (2003) Antifungals from fluorescent pseudomonads: biosynthesis and regulation. Curr Sci 85:693–1703
Fernando WGD, Pierson LS (1999) The effect of increased phenazine antibiotic production on the inhibition of economically important soil–borne plant pathogens by Pseudomonas aureofaciens 30–84. Arch Phytopathol Plant Prot 32:491–502
GaoFen ML, Qiao XW, Hao BQ (2004) Preliminary purification and characterization of antifungal substance produced by Bacillus cereus strain, BC98-I. Acta Phytophylacica Sinica 31:65–370
Harish S, Manjula K, Podile AR (1998) Fusarium udum is resistant to the mycolytic activity of a biocontrol strain of Bacillus subtilis AF 1. FEMS Microbiol Ecol 25:385–390
Kajimura Y, Sugiyama M, Kaneda M (1995) Bacillopeptins, new cyclic lipopeptide antibiotics from Bacillus subtilis FR-2. J Antibiot 48:1095–1103
Kiprop EK, Baudoin JP, Mwang’ombe AW, Kimani PM, Mergeai G, Maquet A (2002) Characterization of Kenyan isolates of Fusarium udum form pigeon pea [Cajanus cajan (L.) Millsp.] by cultural characteristics, aggressiveness and AFLP analysis. J Phytopathol 150:517–525
Kloepper JW, Zablotowiz RM, Tipping EM, Lifshitz R (1991) Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB (eds) The rhizosphere and plant growth. Kluwer Academic Publishers, Dordrecht, pp 315–326
Kumar RS, Ayyadurai N, Pandiaraja P, Reddy AV, Venkateswarlu Y, Prakash O, Sakthivel N (2005) Characterization of antifungal metabolite produced by a new strain P. aeruginosa PUPa3 that exhibits broad-spectrum antifungal activity and biofertilizing traits. J Appl Microbiol 98:145–154
Lugtenberg B, Kamilova F (2009) Plant growth promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616–2624
Moyne AL, Shelby R, Cleveland TE, Tuzun S (2001) Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90:622–629
Perez JC, Arango JS, Uribe LFP (2012) Returning to our roots. In: Proccedings of the 9th international and 1st Latin American PGPR workshop. Quirama, Medellín, Colombia, 3–9 June 2012
Raaijmakers JM, Vlami M, De Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhock 81:537–547
Reddy MV, Sharm SB, Nene YL (1990) Pigeonpea: disease management. In: Nene YL, Hall SD, Shiela VK (eds) Pigeon pea. CA B Int, UK, pp 303–307
Sadfi N, Cherif M, Hajlaoui MR, Boudabbous A, Belanger R (2002) Isolation and partial purification of antifungal metabolites produced by Bacillus cereus. Ann Microbiol 52:323–337
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Sharma A, Johri BN (2003) Combat of iron deprivation through a plant growth promoting fluorescenst Pseudomonas strain GRP3 in mung bean (Vigna radiata L. Wilzeck). Microbiol Res 158:1–5
Srivastava AK, Singh T, Jana TK, Arora DK (2001) Induced resistance and control of charcoal rot in Cicer areitinum (chickpea) by Pseudomonas fluorescens. Can J Bot 79:787–795
Vasudeva RS, Subbaiah TV, Sastry MLN, Rangaswamy G, Iyengar MRS (1958) ‘Bulbiformin’, an antibiotic produced by Bacillus subtilis. Ann Appl Biol 46:336–345
Yu GY, Sinclair B, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Zhang F, Dashti N, Hynes RK, Smith DL (1997) Plant growth promoting rhizobacteria and soybean (Glycine max L. Merr.) growth and physiology at suboptimal root zone temperatures. Ann Bot 79:243–249
Acknowledgments
The authors are thankful to Dr. P. G. Rao, Director, North East Institute of Science and Technology (CSIR), Jorhat for his keen interest and help in this work. Dutta and Morang thank Department of Science and Technology, Government of India and University Grant Commission, New Delhi, respectively for the financial assistance to carry out a part of this work. Dileep Kumar thanks Dr. Suresh Das, Director and Dr. A. Sundaresan, Head, Agroprocessing and Natural Products Division of National Institute for Interdisciplinary Science and Technology (CSIR), Thiruvanathapuram for their help to publish this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dutta, S., Morang, P., Nishanth Kumar, S. et al. Fusarial wilt control and growth promotion of pigeon pea through bioactive metabolites produced by two plant growth promoting rhizobacteria. World J Microbiol Biotechnol 30, 1111–1121 (2014). https://doi.org/10.1007/s11274-013-1532-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1532-9