Abstract
Our study investigated how environmental factors affect the zooplankton community in different seasons. In 2017, we conducted a spring (May) and summer (August) survey in Changli near shore area of Hebei province, China, to measure the abundance, biomass, and temporal and spatial distribution of the zooplankton community, and monitored 12 conventional environmental factors. The results showed that a total of 19 species (genus) of 6 groups of zooplankton were identified in 2017, among which copepods were the most abundant with 9 species, accounting for 47.4% of the number of zooplankton species. There were 5 species of Pelagic larvae, accounting for 26.2% of the number of zooplankton species. Two species of coelenterates, accounting for 10.5% of the number of zooplankton species. In addition, there are 1 species of mysidae, 1 species of chaetognathe and 1 species of tunicata, accounting for 5.3% of the number of zooplankton species. In spring, the total abundance of zooplankton was 43,463 ind./m3. In summer, the total abundance of zooplankton was 21,317 ind./m3. The spatial distribution of zooplankton in spring and summer is generally higher than the buffer zone near the shore and the buffer zone around the core zone. The evaluation of biological habitat quality by biodiversity index showed that the quality level of habitat is poor in spring and general in summer. Canonical correspondence analysis(CCA) indicated that the salinity, temperature, nutrient and Chl a were the main environmental factors which affected the zooplankton community structure. Finally, we compared our results with historical data and found that zooplankton abundance and biomass has declined in Changli near shore area, China, but the dominant species have remained relatively stable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooplankton is a group of heterotrophic invertebrates and chordate larvae that often swim in water and cannot produce organic substances by themselves. There are many kinds of zooplankton and they are widely distributed in the ocean. They can be used as indicators of warm and cold currents (Andronikova 1996), as well as important baits for fish and other economic animals in the upper and middle waters (Warwick 1981; Ware and Thomson 2005). The environmental factors of near shore area are complex and changeable. The zooplankton community in the ocean is affected by many environmental factors (such as physics, chemistry and biology). The structure and dynamics of the zooplankton community are the result of the synthetical effect of many environmental factors on time and space scales (David et al. 2005). Therefore, species diversity and community characteristics of zooplankton are important indicators for evaluating the nutritional level, resource status, pollution status and stability of marine ecosystems.
Changli Golden Coast Nature Reserve was one of the first five national marine nature reserves approved by the State Council. It is located in the eastern coast of Hebei province, China. It is mainly divided into three parts: core zone, buffer zone and experimental zone. The protected area is not only the main habitat of amphioxus in the Bohai Sea, but also the typical coastal zone for studying ocean dynamic process and land-sea change. It has important ecological value, scientific research value and ornamental value. Previous studies on the species composition and quantity variation of zooplankton in the Bohai Sea focused mainly on the Bohai Bay and the central part of the Bohai Sea (Wang et al. 2002, 2014; Gao et al. 2014; Wei et al. 2015; Xu et al. 2016; Zhang et al. 2016; Wu et al. 2016). To date, there are few studies on the Changli Reserve. Even though lessons can be learned from the previous work of other part of the Bohai Sea, it is not always possible to extrapolate the zooplankton community structure in the near shore area of Changli Reserve, considering their differences in water temperature, transparency, chlorophyll a, pH, ammonia nitrogen, silicate and active phosphate, to mention a few.
The objective of this study was to analyze the seasonal succession of zooplankton community structure in Changli near shore area by investigating the characteristics of zooplankton community structure and monitoring results of environmental factors, and to preliminarily explore the relationship between zooplankton and environmental factors, so as to provide basic data for amphioxus habitat protection in Changli near shore area. It also provides scientific basis for the rational development, utilization and control of biological resources in this area.
Materials and Methods
Sample Collection and Method
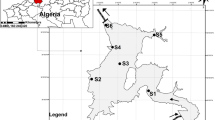
Two surveys were conducted to investigate zooplankton and environmental factors in Changli Reserve, Hebei Province(39°26′30″ N ~ 39°39′00″ N, 119°17′30″ E ~ 119°36′21″ E) in spring (May) and summer (August) in 2017. A total of 14 sampling stations were set up in the area (Fig. 1). Among them, 1 ~ 3 and 11 ~ 14 stations are in buffer zones; 4 ~ 10 stations are in core zones; buffer zones and core zones were prohibited from development, which were the most primitive and natural ecosystems and had important scientific research value.
The shallow water plankton network type II (net length:140 cm, net aperture area:0.08 m2, mesh size:160 μm) was selected. Samples were collected from the bottom to the surface by vertical trawls. They were fixed and stored in 5% neutral formalin solution and brought back to the laboratory for classification, identification and counting. The environmental factors such as water temperature(WT), salinity (S), transparency(T), dissolved oxygen(DO), chemical oxygen demand(CODMn), active phosphate(DIP), silicate(DISi), chlorophyll(Chl a) were investigated synchronously at each monitoring station. All operating steps were strictly in accordance with the ‘Marine Monitoring Standard’(GB17378-2007) (General Administration of Quality Supervision and Inspection, National Standardization Management Committee 2008).
Statistical Analysis
The abundance of zooplankton was the number of individuals per cubic meter of water, and the biomass was the wet weight of fixed samples. The dominant species of zooplankton were determined according to the dominance value (Y) of each species, and the species with Y (> 0.02) were regarded as dominant species (Xu and Chen 1989).
The diversity index of zooplankton was expressed by Shannon–Weaver diversity index (H') and Margalef richness index (D). Shannon–Weaver diversity index and richness index were calculated by Primer 6. According to the reference index of biodiversity index provided by ‘Environmental Monitoring of Coastal Sea Area’(HJ442-2008) (Department of Science and Technology Standards, Ministry of Environmental Protection 2009), the habitat quality grade of H' > 3.0 is excellent, that of 2.0 < H' < 3.0 is general, that of 1.0 < H' < 2.0 is poor, and that of H' < 1.0 is extremely poor.
The correlation between abundance and biomass and environmental factors was analyzed by SPSS 17. The composition of zooplankton community was analyzed by DCA(Dedentred Correspondence Analysis). When the maximum lengths of gradient in four axes were less than 3, the abundance of zooplankton and environmental factors were analyzed by CCA(Canonical Correspondence Analysis) in CANOCO 4.5.
Results
Species Composition and Dominant Species
A total of 19 species (genera) of 6 groups of zooplankton were found in 2017. It can be seen intuitively that the proportion of zooplankton groups to the total number of zooplankton species (Fig. 2). Copepods were the most abundant species, accounting for 47.4% of zooplankton species, followed by 5 species of zooplankton larvae, accounting for 26.2% of zooplankton species, 2 species of coelentera, accounting for 10.5% of zooplankton species, and 1 species of bran shrimp, 1 species of Chaetognatha and 1 species of capsule, accounting for 5.3% of zooplankton species.
Dominant species in spring included Calanus sinicus, Paracalanus parvus, Acartia bifilosa, Acartia Pacifica and Oithona similis. Dominant species in summer included Sagitta crassa, Oikopleura longicauda, Paracalanus parvus, Acartia bifilosa and Oithona similis, which were slightly different from those in spring (Table 1).
Zooplankton Abundance & Biomass Horizontal Distribution
The temporal and spatial distribution of zooplankton abundance in Changli near shore area are shown in Fig. 3. In spring, the total abundance of zooplankton was 43,463 ind./m3, and the range of abundance varied from 287 to 19,188 ind./m3. The average abundance of zooplankton was 3105 ind./m3. The highest total abundance appeared at 2 station(19,188 ind./m3), and the lowest total abundance appeared at 12 station(287 ind./m3). In summer, the total abundance of zooplankton was 21,317 ind/m3, and the range of abundance varied from 252 to 8288 ind./m3. The average abundance of zooplankton was 1522 ind./m3. The highest total abundance appeared at 2 station(8288 ind./m3), and the lowest was at 7 station (252 ind./m3).
The spatial distribution of dominant species in spring and summer are shown in Figs. 4 and 5, respectively. Generally, the distribution characteristics were consistent with the distribution characteristics of total abundance. In spring, the high value areas appeared in the buffer zone, while in summer, the high value areas appeared in the core zone and its surrounding buffer zone, but the abundance of dominant species in spring was higher than that in summer.
The horizontal distribution of zooplankton biomass are shown in Fig. 6. In spring, the total wet-weight biomass of zooplankton was 9470 mg/m3, the range of wet-weight biomass at each station ranged from 177.6 to 2573.8 mg/m3, and the average wet-weight biomass of zooplankton was 676.4 mg/m3. The highest wet weight biomass appeared at 2 station (2573.8 mg/m3), and the lowest wet weight biomass appeared at 12 station (177.6 mg/m3). In summer, the total wet-weight biomass of zooplankton was 9299.9 mg/m3. The range of wet-weight biomass at each station ranged from 33.9 to 3907.8 mg/m3, and the average wet-weight biomass of zooplankton was 1378.6 mg/m3. The highest wet weight biomass appeared at 9 station(3907.8 mg/m3), and the lowest wet weight biomass appeared at 7 station(33.9 mg/m3).
Biodiversity Index and its Change
In spring, the values of indices in the core and buffer zones of the sea area did not change significantly, except for the evenness index, the other diversity indices showed that the buffer zone was slightly higher than the core zone. This was similar to that of spring zooplankton abundance. In summer, the values of indices in the core and buffer zones of the sea area changed significantly, and the diversity indices in the core zone were higher than those in the buffer zone. According to the reference index of biodiversity index provided by "Environmental Monitoring of Coastal Sea Area"(HJ442-2008), the quality grade of habitats in spring was poor and that in summer was general.
Based on the average of diversity index in Table 2, the tendency of the index changes of zooplankton at different stations in spring and summer were drawn (Fig. 7). It is obvious that the maximum of zooplankton richness index (D) appears at station 6 and the minimum appears at station 4 in spring. The maximum Shannon–Weaver biodiversity index (H') appeared at station 6 and the minimum appeared at station 8. The maximum of the evenness index (J) appears at station 4, and the minimum value appears at station 10. It can be seen that there is a similarity between zooplankton richness index (D) and Shannon–Weaver biodiversity index (H') in spring, and the maximum values appear at station 6 (which belongs to the core of sea area).
In summer, the maximum of zooplankton richness index (D) appeared at station 9 and the minimum appeared at station 2. The maximum Shannon–Weaver biodiversity index (H') appeared at station 10 and the minimum appeared at station 2. The maximum of the evenness index (J) appears at station 10, and the minimum value appears at station 2. Similarly, the trends of zooplankton richness index (D), Shannon–Weaver biodiversity index (H') and evenness index (J) are similar in summer, and the maximum values of evenness index (J) and Shannon–Weaver biodiversity index (H') appear at station 10 (which belongs to the core of sea area), and richness index (D). The minimum values of evenness index (J) and Shannon–Weaver biodiversity index (H') appeared at station 2 (which belongs to the sea buffer zone). The overall trend is that the highest value occurs in the core area, and the lowest value appears in the buffer zone. The results showed that the water body fluctuated frequently in the buffer zone, and the species, abundance and biomass of zooplankton continued to succession, while the water body fluctuated slightly in the core zone, and the indexes tended to be stable.
On the whole, the Shannon–Weaver biodiversity index of zooplankton in summer was significantly higher than that in spring (P < 0.05), while the richness index and evenness index did not change significantly (P > 0.05).
Correlation Analysis Between Zooplankton Abundance and Environmental Factors
The correlation between the abundance of zooplankton and environmental factors, such as water temperature, salinity, nutrients and so on, was shown in Table 3. In spring, the abundance of zooplankton was negatively correlated with salinity, dissolved oxygen and nitrite nitrogen (P < 0.05), but not with water temperature, transparency, chlorophyll a, pH, ammonia nitrogen, silicate and active phosphate (P > 0.05). In summer, the abundance of zooplankton was positively correlated with water temperature and dissolved oxygen (P < 0.05), but not with salinity, transparency, chlorophyll a, ammonia nitrogen, silicate and nitrate nitrogen (P > 0.05).
CCA of zooplankton and environmental factors in Hebei Changli reserve area was carried out. The results of spring CCA analysis were shown in Table 4 and Fig. 8. According to Monte Carlo permutation test results, the ranking effect was ideal, showing significant levels (P < 0.005). The results showed that the first and second axes can explain most of the accumulated change information of zooplankton community, accounting for 55.81% of the total, and the eigenvalues were 0.0957 and 0.0601, respectively. Axis 1 was negatively correlated with silicate and dissolved oxygen, while Axis 2 was positively correlated with water temperature, chlorophyll a and active phosphate, and negatively correlated with nitrite and pH.
The results of summer CCA analysis were shown in Table 5 and Fig. 9. According to Monte Carlo permutation test results, the ranking effect was ideal, showing significant levels (P < 0.005). The results showed that the first and second axes can explain most of the accumulated change information of zooplankton community, accounting for 54.78% of the total, and the eigenvalues are 0.1828 and 0.0837, respectively. Axis 1 was positively correlated with pH, and Axis 2 was positively correlated with COD, negatively correlated with water temperature, active phosphate and nitrate nitrogen.
In conclusion, salinity, water temperature, chlorophyll a and nutrients were the main environmental factors affecting the community structure of zooplankton in the coastal waters of Changli, and the influencing factors varied with seasons.
Discussion
Zooplankton Population Structure and its Dynamic Changes
Nineteen species of zooplankton were identified in two surveys. Among them, copepods were the main group in Changli Reserve, and larvae were the second largest group. Compared with previous surveys, zooplankton major composition groups were more consistent (Wang and Ma 2005; Liang 2018; Li et al. 2016). The number of zooplankton species was significantly lower than that in the previous studies, but the dominant species and constituent groups were similar to previous studies. The reason may be that the survey area was only in the small protected area, resulting in the small number of zooplankton species.
The seasonal variation of zooplankton was obvious. The total abundance of zooplankton was 43,463 ind./m3 in spring and 21,317 ind./m3 in summer. The abundance distribution of zooplankton also showed obvious regional characteristics. The abundance of zooplankton in the core of sea area was significantly lower than that in the buffer area. In spring, the surface water temperature rose, the photometry increased, the nutrients were abundant and the phytoplankton multiplied rapidly. In summer, the rainfall increased, the runoff of land source increased and the feeding of large amount of bait in the aquaculture area brought abundant nutrients, while zooplankton also multiplied in large quantities due to the suitable water temperature and rich food. However, the abundance of zooplankton in the core area was low, which may be due to the decrease of zooplankton species abundance caused by excessive nutrients, or the decrease of zooplankton predation efficiency caused by wind and waves (Zhou and Qin 2018).
We also investigated the sea in spring and summer of 2016, and the results showed that the number of zooplankton species in 2016 increased compared with that in 2017, but the main groups were similar (Li et al. 2016). In addition, the biomass and abundance of zooplankton in 2016 were slightly lower than those in 2017, but they were higher in summer. The fluctuation of species number may be due to the contingency of field ecological survey, but compared with the data of other historical periods, the species number is basically stable.
Biodiversity
Diversity index (biodiversity index, evenness index and richness index) is one of the indicators reflecting the characteristics of community structure and indicating organic pollution. The average value of H' was 1.89 in spring and 2.09 in summer. The results of 2016 survey are basically consistent with the results, and there is no significant difference. According to the reference index of biodiversity index, the quality grade of habitat in spring was poor, while that in summer was general. The main reason may be that the high-density raft culture in the coastal waters of Changli has changed the marine hydrodynamic environment, and the rapid development of tourism in the Golden Coast of Changli has influenced the habitat by human factors. In addition, in recent years, a large number of water storage projects have been constructed in the upper reaches of Luanhe River, which has reduced the area of the estuary transition section and insufficient water exchange, thus affecting the succession of zooplankton communities and gradually reducing biodiversity (Shi et al. 2011).
Influence of Environmental Factors on Zooplankton
Water environment determined the characteristics of biological population or community structure. On the contrary, different zooplankton community structure had a certain feedback effect on the environment. Zooplankton was sensitive to environmental changes. The changes of individual, population or community can objectively reflect the changes of water quality. Most studies had shown that zooplankton community structure was closely related to many abiotic factors. Among them, temperature and salinity were the most important factors affecting zooplankton distribution (Jiang et al. 2017; Luo et al. 2016; Marques et al. 2006). The survival and reproduction of zooplankton will also be limited by temperature and salinity (Devreker et al. 2005; Lenz et al. 2005).
Temperature was the key factor for individual growth, development and reproduction. Appropriate elevation will increase the intensity of zooplankton metabolism and accelerate zooplankton generation replacement so as to speed up reproduction. Because different zooplankton had different tolerance to temperature, their species composition and quantity distribution will vary with the change of temperature and salinity, and then the seasonal succession of zooplankton community structure appeared. At the same time, zooplankton also improved their resistance to environmental change through their own oxygen consumption and metabolic intensity. Salinity, as another important environmental factor affecting zooplankton community structure, mainly affected its osmotic pressure, and zooplankton adapted to it through the ion regulation mechanism of osmotic pressure. With the increase of salinity, the number of copepods increased gradually. In different salinity regions, the number distribution of copepods was different (Zervoudaki et al. 2009). From spring (May) to summer (August) in Changli Reserve, the temperature gradually increased from 13.54 to 28.18 °C to promote the rapid reproduction of zooplankton, which mainly fed on phytoplankton. There was no obvious gradient change of salinity in seasons, when the average salinity was 31.06 in spring and 30.52 in summer. The results showed that the protected area was suitable for large-scale reproduction of broad-temperature and wide-salt zooplankton.
In addition to salinity and water temperature, chlorophyll a and nutrients were also correlated with zooplankton community distribution. Chlorophyll a content was an important indicator of the present biomass of phytoplankton in the sea area (Gao et al. 2017). Phytoplankton growth and standing biomass were especially affected by nutrient content (Wang et al. 2018). Chlorophyll a and nutrients were indirectly controlled by phytoplankton in the community structure of zooplankton. Overall, the cumulative contribution rate of environmental factors to the explanation of zooplankton species variables was only 55.3%. It showed that besides environmental factors, the distribution of zooplankton community was also affected by phytoplankton distribution, aquaculture activities, tourism activities and other aspects. To further explore the change mechanism of zooplankton community structure, it is necessary to conduct large-scale spatial and temporal surveys of zooplankton and to further analyze the endogenous dynamics and external environment that affect the changes of zooplankton community.
Since the investigated area is one of the important national nature reserves in China, there is no human development activities, and there is no abnormal climate impact during the 2016 and 2017 investigation period, so the index of environmental factors is basically stable.
Conclusion
-
1.
Nineteen species of zooplankton were identified in 2017, of which copepods accounted for the most, followed by five species of planktonic larvae, two species of coelentera, and one species of bran shrimp, one species of maxilla and one species of capsule. In spring, the total abundance of zooplankton was 43,463 ind./m3, and the total abundance of zooplankton was 21,317 ind./m3 in summer.
-
2.
The average value of H' in Changli Reserve was 1.89 in spring and 2.09 in summer. According to the reference index of biodiversity index, the quality grade of habitat was poor in spring and that was general in summer.
-
3.
Canonical correspondence analysis showed that salinity, water temperature, chlorophyll a and nutrients were the main environmental factors affecting the community structure of zooplankton in the coastal waters of Changli, and the influencing factors varied with seasons.
References
Andronikova IN (1996) Zooplankton characteristics in monitoring of Lake Ladoga[J]. Hydrobiologia 322(1–3):173–179
David V, Sautour B, Chardy P et al (2005) Long-term changes of the zooplankton variability in a turbid environment: The Gironde estuary (France)[J]. Estuar Coast Shelf Sci 64(2):171–184
Department of Science and Technology Standards, Ministry of Environmental Protection (2009) Technical Specifications for Environmental Monitoring in Coastal Sea Areas: HJ 442–2008 [S]. China Environmental Science Press, Beijing
Devreker D, Souissi S, Seuront L (2005) Effects of chlorophyll concentration and temperature variation on the reproduction and survival of Temora longicornis, (Copepoda, Calanoida) in the Eastern English Channel[J]. J Exp Mar Biol Ecol 318(2):145–162
Gao L, Yao HY, Zhang MM et al (2017) Temporal and spatial variation of sea water transparency and its relationship with environmental factors in Qingdao coastal waters [J]. J Mar Sci 35(3):79–84
Gao WS, Liu XB, Zhang QF et al (2014) Zooplankton diversity in the coastal waters of Bohai Bay[J]. Mar Sci 38(4):55–60
General Administration of Quality Supervision and Inspection, National Standardization Management Committee (2008) Marine Monitoring Code Part 4: Seawater Analysis: GB 17378.4–2007[S]. Beijing: China Standards Press
Jiang HC, Liu N, Gao JQ et al (2017) Community characteristics of zooplankton and their relationship with environmental factors in Forty Mile Bay, Yantai [J]. Ecological Journal 37(4):1318–1327
Lenz PH, Hower AE, Hartline DK (2005) Temperature Compensation in the Escape Response of a Marine Copepod, Calanus finmarchicus (Crustacea)[J]. Biol Bull 209(1):75–85
Li DL, Liu XB, Liu ZG et al (2016) Zooplankton community structure in relation to environmental factors in the coastal water near Luan River Estuary, China[J]. Mar Sci Bull 18(2):41–55
Liang M (2018) Spatial niche of dominant species of zooplankton in Caofeidian coastal waters [J]. Journal of Ecological Environment 27(7):1241–1250
Luo X, Zeng JN, Xu XQ et al (2016) Distribution characteristics of zooplankton and their relationship with environmental factors in summer and autumn in Zhoushan sea area [J]. Ecological Journal 36(24):8194–8204
Marques SC, Azeiteiro UM, Marques JC et al (2006) Zooplankton and ichthyoplankton communities in a temperate estuary: spatial and temporal patterns[J]. J Plankton Res 28(3):297–312
Shi YT, Wen HY, Qiao GJ (2011) Impact of human activities on ecological environment of Luanhe River Estuary Wetland[J]. South to North Water Diversion and Water Conservancy Technology 9(3):124–128
Warwick RM (1981) The nematode/copepod ratio and its use in pollution ecology[J]. Mar Pollut Bull 12(10):329–333
Ware DM, Thomson RE (2005) Bottom-up ecosystem trophic dynamics determine fish production in the Northeast Pacific[J]. Science 308(5726):1280–1284
Wang K, Zhang WC, Wang R et al (2002) Zooplankton community structure in central and southern Bohai in spring and autumn[J]. Marine Science Quarterly 44(00):42–50
Wang SB, Yu JS, Cao YM et al (2018) Effects of light and nutrients on biomass and nutrient linkages of zooplankton and phytoplankton[J]. J Ecol Environ (6):1122–1127
Wang Y, Fang NJ, Guo H et al (2014) Community structure of zooplankton and its relationship with environmental factors in the Tianjin Bay of Bohai Bay in spring [J]. Marine Fisheries 36(4):300
Wei ZL, Cai ZY, Shi HH et al (2015) Species composition and temporal spatial distribution of zooplankton in Long Island waters of Bohai[J]. Journal of Shanghai Ocean University 24(4):550–559
Wang ZL, Ma MH (2005) Zooplankton ecology in the Gold Coast National Nature Reserve, Changli, [J]. Mar Environ Res 24(2):43–46
Wu D, Zhou XB et al (2016) Characteristics of zooplankton communities in coastal waters of Bohai Bay[C]. Papers of the Annual Meeting of the Chinese Society of Environmental Sciences (Volume II)
Xu DH, Sun XM, Chen BH et al (2016) Ecological characteristics of zooplankton in central Bohai [J]. Progress in Fishery Sciences 37(4):7–18
Xu ZL, Chen YD (1989) Relationship between dominant species aggregation intensity of autumn zooplankton and mackerel fishery in east the Yellow Sea[J]. J Ecol (19):13–15.
Zhang DJ, Sun YB, Bi XD et al (2016) Community structure of zooplankton in the vicinity of Lingang industrial area, Tianjin[J]. Fisheries Science 35(4):431–435
Zhou J, Qin BQ (2018) Advances in research on effects of wind waves on plankton in lakes[J]. Progress in Water Science 29(2): 293–300
Zervoudaki S, Nielsen TG, Carstensen J (2009) Seasonal succession and composition of the zooplankton community along an eutrophication and salinity gradient exemplified by Danish waters[J]. J Plankton Res 31(12):1475–1492
Acknowledgements
Thanks to the staff of Changli Golden Coast National Nature Reserve for their help in collecting samples. This research was financially supported by the University Innovation Team Training Program for Tianjin (TD12-5003), the Tianjin 131 Innovation Team Program (20180314) to Jun Sun.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., Yang, S., Zhao, X. et al. Zooplankton Community Structure in Changli Near Shore Area of Hebei Province, China. Thalassas 38, 501–510 (2022). https://doi.org/10.1007/s41208-021-00379-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-021-00379-0