Abstract
The stem-end rot of avocado, caused predominantly by Lasiodiplodia theobromae, can result in heavy postharvest losses. Application of a cell suspension of Aureobasidium pullulans at the stem-end of unripe fruit delayed (P < 0.05) disease incidence by 2 days compared to controls which had either stalk intact or not. Presence of stalk reduced the progression of symptoms in both treated and control fruits, but did not significantly (P > 0.05) affect disease incidence. L. theobromae, when cultured together with A. pullulans, showed significantly (P < 0.05) reduced radial growth of colony towards A. pullulans at 48 and 72 h and shortened aerial mycelium compared to controls. Neither of the organisms overgrew on each other but their colony margins were in close proximity. Presence of A. pullulans significantly (P < 0.001) reduced germination of conidia in water, and the germ tubes were shorter and showed only emergence. Chitinase, β-1,3-glucanase and antifungal activity of the peel of control fruit declined during ripening. However, there was increased chitinase and β-1,3-glucanase activity in A. pullulans-treated fruits or fruits that were inoculated with L. theobromae after treatment with A. pullulans. β-1,3-glucanase activity increased only slightly in fruits that were inoculated with L. theobromae without treatment. Greater preformed antifungal activity was retained in A. pullulans-treated fruits during ripening. Enhanced activity of chitinase and β-1,3-glucanase and greater retention of preformed antifungal activity may have contributed to the delayed stem-end rot incidence in A. pullulans-treated avocados. Application of A. pullulans, 2 days prior to inoculation and retention of stalk at harvest, appears to have allowed better establishment of A. pullulans on the fruit surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
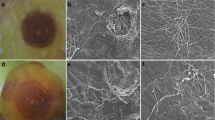
Ripe avocado fruits are prone to several postharvest diseases of which anthracnose caused by Colletotrichum species and the stem-end rot (SER) are considered the most destructive. The SER is known to be caused by Lasiodiplodia theobromae (Syn. Botryodiplodia theobromae), Pestalotiopsis sp. and Phoma spp. in Sri Lanka [3]. The SER begins as a slight shrivelling around the stem button in the ripening fruit, and fungal mycelium may often be visible on the scar when the button is removed. Conspicuous dark rot with a well-defined margin develops downwards from the stem-end. Rotting extends further with ripening covering most of the fruit which becomes shrivelled. Affected fruit flesh becomes soft and pale in colour (Fig. 1), and the vascular strands running vertically turn dark brown to black.
In situations where the SER is a serious problem, the use of synthetic fungicides may be necessary. SER can be controlled by good field sanitation, optimal cultural and harvesting practices coupled with prompt cold storage after harvest [33]. In warm and humid tropics, however, the use of refrigeration is costly and often unaffordable. Most of the commercially available synthetic fungicides are toxic and can bring about adverse effects on human health and environment. More attention is therefore required to be focused on other control methods. Biological control using microbial antagonists is also a viable option [14, 15, 23, 28, 29].
Aureobasidium pullulans (de Bary) Arnaud is a yeast-like saprophytic fungus that occurs commonly in the phyllosphere of many crop plants and on various tropical fruits [19, 20]. The yeast has been identified as an effective biocontrol agent against leaf and postharvest diseases [4, 11, 14, 27, 28, 34]. It has also been found as a non-pathogenic, surface colonizer on avocado fruit surface [17].
Several biocontrol products are available in the market for postharvest usage, and they represent only a small fraction of the potential market for control of postharvest diseases [12]. Apart from some of the earliest biocontrol products such as Bio-Save®10 LP and Bio-Save®11 LP which are based on strains of the bacterium, Pseudomonas syringae [5], there are also commercial products based on plant-inhabitant yeasts. For example, BoniProtect® and BoniProtect®Forte which are developed and marketed in Germany are mixtures of two strains of A. pullulans. BoniProtect®Forte is applied at bloom to strawberries and protects them during postharvest storage against grey mould caused by Botrytis cinerea [32].
Several modes of action have been suggested to explain the biocontrol activity of microbial antagonists such as competition for space and nutrients, antibiosis, direct parasitism and induction of host defences [6, 31]. A. pullulans was found to increase chitinase, β-1,3-glucanase and peroxidase activity in apple fruits [14]. Enhanced natural defences in green strawberry fruits by A. pullulans were also recorded [4]. Understanding of the mode of action of the microbial antagonists will help in developing more effective procedures for better results from the known antagonists, and it will also help in selecting desirable antagonists [29]. The effect of postharvest application of A. pullulans for the control of avocado SER has not been examined. Hence, the present study investigated the biological control activity of A. pullulans on avocado SER caused by L. theobromae and its mode of action.
Materials and methods
Fruits
Avocado fruits (unknown variety) at harvesting maturity and of uniform size and shape without any defects or disease symptoms were handpicked from trees grown in the Kandy area and immediately delivered to the Plant Pathology Laboratory, Department of Botany, University of Peradeniya.
Isolation of SER pathogens
Ripe fruit samples showing characteristic symptoms of SER were obtained from different market places in Kandy. To isolate causal agents, peel segments (0.5 cm2) were cut from the diseased areas, surface sterilized in 1% sodium hypochlorite for 1 min and placed aseptically on PDA. The SER isolates were subcultured and identified by their cultural and spore morphology. L. theobromae, the most commonly isolated pathogen from SER, was used for subsequent experiments. Six unripe fruits without any visible damage or disease symptoms were re-inoculated with L. theobromae isolates and incubated using the procedure described below.
Aureobasidium pullulans
Aureobasidium pullulans was obtained from the culture collection of the Department of Botany and previously isolated from the surface of mandarin fruit. The yeast was maintained on PDA throughout the research period. To prepare a cell suspension, the mycelium was scraped from a 7-day-old colony and suspended in sterile distilled water (SDW). The suspension was filtered through glass wool, and the filtrate which contained yeast cells was retained. After adjusting the concentration of yeast cells to 1 × 107 cells/ml, the suspension was used for fruit treatment.
Fruit inoculation and disease assessment
A suspension of conidia of L. theobromae was prepared by crushing the pycnidia of a 21-day-old colony and suspending them in SDW. After shaking vigorously to release conidia, the suspension was filtered through glass wool, the concentrations of conidia in the filtrate was adjusted to 1 × 106 conidia/ml.
Four sets, each containing eight unripe fruits soon after harvest, were prepared for four treatments. Drops (50 µl) of a suspension (1 × 107 cells/ml) of A. pullulans were applied at the stem-end of two sets of fruits, in one set the stalk remained intact and in the other set, the stalk was removed. Two sets of untreated controls were also maintained, one with and another without stalks.
The treated and control fruits were incubated at room temperature (RT, 26 ± 2 °C), and after 2 days, they were inoculated by adding a drop (35 µl) of conidia of L. theobromae on to the stem-end of each fruit. The inoculated fruits were returned to incubation at RT (26 ± 2 °C). Percentage area of SER was assessed using a self-prepared scale. The experiment was performed twice using eight replicate fruits for each treatment.
The extent of SER was assessed as the percentage of fruit surface covered by the lesion by visually comparing with a scale prepared from photographs of fruits showing different disease levels, 5, 10, 20, 30, 40 and 50%.
Dual culture of A. pullulans and L. theobromae
The effect of A. pullulans on the growth of L. theobromae was tested by dual culture with L. theobromae according to the method described by Korsten and Jager [18] with slight modification. Replicate PDA plates (9 cm diameter) were divided into two halves, and A. pullulans was streaked on one half of agar medium. After 3 days, L. theobromae was streaked on the other half of each petri dish, 5 cm apart from the antagonist streak line. Control plates were streaked only with L. theobromae. All plates were incubated at RT (26 ± 2 °C), and the radii of L. theobromae colonies were measured daily for 14 days. Percentage reduction in colony growth (RCG) was calculated using the equation % RCG = (R c − R t) × 100/R c where R c = distance from the point of inoculation to the colony margin of the control, R t = distance from the point of inoculation to the colony margin in the direction of the antagonist. The experiment was repeated twice each with eight replicates.
Germination of L. theobromae conidia in the presence of A. pullulans
The possibility of inhibition of germination of conidia of L. theobromae by A. pullulans was tested in a mixed suspension using the method described by Adikaram et al. [4] with slight modifications. Aliquots (20 µl) of suspensions of A. pullulans (1 × 107 cells/ml) and conidia of L. theobromae (1 × 106 conidia/ml) were mixed together on a sterile glass slide. Controls had drops (20 µl) of conidia of L. theobromae and SDW placed on the same slide. Eight replicate slides were prepared. Conidia were allowed to germinate by placing the slides in a high-humidity moist chamber at RT for 14 h. One hundred randomly selected conidia of L. theobromae were counted under light microscope (×400), and percentage germination was calculated. The experiment was repeated twice each time with eight replicates.
Chitinase assay
Chitinase activity of the fruit peel was assayed using CM-Chitin-RBV as substrate as described by Saborowski et al. [26] with slight modifications. Peel samples (1 g) were homogenized for 2 min at 11,000 rpm in a pre-cooled centrifuge tube with 5 ml of 0.2 M citrate-Na2HPO4 buffer (pH 5) using an ultrasonic homogenizer (Ultra Turrax®T25 basic, IKA Labortechnik). The homogenate was cooled to 4 °C and clarified by centrifugation at 6000g (Sigma 3K30 Laboratory centrifuge) for 10 min. The supernatant was used for the enzyme assay.
An aliquot (600 µl) of 0.2 M citrate-Na2HPO4 buffer (pH 5) was added to 300 µl of CM-Chitin-RBV (2 mg/ml, Loewe Biochemica GmbH, Otterfing, Germany). After pre-incubation for 5 min at 37 °C, the enzymatic reaction was started by adding 300 µl of crude extract. After incubation for 1 h, the reaction was terminated by adding 300 µl of 1.0 N HCl. Reaction tubes were cooled in an ice water bath for at least 10 min to ensure complete precipitation of the non-degraded substrate. After centrifuging (20,000g, 5 min), the absorbance of the supernatant was measured in a spectrophotometer (Cam Spec M302, Spectronic Camspec Ltd, UK) at 550 nm in triplicates. A blank containing the reaction mixture without the enzyme was run in parallel.
β-1,3-glucanase assay
Activity of β-1,3-glucanase was determined using the method of Dann and Deverall [9] with slight modifications. Peel samples (1 g) were homogenized (Ultra Turrax® T25 basic, IKA Labortechnik) at 11,000 rpm in a pre-cooled centrifuge tube with 1% (w/w) polyvinyl polypyrrolidone and 5 ml potassium acetate buffer (50 mM, pH 5), containing 1 mM EDTA and 5 mM reduced glutathione. The extracts were cooled to 4 °C and centrifuged (Sigma 3K30 Laboratory centrifuge) at 9000g for 5 min. The supernatant was used for the enzyme assay.
Potassium acetate buffer (1.6 ml, 10 mM, pH 5) and the crude extract (0.4 ml) were allowed to equilibrate to 30 °C for 3 min. The reaction was initiated by adding 0.4 ml of the substrate (pachyman) suspension and was stopped after 10 min by adding 2.8 ml of 20% (w/v) Tris. The tube was vortexed for 5 min and centrifuged at 9000g for 3 min. Aliquots (3.0 ml) of the supernatant were transferred to cuvettes (Optiglass Ltd, England), and the absorbance was measured at 610 nm against a blank containing the substrate devoid of enzyme extract, using a spectrophotometer (Cam Spec M302, Spectronic Camspec Ltd, UK). The relative enzyme activity was expressed as absorbance at 610 nm. Two replicates were used.
TLC bioassay for antifungal activity
Antifungal activity was assessed according to the method described by Sivanathan and Adikaram [30] with slight modifications. Peel tissues (2 g) were cut out from treated and control fruits and immediately transferred to the deep freezer and stored at −20 °C for 2 days. Peel tissues were homogenized (Ultra Turrax® T25 basic, IKA Labortechnik) in three successive portions (45 ml) of fresh diethyl ether at 11,000 rpm. The extracts were combined, filtered through Whatman No. 1 filter paper and evaporated in vacuo at 40 °C (Stuart RE300). Crude residue was collected in 600 μl of diethyl ether. Aliquots (100 μl) of the extracts were spotted on a thin layer chromatography (TLC) plate coated with silica gel (Kieselgel GF254, 13% CaSO4, BDH). The plate was developed in chloroform: methanol (98:2 v/v) and air-dried overnight. The plate was carefully sprayed with a thick suspension of conidia of Cladosporium cladosporioides in Czapek-Dox nutrient solution and incubated for 2–3 days in a moist chamber at RT (26 °C ± 2). C. cladosporioides was used because of its better growth on TLC than L. theobromae [16] and was previously used to detect antifungal activity of avocado fruit peel [2, 30]. The area (using image j software) and Rf values of inhibition zones were recorded. The experiment was performed with four replicates. The antifungal compound responsible for each inhibition zone was determined as described previously [1].
Experimental design and data analysis
Experimental layout was according to complete randomized design (CRD), and the data (except for β-1,3-glucanase assay) were statistically analysed using Minitab Version 14. Spore germination data were analysed using a paired t test. Disease development data were subjected to Nested ANOVA followed by arc sign transformation. Dual culture and chitinase assay data were analysed by two-way ANOVA. Treatment mean differences were compared using Tukey’s multiple range test. β-1,3-glucanase assay data were subjected to a regression analysis [21] using SAS 9.1 version. Regression lines were drawn for all treatments, and the range of values for intercepts and slopes was calculated.
Results
Isolation of SER fungi
Among the fungi isolated on PDA from the SER of ripe avocado fruits, over 75% isolates were identified as L. theobromae. Pestalotiopsis sp. was also isolated from the SER. Re-inoculation of fruits with L. theobromae alone produced all SER symptoms.
Treatment of fruits with A. pullulans
Application of A. pullulans at the stem-end region of unripe fruits, 2 days prior to inoculation with L. theobromae, delayed the incidence of SER at ripe stage by 2 days.
In both, A. pullulans-treated fruits and control, the development of SER symptoms was observed following artificial inoculation with L. theobromae, irrespective of the presence of stalk or not. Disease symptoms first appeared in untreated fruits only 2 days after inoculation (Fig. 2) either with or without stalks. Fruits treated with A. pullulans (both with and without stalk) prior to inoculation with L. theobromae showed symptoms only after 4 days of storage (Fig. 2). The SER incidence, following artificial inoculation with L. theobromae, was significantly (P < 0.05) delayed by A. pullulans treatment. The presence of a stalk has only slowed down the disease progression in both treated and control fruits, but did not significantly affect the disease incidence (P > 0.05). In the control fruits that were kept without stalk, 100% disease incidence was observed on the eighth day after harvest, while the fruits with stalk intact reached 100% disease on the ninth day after harvest. Treated fruits with stalk intact showed a slower disease progression within the first 3 days of disease development than in those fruits that were treated without stalks intact. However, A. pullulans-treated fruits, both with stalks intact and removed, the disease development reached 100% on the tenth day after harvest.
Incidence of SER in ripe avocados, pre-treated with A. pullulans at unripe stage, with stalk (grey line) or without stalk (black dash line), control with stalk (grey dash line) and control without stalk (dotted line). SER incidence and severity figures were recorded following artificial inoculation of treated fruits with L. theobromae
Dual culture
When A. pullulans and L. theobromae were grown together on the same agar plate, colonies of both fungi initially grew somewhat freely towards each other. Growth of L. theobromae colony was slightly slower towards A. pullulans growth with only a 2.4% reduction in colony growth after 24 h, and the radial growth of L. theobromae colony was not significantly (P > 0.05) different at this stage from that of the controls. After 48 and 72 h, however, the increase in radial growth of L. theobromae colony, in the presence of A. pullulans, was significantly (P < 0.05) smaller compared to the controls (Table 1). There was a greater reduction (35.6%) in colony growth of L. theobromae after 72 h compared to that at 48 h (Table 1). At this stage (72 h), the margins of the two colonies were in close proximity to each other, without any area of lack of visible mycelium growth in between.
Growth of the colonies continued to be observed for 14 days, and there was no overgrowth of L. theobromae or A. pullulans on each other observed during this 14-day period. Somewhat shorter aerial mycelium of L. theobromae was also observed in the newly grown portion of the colony towards that of A. pullulans.
Conidia germination assay
The presence of A. pullulans cells significantly (P < 0.001) reduced germination of L. theobromae conidia compared to the controls, and the germ tubes showed only their emergence.
Only 25% of conidia of L. theobromae germinated in the presence of A. pullulans in the drop compared to the 98% germination of conidia, with elongated germ tubes, in controls within 14 h (Table 2).
Chitinase and β-1,3-glucanase activity
The peel of freshly harvested, untreated fruit displayed some chitinase and β-1,3-glucanase activity which declined with time, during ripening (Fig. 3). Treatment with A. pullulans resulted in a significant (P < 0.05) increase in β-1,3-glucanase activity during the first 48 h, and the activity remained more or less at the same level thereafter (Fig. 3). Inoculation of treated fruits with L. theobromae caused an increase in β-1,3-glucanase activity, 48 h after inoculation. Chitinase activity also increased steadily following treatment with A. pullulans and also after inoculation with L. theobromae over the 96 h period, and the increase was significant (P < 0.05) compared to controls.
Chitinase (A) and β-1,3-glucanase activity (B) of avocado fruit peel in untreated control (dark grey box), treated with A. pullulans (white box), treated and inoculated with L. theobromae (black box) and inoculated with L. theobromae (light grey box). Values in bars in A with same letters, within each time point, are not significantly different (P > 0.05). Differences in β-1,3-glucanase activity among treatments are significant (P < 0.05)
Fruits that were inoculated with L. theobromae, without prior treatment, showed a decline in both chitinase and β-1,3-glucanase activity initially, followed by a slight increase in only β-1,3-glucanase within the next 48 h. The chitinase activity, however, increased substantially during the next 48 h reaching a level closer to that in fruits inoculated with L. theobromae after A. pullulans treatment (Fig. 3).
Antifungal activity
Diethyl ether extract of the peel of unripe avocado fruit, on TLC bioassay, produced four inhibition areas at Rf 0.12, 0.35, 0.60 and 0.91. The inhibition zones produced at Rf 0.91 and 0.60 were determined to be due to antifungal monoene (1-acetoxy-2,4-dihydroxy-n-heptadeca-16-ene) and diene (1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15-diene) [24, 25]. The inhibition caused by the diene at Rf 0.60 was by far the largest and most intense (Fig. 4). The other two inhibition zones were produced by a-acetoxy-2,4-dihydroxyheptadec-16-yne (Rf. 0.35), 1,2,4-trihydroxyheptadec-16-yne and 1,2,4-trihydroxyheptadex-16-ene (Rf 0.12) [1].
Total area of inhibition zones at a Rf 0.12 produced by 1,2,4-trihydroxyheptadec-16-yne and 1,2,4-trihydroxyheptadex-16-ene (black box), b Rf 0.35 by a-acetoxy-2,4-dihydroxyheptadec-16-yne (white box) and c Rf 0.60 by diene, 1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15-diene (grey box) in the extracts of peels of treated fruits resulted in TLC bioassay with C. cladosporioides. C—control, A—A. pullulans treated, A + P—A. pullulans treated and L. theobromae inoculated and P—L. theobromae inoculated
The size of antifungal areas declined with ripening in both treated fruits and controls. However, the rate of natural decline of antifungal activity was slower in A. pullulans-treated fruits during ripening resulting in greater retention of antifungal activity at ripe stage of fruit (Fig. 4).
Discussion
Few studies have been conducted on biological control of avocado SER and anthracnose. Bacterial species such as B. subtilis and B. lichineformis were proven to be antagonistic to C. gloeosporioides, Nigrospora sphaerica and Fusarium solani [17], and Pestalosiopsis neglecta was antagonistic to Phoma sp. [3]. Another study conducted by Prusky et al. [22] showed that a non-pathogenic mutant strain of Colletotrichum magna induced resistance against C. gloeosporioides on avocado.
Application of a cell suspension of A. pullulans at the stem-end of freshly harvested avocado fruit and artificial inoculation with L. theobromae after a lapse of 2 days significantly delayed the incidence of SER in ripe fruits. This indicates a direct or indirect delaying effect of A. pullulans on the SER pathogen. The delay in SER incidence was observed in yeast-treated fruits irrespective of the presence of stalk intact or not. The presence of stalk, however, slowed down the expansion of the SER in both treated and controls. Removal of stalk exposes the stem-end scar to the outer environment providing a point of easy access for SER pathogens. The avocado fruit health can be improved by leaving longer stalks [10]. The present study also suggests that the presence of stalk provides some protection to the ripe avocado fruit against SER fungi.
The mode of biological control action of beneficial microorganisms on plant pathogenic fungi can be either direct [29] or indirect antagonism. Direct antagonism comprises the mechanisms that are a direct result of the action of the biocontrol agent (BCA) such as competition for nutrients and space, secretion of lytic enzymes such as chitinases and β-1,3-glucanases that degrade the polymers of the pathogen cell wall and mycoparsitism which often requires direct physical interaction between the BCA and fungal hyphae [7, 15]. There was no mutual overgrowth of colonies of L. theobromae and A. pullulans observed over a period of 14 days on dual culture plates, and this may possibly rule out any mycoparasitism. Reduced germination of conidia of L. theobromae and germ tube growth observed in the slide germination assay, in the presence of A. pullulans, suggests antibiosis or competition by the two organisms for space or nutrients. It was, however, quite clear from dual culture that there had not been strong antibiosis as no clear area of lack of mycelial growth was observed between the colonies of the two organisms when they were cultured together on PDA in the present study. The reduced colony growth of L. theobromae towards the A. pullulans colony and shortened aerial mycelium observed could be a sign of limited antibiosis from A. pullulans. According to a previous report, A. pullulans does not appear to produce antibiotic substances [6].
Chitinase and β-1,3-glucanase are PR proteins produced as defence responses in plants and are capable of hydrolysing chitin and glucan, respectively, in the fungal hyphae. Our experiments have shown increased chitinase and β-1,3-glucanase levels retained at ripe stage of avocado fruit, pre-treated with A. pullulans. TLC bioassay of tissue extracts for antifungal activity revealed the presence of considerable antifungal activity in treated tissue extracts, even 96 h after harvest. This is comprised of five previously identified antifungal compounds from the unripe avocado peel [1, 2, 24, 25]. These findings support enhanced resistance in A. pullulans-treated fruits at ripe stage.
It has been suggested that the establishment of a biocontrol agent before a pathogen arrival would be a good strategy to prevent fruits from infections [13]. Here the artificial inoculation of fruits with L. theobromae was done 2 days after the treatment with A. pullulans which may have allowed A. pullulans to establish on the stem-end region. Comparable levels of decay control with A. pullulans have been reported previously against wound pathogens on different commodities such as B. cinerea and P. expansum in apples [14], B. cinerea in strawberries [4] and M. laxa in sweet cherries [28].
Biocontrol of a plant disease involves a three-way interaction of the pathogen, plant tissue(s) and BCA. The plant tissue does not passively produce space for the pathogen and BCA interaction, but it appears to perceive the presence of the biocontrol agent as well [8].
Aureobasidium pullulans has been previously isolated as a non-pathogenic, surface colonizer on avocado fruit surface [17]. Thus, it is well adapted for the survival and growth on the avocado fruit surface under natural conditions. This might have led to the successful SER control on avocado.
This study concludes that the combined effect of enhanced activity of chitinase, β-1,3-glucanase and reduced rate of decline of antifungal activity during ripening would be the basis for delayed incidence of SER in A. pullulans-treated avocado fruit.
References
Adikaram, N. K. B., Egodawela, N. A., & Karunaratne, A. (1993). Antifungal compounds in the avocado fruit peel and their relation to anthracnose development. Acta Horticulturae, 343, 25–28.
Adikaram, N. K. B., Ewing, D. F., Karunaratne, A. M., & Wijeratne, E. M. K. (1992). Antifungal compounds from immature avocado peel. Phytochemistry, 31(1), 93–96.
Adikaram, N. K. B., & Karunaratne, A. (1998). Suppression of avocado anthracnose and stem end rot pathogens by endogenous antifungal substances and a surface inhabiting Pestalotiopsis sp. In G. I. Johnson, E. Highley, & D. C. Joyce (Eds.), ACIAR proceedings of the disease resistance of fruit (Vol. 80, pp. 72–77).
Adikaram, N. K. B., Joyce, D. C., & Terry, L. A. (2002). Biocontrol activity and induces resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australasian Plant Pathology, 31(3), 223–229.
Al-Mughrabi, K. I., Vikram, A., Peters, R. D., Howard, R. J., Grant, L., Barasubiye, T., et al. (2013). Efficacy of Pseudomonas syringae in the management of potato tuber diseases in storage. Biological Control, 64(3), 315–322.
Castoria, R., De Curtis, F., Lima, G., Caputo, L., Pacifico, S., & De Cicco, V. (2001). Aureobasidium pullulans (LS-30), an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biology and Technology, 22(1), 7–17.
Castoria, R., De Curtis, F., Lima, G., & De Cicco, V. (1997). β-1,3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases. Postharvest Biology and Technology, 12, 293–300.
Castoria, R., & Wright, S. A. I. (2010). Host responses to biological control agents. In M. L. Gullino & D. Prusky (Eds.), Postharvest pathology (pp. 171–181). New York: Springer. ISBN 978-1-4020-8931-2.
Dann, E. K., & Deverall, B. J. (2000). Activation of systemic disease resistance in pea by an avirulent bacterium or a benzothiadiazole, but not by a fungal leaf spot pathogen. Plant Pathology, 49(3), 324–332.
Everett, K. R. (2002). Avocado fruit rots: A review of industry funded research. New Zealand Avocado Growers Association Annual Research Report, 2, 2–16.
Faquihi, H., Mhand, R. A., Ennaji, M., Benbouaza, A., & Achbani, E. (2014). Aureobasidium pullulans (De Bary) G. Arnaud, a biological control against soft rot disease in potato caused by Pectobacterium carotovorum. International Journal of Science and Research, 3(10), 1779–1786.
Fravel, D. R. (2005). Commercialization and implementation of biocontrol. Annual review of Phytopathology, 43, 337–359.
Guijarro, B., Melgarejo, P., Torres, R., Lamarca, N., Usall, J., & Cal, A. D. (2007). Effects of different biological formulations of Penicillium frequentans on brown rot of peaches. Biological Control, 42(1), 86–96.
Ippolito, A., Ghaouth, A. E., Wilson, C. L., & Wisniewski, M. (2000). Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biology and Technology, 19, 265–272.
Janisiewicz, W. J., & Korsten, L. (2002). Biological control of postharvest diseases of fruits. Annual review of Phytopathology, 40, 411–441.
Karunanayake, K. O. L. C. (2008). Natural defence mechanisms in mango fruit and their potential in management of postharvest diseases. Ph.D Thesis, University of Peradeniya, Sri Lanka p. 296.
Korsten, L., Bezuidenhout, J. J., & Kotze, J. M. (1988). Biological control of postharvest diseases of avocado. South African Avocado Growers’ Association Yearbook, 11, 75–78.
Korsten, L., & Jager, E. E. D. (1995). Mode of action of Bacillus subtilis for control of avocado postharvest pathogens. South African Avocado Growers’ Association Yearbook, 18, 124–130.
Kudanga, T., & Mwenje, E. (2005). Extracellular cellulase production by tropical isolates of Aureobasidium pullulans. Canadian Journal of Microbiology, 51(9), 773–776.
Loncaric, I., Oberlerchner, J. T., Heissenberger, B., & Moosbeckhofer, R. (2009). Phenotypic and genotypic diversity among strains of Aureobasidium pullulans in comparison with related species. Antonie van Leeuwenhoek, 95(2), 165–178.
Noronha, E. F., & Ulhoa, C. J. (2000). Characterization of a 29-kDa β-1,3-glucanase from Trichoderma harzianum. FEMS Microbiology Letters, 183, 119–123.
Prusky, D., Freeman, S., Rodriguez, R. J., & Keen, N. T. (1994). A nonpathogenic mutant strain of Colletotrichum magna induces resistance to C. gloeosporioides in avocado fruits. Molecular Plant Microbe Interactions, 7(3), 326–333.
Prusky, D., & Keen, N. T. (1993). Involvement of preformed antifungal compounds in the resistance of subtropical fruits to fungal decay. Plant Disease, 77(2), 114–118.
Prusky, D., Keen, N. T., Sims, J. J., & Midland, S. L. (1982). Possible involvement of an antifungal di-ene in the latency of Colletotrichum gloeosporioides on unripe avocado fruits. Phytopathology, 72(12), 1578–1582.
Prusky, D., Kobiler, I., Fishman, Y., Sims, J. J., Midland, S. L., & Keen, N. T. (1991). Identification of an antifungal compound in unripe avocado fruits and its possible involvement in the quiescent infections of Colletotrichum gloeosporioides. Journal of Phytopathology, 132(4), 319–327.
Saborowski, R., Buchholz, F., Vetter, R. A. H., Wirth, S. J., & Wolf, G. A. (1993). A soluble dye-labelled chitin derivative adapted for the assay of krill chitinase. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 105(3), 673–678.
Schena, L., Ippolito, A., Zahavi, T., Cohen, L., Nigro, F., & Droby, S. (1999). Genetic diversity and biocontrol activity of Aureobasidium pullulans isolates against postharvest rots. Postharvest Biology and Technology, 17(3), 189–199.
Schena, L., Nigro, F., Pentimone, I., Ligorio, A., & Ippolito, A. (2003). Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biology and Technology, 30(3), 209–220.
Sharma, R. R., Singh, D., & Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control, 50(3), 205–221.
Sivanathan, S., & Adikaram, N. K. B. (1989). Biological activity of four antifungal compounds in immature avocado. Journal of Phytopathology, 125(2), 97–109.
Spadaro, D., & Droby, S. (2016). Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends in Food Science & Technology, 47, 39–49.
Sylla, J., Alsanius, B. W., Krüger, E., Reineke, A., Bischoff-Schaefer, M., & Wohanka, W. (2013). Introduction of Aureobasidium pullulans to the phyllosphere of organically grown strawberries with focus on its establishment and interactions with the resident microbiome. Agronomy, 3(4), 704–731.
UC IPM Pest Management Guidelines. (2008). Integrated pest management for avocados. University of California. UC ANR Publication 3503:222. http://ipm.ucanr.edu/PMG/selectnewpest.avocado.html. Accessed 25 Jan 2016.
Wachowska, U., & Glowacka, K. (2014). Antagonistic interactions between Aureobasidium pullulans and Fusarium culmorum, a fungal pathogen of winter wheat. BioControl, 59(5), 635–645.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhupani, Y.D.S., Adikaram, N.K.B. Delayed incidence of stem-end rot and enhanced defences in Aureobasidium pullulans-treated avocado (Persea americana Mill.) fruit. J Plant Dis Prot 124, 227–234 (2017). https://doi.org/10.1007/s41348-017-0086-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0086-8