Abstract
Shoot blight and branch dieback of English walnut has been associated with a broad diversity of Botryosphaeriaceae and Diaporthe fungi worldwide. These pathogens affect both wood and fruit tissues, infecting the tree through mechanical or natural wounds. Fruit infections can play an important role in the life cycle of the disease. Thus, the effects of cultivar, and fruit maturity on English walnut fruit infection by Botryosphaeriaceae (Botryosphaeria dothidea, Neofusicoccum mediterraneum and N. parvum) and Diaporthe (Diaporthe neotheicola and Dia. rhusicola) fungi were evaluated. The infection and disease progress from inoculated attached fruits or by inoculating fruit abscission wounds was evaluated both under laboratory and field conditions. An initial experiment evaluating two inoculation methods was conducted, but there were not significant differences in disease severity between inoculations with mycelial plugs or conidial suspensions. A total of eight cultivars were selected to evaluate their susceptibility to fruit infection, with ‘Chandler’ being the most susceptible for all the pathogens tested compared to the other cultivars. Botryosphaeriaceae showed higher aggressiveness on fruit collected at beginning- or middle summer, while Diaporthe showed similar aggressiveness regardless of fruit maturity stage. Botryosphaeriaceae fungi were able to colonize the entire surface of the inoculated fruit, reaching the peduncle and infecting the attached shoot; while Diaporthaceae fungi were not able to colonize the surface of the inoculated fruit quickly enough to infect the attached shoot before the peduncle was naturally separated from the shoot. Finally, we demonstrated that both Botryosphaeriaceae and Diaporthe fungi can infect shoots by inoculating the natural fruit abscission wounds in the field. This study generates new insights into the influence of fruit infection leading to shoot blight and branch dieback of English walnut caused by Botryosphaeriaceae and Diaporthe fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The English walnut (Juglans regia L.) is a crop of a major economic importance in China, USA and in Mediterranean countries such as France and Spain (INC 2017; López-Larrinaga et al. 2017; FAO 2020). The global harvested surface is 1,021,391 ha and the total world production of walnuts in shells is 3,323,964 t (FAO 2020). Among the Mediterranean walnut producer countries, Spain counts with a total harvested surface of 12,781 ha and 18,883 t of production of walnuts in shell (MAPA 2021). In this country, the main walnut growing region is Andalusia (southern Spain), with 2,450 ha and 4,052 t of walnut production in shell (MAPA 2021).
The most important fruit disease of this crop described in Spain is walnut blight, which is caused mainly by Xanthomonas arboricola pv. juglandis. This bacterium is also sometimes associated with many other bacteria and fungi that co-infect fruits increasing the severity of the disease (Lovera et al. 2017a, b). Recently, Botryosphaeriaceae (Botryosphaeria dothidea, Diplodia seriata, Dothiorella sarmentorum, Neofusicoccum mediterraneum and N. parvum) and Diaporthe (Diaporthe amygdali, Dia. neotheicola and Dia. rhusicola) fungi have been described in southern Spain associated with canker, branch dieback and shoot blight of English walnut (López-Moral et al. 2020). Although Botryosphaeriaceae and Diaporthe fungi have been reported in the main walnut growing areas worldwide, i.e., California (Chen et al. 2014; Michailides and Hasey 2010; Trouillas et al. 2010), China (Li et al. 2015), Chile (Díaz et al. 2018), Spain (López-Moral et al. 2020), Czech Republic (Eichmeier et al. 2020), and Italy (Gusella et al. 2020), causing damages in shoots and branches, previous studies suggest that these pathogens can also infect walnut fruit (Agustí-Brisach et al. 2019; Chen et al. 2014; López-Moral et al. 2020). These pathogens can survive in the hulls playing a key role in subsequent outbreaks of the disease (Michailides and Hasey 2010; López-Moral et al. 2020). In fact, the pathogenicity of Botryosphaeriaceae and Diaporthe fungi to walnut fruit has been already demonstrated in California (Chen et al. 2014) and in Spain (López-Moral et al. 2020) as well as the effect of co-infections of N. mediterraneum and Dia. neotheicola on fruit infection (Agustí-Brisach et al. 2019).
Botryosphaeriaceae and Diaporthe fungi are characterized by the production of fruiting bodies (pycnidia) on old or dead tissues that remain in the tree canopy or in the soil (i.e., pruning and harvest debris), where produce a long-viable spore. The spores are the primary inoculum of the pathogens which are spread mainly by wind and rain splash causing infections through wounded tissues from late-winter to summer (Michailides and Hasey 2010; Moral et al. 2019; López-Moral et al. 2020). It is well known that the environmental (i.e., temperature, relative humidity, rainfall, etc.) and agronomic factors (i.e., cultivar, cultural practices, etc.) can influence the tree infection by these pathogens and the disease progress (Moral et al. 2019). In this way, the effects of cultivar, shoot-branch age, and temperature during shoot infections by Botryosphaeriaceae and Diaporthe fungi has been recently studied on English walnut (López-Moral et al. 2022). These authors showed that i) the cultivar susceptibility varies depending on the fungal species tested, with ‘Chandler’ being the most tolerant cultivar; ii) one-year-old shoots are more sensitive to N. parvum and Dia. neotheicola infections compared to 2- to 4-years-old branches; and iii) the optimum temperature for shoot infection is around 26 ºC for N. parvum and 21 ºC for Dia. neotheicola. This study was helpful to improve our knowledge on the biology and epidemiology of the disease with regards to shoot infection.
Nevertheless, the influence in the fungal infection of several factors with regards to fruit such as walnut cultivar or fruit maturity stage is still poorly understood. Although walnut fruit infections by Botryosphaeriaceae and Diaporthe are not of major economic importance regarding fruit production, elucidating the role of fruit infection in the disease progress would be essential to continue understanding the life cycle of the pathogens associated with shoot blight and branch dieback of English walnut. Therefore, the goals of this study were (i) to evaluate the cultivar susceptibility and the effect of fruit maturity on the infection of walnut fruit by Botryosphaeriaceae and Diaporthe fungi, and (ii) to determine the role of natural fruit abscission wounds in infection of shoots by these pathogens in laboratory and field conditions.
Materials and methods
Fungal isolates and inoculum preparation
Four representative isolates belonging to Botryosphaeriaceae (isolates Botryosphaeria dothidea ColPat-443, Neofusicoccum mediterraneum ColPat-556, and N. parvum ColPat-526 and ColPat-554); and three belonging to Diaporthe (isolates Diaporthe neotheicola ColPat-448 and ColPat-532, and Dia. rhusicola ColPat-444) were selected for this study. They all were previously characterized as causal agents of shoot blight and branch dieback of English walnut in southern Spain (López-Moral et al. 2020), and they are maintained as single spore cultures (Dhingra and Sinclair 1995) in the collection of the Department of Agronomy at the University of Cordoba (Spain). Fresh colonies of all the isolates were obtained from the collection stock cultures by growing them onto potato dextrose agar (PDA; Difco Laboratories®, Detroit, USA) at 23 ± 2 °C under a 12-h diurnal photoperiod of cool fluorescent light (350 µmol m–2 s–1) for 10 days, and served as inoculum for the experiments described below. Additionally, pistachio leaf agar (PLA) was used to induce the formation of pycnidia of each fungal species towards preparing conidial suspensions (5 × 104 conidia mL− 1) for further inoculations as described by López-Moral et al. (2022).
Plant material
Detached healthy and unwounded fruit (hulls) of the six commercial cultivars, Chandler, Hartley, Howard, Serr, Sundland, and Tulare, were used in this study to evaluate their susceptibility against all the fungal species described above under controlled laboratory conditions. ‘Chandler’ was the only cultivar used for the remaining experiments conducted in this study in both laboratory and field conditions. The plant material was collected from a 10-year-old experimental field located in Alcalá del Río (Sevilla Province, Andalusia region, southern Spain), which belongs to the Andalusian Institute for Research and Formation in Agriculture and Fishery (IFAPA in Spanish). This experimental field was used also to conduct the experiments under field conditions. The detached fruit used for inoculations in the laboratory were superficially cleaned by dipping in a 0.02% Tween 20 solution for 1 min, surface-disinfected for 1 min in 20% sodium hypochlorite solution (Cl at 5 g L− 1), washed twice with distilled water, and air dried for 30 min; whereas the attached fruit used for field inoculations were superficially disinfested by spraying them with 70% ethanol solution.
Effect of inoculation method on detached walnut fruit
The isolates N. parvum ColPat-554 and Dia. neotheicola ColPat-448 and green fruit (1 month after fruit set) of walnut cv. Chandler collected at the end of spring (June 5th 2019) were used in this experiment. After surface disinfection as described above, the fruit were placed in humidity chambers, and a wound of 6.0-mm diameter × 4.0-mm depth was done in the center of each fruit hull cheek, using a sterile cork-borer. Inoculations were conducted by placing a 6.0-mm in diameter mycelial plug or with a 20-µl drop of 5 × 104 conidia ml− 1 conidial suspension inside the hole; and the inoculated fruits were incubated at 27 °C and 100% relative humidity (RH) for three weeks. There were three replicated humidity chambers per fungal isolate and inoculation method combination with 10 fruits per humidity chamber. Additionally, two lots of 30 fruits each (three humidity chambers, 10 fruits in each) were treated with a 6.0-mm diameter PDA plug or with 20-µl drop of sterile distilled water (SDW) and used as controls. A factorial block design was used with the fungal isolate, the inoculation method and their interaction as the independent variables, the fruits as replications and the humidity chambers as repetitions. Disease Severity (DS) was evaluated by measuring both the smallest and largest diameters of the necrotic lesions developed on fruit surface. Evaluations were conducted every three days until most of the fruits inoculated with the most aggressive pathogen reached a 90 to 100% surface affected. The percentage of the affected fruit surface of the 10 fruits of each block (humidity chamber) were averaged, and the relative area under the disease progress curve (RAUDPC) was estimated for each inoculated or control fruit as described by López-Moral et al. (2017, 2020).
Symptoms of fruit rot were reproduced on inoculated fruit using both inoculation methods, with those on fruits inoculated by wounding and a mycelial plug deposition method as the most aggressive. For this reason, the wounding and mycelial plug deposition method was selected for further analysis.
Suceptibility of six English walnut cultivars to Botryosphaeriaceae and Diaphorte fungi on detached walnut fruit
The isolates B. dothidea ColPat-443, N. mediterraneum ColPat-556, N. parvum ColPat-554, Dia. neotheicola ColPat-448, and Dia. rhusicola ColPat-444 were used. Green walnut fruit (1 month after fruit set) of the six commercial cvs. listed above were collected at the end of spring (June 4th 2020). After surface disinfection, the fruit were inoculated under controlled laboratory conditions by the wounding and mycelial plug deposition method as described above. Humidity chambers containing the inoculated walnut fruits were incubated as described above. There were three replicated humidity chambers per fungal isolate and cultivar combination, and 10 fruits per humidity chamber. One additional lot of 30 fruits per commercial cultivar (three humidity chambers per lot, 10 fruits in each) treated with a 6.0-mm in diameter PDA plug were included as controls. A factorial block design was used with the fungal isolate, the cultivar and their interaction as the independent variables, the fruits as replications and the humidity chambers as repetitions. DS, the percentage of the affected fruit surface, and the RAUDPC for each inoculated or control fruit were assessed as described above.
Effect of fruit maturity on disease development
The isolates N. parvum ColPat-526 and ColPat-554 and Dia. neotheicola ColPat-448 and ColPat-532 were used. Green walnut fruit of cv. Chandler were harvested at different maturity stages during fruit setting: (i) one month after fruit set stage (MAFS; maturity stage 1); (ii) two MAFS (maturity stage 2); (iii) three MAFS (maturity stage 3); (iv) four MAFS (maturity stage 4); and (v) five MAFS, just before the natural hull split (maturity stage 5). The fruit were washed, disinfested, and placed in humidity chambers as described before. There were three replicated humidity chambers per fungal isolate and fruit maturity stage, and 10 fruits per humidity chamber. One additional lot of 30 fruits per fruit maturity stage (three humidity chambers per lot, 10 fruits in each) treated with a 6.0-mm in diameter PDA plug were included as controls. A factorial block design was used with the fungal isolate, the fruit maturity stage and their interaction as the independent variables, the fruits as replications and the humidity chambers as repetitions. DS, the percentage of the affected fruit surface and the RAUDPC for each inoculated or control fruit were assessed and obtained as described before.
Infection and disease progress on shoots from inoculated attached fruits under controlled laboratory conditions
The isolates B. dothidea ColPat-443, N. mediterraneum ColPat-556, N. parvum ColPat-554, Dia. neotheicola ColPat-448, and Dia. rhusicola ColPat-444 were used. Apical shoots with two attached fruits of cv. Chandler (1 month after fruit set) were carefully cut at the end of spring (June 4, 2020). The cut shoots were immediately wrapped with Parafilm® at the end, and kept at 4 ºC until being processed in the laboratory. Once in the laboratory, plant material was disinfested as described before and placed in humidity chambers (56 × 18 × 41 cm plastic containers). Inoculations were conducted in the center of the hull by the wounding and mycelial plug deposition method as described before; and the humidity chambers were incubated at 27 ºC in the dark at 100% RH. There were three replicated humidity chambers per fungal isolate, and five shoots with 10 attached fruits per humidity chamber. Additionally, 15 shoots with two attached fruits (three humidity chambers, five shoots and 10 fruits in each) treated with a 6.0-mm in diameter PDA plug were included as controls. A randomized complete block design was used with the fungal isolate as the independent variables, the shoots with two attached fruits as replications and the humidity chambers as repetitions. The disease progress was observed periodically until three weeks after inoculation, and the internal discoloration developed in the terminal end of the shoot, close the peduncles, was measured at the end of the experiment, and averaged for each five shoots of each repetition.
To check that shoot infections were caused by the pathogen used to inoculate the attached fruit, reisolations from inoculated attached fruit and from infected fruit were conducted. To this end, small fragments of tissues from the margin of the affected area in both shoot and their respective attached fruit, and from asymptomatic wood tissues 2-cm beyond the margin of the shoot lesion were surface disinfested by dipping the fragments into a commercial bleach (Cl at 50 g liter–1) solution at 10% (vol/vol) in sterile water for 2 min, rinsed with SDW, air dried for 15 min, and plated on PDA acidified with lactic acid [2.5 ml of 25% (vol/vol) per liter of medium] (APDA). Petri dishes were incubated for 5 to 14 days at 25 °C in the dark until colonies were large enough to be examined, and the percentages of isolation (%) were determined. Three shoots with their respective inoculated attached fruit per fungal species or control were selected for reisolations, and two Petri dishes [7 tissue pieces (attempts of isolation) in each] per tissue and fungal species or control combination were used. The reisolated fungal colonies were morphologically compared with the original ones to verify that they were the same isolate used in the inoculation experiment.
Infection and disease progress on shoots from natural wounds in the field
The isolates N. parvum ColPat-554, and Dia. neotheicola ColPat-448 were used in this experiment. Six walnut trees of cv. Chandler were randomly selected in the experimental field described above. At middle autumn, two opposite apical shoots with natural fruit-abscission wound were selected per tree, also considering one in the north and the second one in the south sides of the tree canopy (12 inoculated attached shoots per isolate). The shoots were inoculated by spraying the abscission wound with 1 mL of conidial suspension of the pathogens obtained as described above. Subsequently, to favor conidial germination on the inoculated tissues, the pruned and inoculated shoots were covered for 72 h with a plastic bag sprayed into with SDW as humidity chamber, that was lined with a paper bag to prevent desiccation by sunlight. Prior to inoculation, the inoculated area was surface-disinfected by spraying with 70% ethanol solution, and air-dried for 15 min. Additionally, 12 wounded shoots were treated with 1 mL of SDW as a control. A randomized complete block design was used with fungal isolates as the independent variable, the shoots as replications and the trees as blocks. DS was assessed by measuring the lesion length from the inoculated point at 6 months after inoculation. Reisolations were also conducted from the margin of the necrotic lesions as described above when it was possible.
Data analysis
For each experiment, data were tested for normality and homogeneity of variances, and logarithmical transformed when necessary. Factorial ANOVA were conducted in all the experiments of this study according to their experimental design and subsequently, independent ANOVA were conducted when significant interaction (P ≤ 0.05) between independent variables occurred to determine the effect of the inoculation methods on the infection, differences in susceptibility of walnut cultivars and their interaction with fungal species, and the effect of fruit maturity on disease development on inoculated fruit, with RAUDPC (%) being the dependent variable. For the inoculation methods experiment, independent ANOVA and means comparison tests were conducted for each fungal species to determine the differences in DS between inoculation methods. For the interaction between walnut cultivars and fungal species, independent ANOVA and means comparison tests were conducted for each fungal species to determine differences in susceptibility between cultivars. For the experiments evaluating the effect of maturity stage on fruit infection, data of RAUDPC (%) from each fungal species were subjected to independent ANOVA. Finally, ANOVA was also conducted to determine the difference in aggressiveness between fungal species on the disease progress [RAUDPC (%); lesion length (mm)] in shoots from inoculated attached fruits in both laboratory and field conditions or in shoots inoculated in the abscission point in the field. In addition, in the experiment evaluating the infection from inoculated fruit to the attached shoot in the laboratory, the Pearson correlation coefficient (r) between RAUDPC (%) on inoculated fruit and lesion length (mm) on the respective infected shoot was obtained using the average of the values of both disease parameters for each of the five fungal species tested (n = 5). In all cases, means were compared using Fisher’s protected LSD test at α = 0.05 (Steel et al. 1985). All data were analyzed using Statistix 10 (Analytical software 2013).
Results
Effect of inoculation method on detached walnut fruit
There were not significant differences between inoculation methods in fruit infection for both N. parvum ColPat-554 (P = 0.1994) and Dia. neotheicola ColPat-448 (P = 0.2254). For N. parvum ColPat-554, RAUDPC values were 98.4 ± 0.5 and 83.8 ± 8.2% for inoculations with mycelial plugs and conidial suspensions, respectively. For Dia. neotheicola ColPat-448, RAUDPC values were 70.3 ± 2.1 and 65.7 ± 1.3% for inoculations with mycelial plugs and conidial suspensions, respectively (Fig. 1).
Relative area under the disease progress curve (RAUDPC; %) for detached walnut fruit of cv. Chandler inoculated with mycelial plugs (dark gray columns) or conidial suspensions (light gray columns) of Neofusicoccum parvum ColPat-554 and Diaporthe neotheicola ColPat-448. Columns are the mean of three replicated humidity chambers, and 10 fruit per humidity chamber. Vertical bars are the standard error of the means. In each graph, columns with the same letter do not differ significantly according to Fisher’s LSD test at P = 0.05
Suceptibility of six English walnut cultivars to Botryosphaeriaceae and Diaphorte fungi on detached walnut fruit
Botryosphaeriaceae fungi were more aggressive than Diaporthe species in all the cultivars evaluated, with N. parvum isolate ColPat-554 being the most aggressive in all cases. The cultivar susceptibility for fruit infection was analyzed separately for each fungal species since factorial ANOVA showed significant differences between cultivars, fungal species, and their interaction (P ≤ 0.0001 in all cases). There were significant differences between cultivars for all the fungal species tested (P ≤ 0.0001). The cultivar susceptibility varied depending on the fungal species, with ‘Chandler’ showing significantly higher values of RAUDPC for all the fungal species tested. On the contrary, ‘Serr’ was the most tolerant cultivar for all the fungal species tested except for Dia. rhusicola. ‘Hartley’ was among the most tolerant cultivars for B. dothidea, N. mediterraneum and Dia. rhusicola; and ‘Sundland’ resulted tolerant for N. parvum, Dia. neotheciola and Dia. rhusicola. ‘Tulare’ or ‘Howard’ were also tolerant for B. dothidea or N. mediterraneum, respectively. Finally, ‘Howard’ and ‘Sundland’ showed intermediate susceptibility for B. dothidea; ‘Sundland’ and ‘Tulare’ for N. mediterraneum; and ‘Hartley’ and ‘Howard’ for N. parvum. ‘Howard’ and ‘Tulare’ also showed intermediate susceptibility for both Dia. neotheicola and Dia. rhusicola (Table 1).
Effect of fruit maturity on disease development
The effects of maturity stage on disease development of walnut fruit of cv. Chandler inoculated with N. parvum isolates ColPat-526 and ColPat-554 and Dia. neotheicola ColPat-448 and ColPat-532 are shown in Fig. 2. No significant differences in RAUDPC for the variable fungal species (P = 0.2198) were observed, but the variable maturity stage (P = 0.0343), and the interaction between fungal species and maturity stages (P ≤ 0.0001) showed significant differences in RAUDPC. Therefore, the effect of maturity stage on fruit infection was analyzed separately for each fungal species. Also, data of the mean values of the two isolates of each species could not be combined since significant differences were observed between them as well as for the interaction between fungal isolates and maturity stage variables (P ≤ 0.05). For N. parvum, the most sensitive maturity stages were at 2- or 3 MAFS (middle summer), whereas the less sensitive maturity stages were at 1- or 5 MAFS (late spring-beginning summer, or beginning autumn, respectively). On the contrary, for Dia. neotheicola isolate ColPat-448, the most sensitive maturity stages were at 1-, 4-, or 5 MAFS (late spring-beginning summer, late summer, or beginning autumn, respectively) and the least sensitive maturity stages were at 2- or 3 MAFS (late spring-beginning summer, or late summer-beginning autumn, respectively). Finally, even though no significant differences between maturity stages on fruit infection by Dia. neotheicola isolate ColPat-532 were observed, this isolate showed a similar pattern with that for Dia. neotheicola isolate ColPat-444.
Relative area under the disease progress curve (RAUDPC; %) for detached fruits of walnut cv. Chandler of the five selected maturity stage (1-, 2-, 3-, 4-, and 5- months after fruit set) inoculated with mycelial plugs of (A) Neofusicoccum parvum isolates ColPat-554 and ColPat-526; or (B) with Diaporthe neotheicola isolates ColPat-448 and ColPat-532. For each fungal isolate and maturity stage combination, columns are the mean of three replicated humidity chambers and 10 detached fruits per humidity chamber. Vertical bars are the standard error of the means. For the graphs (A) or (B), columns with the same lower case or capital letter do not differ significantly for RAUDPC between N. parvum or Dia. neotheicola isolates, respectively, according to Fisher’s LSD test at P = 0.05
Infection and disease progress on shoots from inoculated attached fruits under controlled laboratory conditions
All the Botryosphaeriaceae fungi were able to colonize the entire surface of the inoculated fruit with two to three weeks after inoculation, reaching the peduncle and, subsequently, infecting the attached shoot (Fig. 3). However, Diaporthe fungi were not able to colonize the surface of the inoculated fruit quickly enough to infect the attached shoot before the peduncle was naturally separated from the shoot. Consequently, internal wood discoloration with basipetal advance was observed in the infected shoots for the three Botryosphaeriaceae isolates; while no shoot infections occurred by any of Diaporthe fungi. For fruit infections, there were significant differences on RAUDPC between fungal species (P ≤ 0.0001), ranging from 77.8 ± 0.6 to 11.0 ± 0.6% for the isolates N. parvum ColPat-554 and Dia. neotheicola ColPat-448, respectively. Regarding shoot infection, significant differences were observed between Botryosphaeriaceae fungi for the variable lesion length (P = 0.0434), ranging between 39.2 ± 10.6 and 2.2 ± 0.9 mm for the isolates N. parvum ColPat-554 and B. dothidea ColPat-443, respectively. In this last case, Diaporthe isolates were excluded from the analysis since, they were not able to reach the attached shoot from the respective inoculated fruit (Fig. 3). All the fungal species were reisolated from the margin of the necrotic lesions developed on the inoculated fruit, with the frequency of reisolation being higher for Botryosphaeriaceae fungi (85.7 to 100%) than for Diaporthe (50.0 to 78.6%). Species of Neofusicoccum were the only ones that showed positive reisolations from tissues collected from 2-cm beyond the margin of the necrotic shoot lesion, but in a small frequency of isolation (7.1%) (Table 2). In addition, there was a positive significant linear correlation for the aggressiveness of the fungal species tested between fruit colonization (RAUDPC) and shoot infection (lesion length) (r = 0.9999; P = 0.0047).
(A) Relative area under the disease progress curve (RAUDPC, %; dark gray columns) and lesion length (mm; light gray columns) for detached fruits of walnut cv. Chandler inoculated with mycelial plugs of the isolates Botryosphaeria dothidea ColPat-443, Neofusicoccum mediterraneum ColPat-556, N. parvum ColPat-554; and Diaporthe neotheicola ColPat-448, and Dia. rhusicola ColPat-444; and the respective lesion developed on infected shoots, respectively, at three weeks after inoculation. For both disease parameters, columns are the mean of three replicated humidity chambers and five detached shoots with attached fruit per humidity chamber. Vertical bars are the standard error of the means. Columns with the same lowercase or capital letter do not differ significantly for RAUDPC or lesion length, respectively, according to Fisher’s LSD test at P = 0.05. *Data excluded from the statistical analysis (Lesion length = 0.0 mm); (B, C) detail of lesions develops in walnut fruit and shoots (red arrow) at three weeks after inoculation with N. parvum or Dia. neotheicola, respectively
Infection and disease progress on shoots from natural wounds in the field
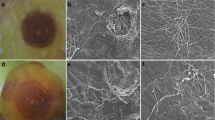
Walnut shoots inoculated with N. parvum isolate ColPat-554 and Dia. neotheicola ColPat-448 showed necrotic lesions and wood discoloration from the inoculated point at 6 months after inoculation (Fig. 4). Non-inoculated control shoots did not show lesions. Although the mean value of the lesions developed in shoots inoculated with N. parvum was larger (48.4 mm) than the mean value observed for isolate Dia. neotheicola (25.1 mm), no significant differences in aggressiveness were observed between the two species (P = 0.5975). Both pathogens were consistently reisolated from the inoculated shoots, with a reisolation success of 100 and 88.1% for N. parvum and Dia. neotheicola, respectively.
Necrotic lesions and internal wood discoloration develop in attached shoots at 6 months after inoculation by spraying the abscission wound with conidial suspensions of Neofusicoccum parvum ColPat-554 (A, B) and Diaporthe neotheicola ColPat-448 (C, D). (red arrow: external margin of the cankers; opposite arrows show infection internally in the pith)
Discussion
This study was conceived to demonstrate the role of fruit infection in the disease progress of shoot blight and branch dieback of English walnut caused by Botryosphaeriaceae and Diaporthe fungi.
Symptoms of fruit rot were reproduced on inoculated fruit using both mycelial plugs and conidial suspension inoculation methods. When fruit inoculations were conducted by mycelial plugs, the onset and development of symptoms were faster than those from inoculations with conidial suspensions. However, there were no significant differences in severity between inoculation methods for each fungal species tested. For these reasons, the wounding and mycelial plug deposition method was selected to facilitate disease assessments in subsequent experiments of this study. Although the mycelial inoculation method does not occur usually in natural conditions, it was selected since results did not differ from those of inoculation with the spore suspension. It should be noted, however, that the inoculation using a mycelial plug bypasses the spore germination process and shortens the incubation time of the infection. Our results are in contrast with those obtained by López-Moral et al. (2022), who evaluated the effect of these two inoculation methods for the same fungal species on the infection of walnut shoots. Our previous work also demonstrated that the lesions caused by N. parvum were significantly greater than those caused by Dia. neotheicola, but the severity of the disease was significantly higher when shoots were inoculated with mycelial plugs. Despite these differences, the mycelial plug inoculation was chosen for further analysis, since similar past studies were also conducted on English walnut using the mycelial plug deposition method, with successful and acceptable results and for consistent parallel comparison with other published studies (Chen et al. 2014; Agustí-Brisach et al. 2019; López-Moral et al. 2020, 2022).
The effect of cultivar susceptibility on walnut fruit infection varied depending on the fungal species, with ‘Chandler’ being the most susceptible cultivar for both Botryosphaeriaceae and Diaporthe fungi. This result contrasts with that of López-Moral et al. (2022), who reported that ‘Chandler’ was the significantly most tolerant cultivar for all the Botryosphaeriaceae and Diaporthe fungi tested in shoot inoculations, both under laboratory and field conditions. Furthermore, Chen et al. (2014) showed that the cv. Chandler developed smaller canker lesions than the cvs. Vina and Tulare whose shoots were inoculated in the field during summer with Botryopshaeriaceae and Diaporthe fungi. These differences in susceptibility between studies could depend on the different inoculation time, since the time of inoculation may represent different age tissues as the experiments of Chen et al. (2014) were conducted in California. In the present study, the cv. Chandler in Spain was compared with cvs. Hartley, Serr, and Sundland, which were not included in the comparative susceptibility study of ‘Chandler’ in California. In the case of fruit infection, the effect of sap obtained from both shoots and hulls, and its composition, should be evaluated to find out why these differences in susceptibility can occur between cultivars. In addition, the composition and the effect of sap extract from different tissues such as shoots and hulls of the same cultivar against Botryosphaeriaceae and Diaporthe infections should be also evaluated.
Interestingly, there were significant differences on the behavior of Botryosphaeriaceae and Diaporthe fungi on fruit infection depending on the maturity stage of walnut fruit. Neofusicoccum parvum isolates were more aggressive in fruit at 2- or 3 MAFS developmental stages, corresponding to fruits collected in middle summer (July or August, respectively). Diaporthe neotheicola isolates did not show important differences in severity on inoculated fruit of different stages of maturity, although highest aggressiveness in fruit was observed at 1- or 5 MAFS developmental stages, corresponding to fruits collected in late spring-beginning summer, or beginning autumn, respectively (June or October, respectively). Our results suggest that the susceptibility of the fruit to N. parvum increases as the fruit ripens until middle summer, and then decreases again as the hull begins to dehydrate. Interestingly, the highest susceptibility observed in the hulls of fruit collected in summer coinciding with the hot temperatures that occur in that season. High temperatures favor fruit infection and disease development by Botryosphaeriaceae fungi more than by Diaporthe fungi. According to the previous results obtained by López-Moral et al. (2020, 2022), in which they showed that warm temperatures, between 25 and 30 ºC, are optimal for mycelial growth and shoot infection by Botryosphaeriaceae fungi, we hypothesized that the hot temperatures that occur in southern Spain between late spring to beginning autumn, can exacerbate the infection and disease progress in walnut trees, mainly by Botryosphaeriaceae fungi.
Finally, the study evaluating the infection and disease progress on shoots from inoculated attached fruits under controlled laboratory conditions showed that Botryosphaeriaceae fungi can colonize quickly the entire surface of the inoculated fruit reaching the peduncle, and subsequently, infecting and colonizing the attached shoot. These results reinforce the hypothesis that Botryosphaeriaceae fungi can infect walnut shoots from the previously infected walnut hulls through the peduncle or probably, through the abscission point (peduncle wound) after natural fruit falling or after harvest. Reisolations from the respective attached shoots infected through fruit inoculation demonstrated that the infection and disease progress occurred for each of the pathogens tested since the three Botryosphaeriaceae species were consistently reisolated (50.0 to 100%) from the margin of the necrotic lesions. In addition, we demonstrated that both Botryosphaeriaceae and Diaporthe fungi can infect shoots through the natural fruit abscission wounds in the field. It is well known that Botryopshaeriaceae fungi can infect abscission wounds (fruit scars, bud, and leaf scars) as it was shown in pistachio, another major nut crop (Michailides 1991). Additionally, leaf scar and catkin scar infections by Botryosphaeriaceae fungi can easily be found in walnuts grown next to Sacramento River in California (T. Michailides, unpublished data).
In summary, this study generates new insights into the influence of fruit infection leading to shoot (spur) blight and branch dieback of English walnut caused by Botryosphaeriaceae fungi. Although Diaporthe fungi infected fruit, they were not able to invade the spur, suggesting that to find under field conditions Diaporthe spp. on spurs, shoots and branches could be the results of other avenues of infection instead of fruit infection. However, because the inoculations with Diaporthe spp. represent results in the laboratory, they did not represent strictly infection conditions of fruits attached on the tree. Therefore, this experiment needs to be expanded to inoculations in the field in a future study. Our results suggest that the natural abscission wounds (scars) caused during harvest serve as potential infection courts by Botryosphaeriaceae and Diaporthe fungi. For these reasons, further studies to evaluate the effectiveness of fungicides preventing infections through the abscission wounds are needed. Finally, evaluating the dehydrated hulls that remain in the soil of the orchard after harvest under dry winter conditions as potential inoculum sources for new outbreaks in the next season should be also studied to continue the elucidation of this disease and the life cycle of pathogens in English walnut orchards.
Conclusions
This study showed the effect of fruit infection by fungal species belonging to Botryosphaeriaceae and Diaporthaceae families on the progress and severity of shoot blight and branch dieback in English walnut. The experiment evaluating cultivar susceptibility suggested that the cv. Chandler was the most susceptible to fruit infection by these pathogens compared to the other cultivars tested. The DS caused by N. parvum was significantly higher in fruits at mature stages comprised in middle summer compared to those at late-spring or beginning-summer; while Dia. neotheicola showed lower aggressiveness in all the mature stages evaluated. Botryosphaeriaceae fungi can infect and colonize quickly the entire surface of the inoculated fruit reaching the peduncle, and subsequently, colonizing the attached shoot. Finally, we demonstrated that both N. parvum and Dia. neotheicola can infect shoots through the natural fruit abscission wounds in the field, with N. parvum showing higher aggressiveness.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Agustí-Brisach C, Moral J, Felts D, Trapero A, Michailides TJ (2019) Interaction between Diaporthe rhusicola and Neofusicoccum mediterraneum causing branch dieback and fruit blight of English walnut in California, and effect of pruning wounds to the infection. Plant Dis 103:1196–1205. https://doi.org/10.1094/PDIS-07-18-1118-RE
Analytical Software (2013) Statistix10. User’s manual. Tallahassee, FL
Chen SF, Morgan DP, Hasey JK, Anderson K, Michailides TJ (2014) Phylogeny, morphology, distribution, and pathogenicity of Botryosphaeriaceae and Diaporthaceae from English walnut in California. Plant Dis 98:636–652. https://doi.org/10.1094/PDIS-07-13-0706-RE
Dhingra OD, Sinclair JB (1995) Basic Plant Pathology Methods, second edn. CRC Press, Boca Raton, FL
Díaz GA, Latorre BA, Ferrada E, Gutiérrez M, Bravo F, Lolas M (2018) First report of Diplodia mutila causing branch dieback of English walnut cv. Chandler in the Maule Region, Chile. Plant Dis 102:1451. https://doi.org/10.1094/PDIS-11-17-1860-PDN
Eichmeier A, Pecenka M, Spetik M, Necas T, Ondrasek I, Armengol J, León M, Berlanas C, Gramaje D (2020) Fungal trunk pathogens associated with Juglans regia in the Czech Republic. Plant Dis 104:761–771. https://doi.org/10.1094/PDIS-06-19-1308-RE
FAO (2020) Food and Agriculture Organization of the United Nations, Statistical Databases. http://www.fao.org/faostat/es/#data/QC(Accessed 27/12/2022).
Gusella G, Giambra S, Conigliaro G, Burruano S, Polizzi G (2020) Botryosphaeriaceae species causing canker and dieback of English walnut (Junglans regia) in Italy. For Pathol 51:e12661. https://doi.org/10.1111/efp.12661
International Nut and Dried Fruit Council (INC) (2017) https://www.nutfruit.org/industry/statistics (Accessed 28/11/2022)
Li GQ, Liu FF, Li JQ, Liu QL, Chen SF (2015) Characterization of Botryosphaeria dothidea and Lasiodiplodia pseudotheobromae from English walnut in China. J Phytopathol 164:348–353. https://doi.org/10.1111/jph.12422
López-Larrinaga F, Mellado-Bermejo E, Valadares de Queirós R, Figueras-Lorenzo M, Gomes-Pires J (2017) Las nuevas plantaciones de nogal en España. Fruticultura 64:45–59
López-Moral A, Raya-Ortega MC, Agustí-Brisach C, Roca LF, Lovera M, Luque F, Arquero O, Trapero A (2017) Morphological, pathogenic and molecular characterization of Colletotrichum acutatum isolates causing almond anthracnose in Spain. Plant Dis 101:2034–2045. https://doi.org/10.1094/PDIS-03-17-0318-RE
López-Moral A, Lovera M, Raya MC, Cortés-Cosano N, Arquero O, Trapero A, Agustí-Brisach C (2020) Etiology of branch dieback and shoot blight of English walnut caused by Botryosphaeriaceae and Diaporthe fungi in southern Spain. Plant Dis 104:533–550. https://doi.org/10.1094/PDIS-03-19-0545-RE
López-Moral A, Lovera M, Antón-Domínguez BI, Gámiz AM, Michailides TJ, Arquero O, Trapero A, Agustí-Brisach C (2022) Effects of cultivar susceptibility, branch age, and temperature on infection by Botryosphaeriaceae and Diaporthe fungi on English walnut (Juglans regia). Plant Dis 106:2920–2926. https://doi.org/10.1094/PDIS-09-21-2042-RE
Lovera M, Rodríguez RA, Arquero O, Trapero A (2017a) Lesiones necróticas en parte aérea de nogal I. Caracterización y etiología. Fruticultura 64:70–89
Lovera M, Rodríguez RA, Arquero O, Trapero A (2017b) Lesiones necróticas en parte aérea de nogal II. Factores que influyen en su incidencia y severidad. Fruticultura 64:90–99
MAPA (2021) https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (Accessed 28/11/2021)
Michailides TJ (1991) Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology 81:566–573. https://doi.org/10.1094/Phyto-81-566
Michailides TJ, Hasey J (2010) Botryosphaeria and Phomopsis cankers of walnuts in California. Walnut husk fly field meeting. University of California cooperative extension, CA
Moral J, Morgan D, Trapero A, Michailides TJ (2019) Ecology and epidemiology of nut crops and olive diseases caused by Botryosphaeriaceae fungi. Plant Dis 103:1809–1827. https://doi.org/10.1094/PDIS-03-19-0622-FE
Steel RGD, Torrie JH, Bioestadística (1985) second ed. McGraw-Hill, Bogotá, Colombia
Trouillas FP, Úrbez-Torres JR, Peduto F, Gubler WD (2010) First report of twig and branch dieback of English walnut (Juglans regia) caused by Neofusicoccum mediterraneum in California. Plant Dis 94:1267. https://doi.org/10.1094/PDIS-06-10-0412
Acknowledgements
The authors thank ‘Crisolar’ and ‘Mañán’ OPFHs and the private companies ‘Almendras Francisco Morales’ and ‘Bain (Borges Group)’ for their collaboration. The authors thank F. Luque, A.M. Gámiz, F. González and J.A. Toro for their skillful technical assistance in the laboratory.
Funding
This research was funded by the ‘Junta de Andalucía’ (projects PPTRATRA-2016.00.6, and UCO-FEDER 20 REF. 1381067-R), co-funded by the European FEDER funds. We acknowledge financial support from the Spanish Ministry of Science and Innovation, the Spanish State Research Agency, through the Severo Ochoa and María de Maeztu Program for Centers and Units of Excellence in R&D (Ref. CEX2019-000968-M).
Author information
Authors and Affiliations
Contributions
AL-M Investigation, Methodology, Formal analysis, Writing – original draft; ML Investigation, Methodology; BIA-D Investigation, Methodology, Visualization; TJM Visualization, Writing – review & editing; OA Funding acquisition, Resources, Visualization; AT Funding acquisition, Project administration, Resources, Writing – review & editing; CA-B Conceptualization, Methodology, Supervision, Formal analysis, Funding acquisition, Resources, Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
López-Moral, A., Lovera, M., Antón-Domínguez, B.I. et al. Effects of cultivar susceptibility, fruit maturity, and natural wounds on the infection of English walnut (Juglans regia L.) fruits by Botryosphaeriaceae and Diaporthe fungi. J Plant Pathol 105, 1391–1401 (2023). https://doi.org/10.1007/s42161-023-01492-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-023-01492-0