Abstract
The effect of environmental factors on zooplankton diversity, abundance, and distribution in the Ghrib Dam water body was investigated for 2 years from June 2013 to May 2015. A total of 61 species of zooplankton were recorded, including 45 rotifers, 13 cladocerans, and 3 copepods. The zooplankton abundance fluctuated between 4 and 3363 ind.L−1, and its Shannon–Wiener index of zooplankton diversity in Ghrib Dam varied from H′ = 1.15 to 3.77 during the 2-year study period. The highest diversity index was recorded at sampling site S1 in January 2015, due to the increase of specific richness (Ni = 27, Dmg = 5.75). Canonical correspondence analysis (CCA) was used to determine the influence of environmental factors in structuring the zooplankton species for each group. The results of CCA revealed that the most significant environmental factors influencing zooplankton community were water temperature (WT), dissolved oxygen (DO), phosphorus (PO4−), and NO3− (p < 0.05, Monte Carlo permutation test). Meanwhile, the appearance of Hexarthra sp., Collotheca pelagica, and Diaphanosoma brachyurum indicated a rise in WT. The existence of Filinia longiseta, Keratella quadrata, and Cyclops strenuus correlated with a rising of DO. Brachionus urceolaris, Filinia passa, Keratella tecta, Notholca acuminata, and Polyarthra dolichoptera positively coexisted in an increased PO4−. Daphnia longispina and Oxyurella tenuicaudis were positively associated with high concentration of NO3−.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In aquatic ecosystems, zooplankton play a fundamental role in the pelagic realm, channelling energy from primary producers to a higher trophic levels (Lampert and Sommer 2007). Rotifera and Crustacea (Cladocera and Copepoda) which are the most common groups of freshwater zooplankton respond rapidly to many environmental stressors, such as hydrological changes and anthropogenic activity-induced water pollution (Dodson et al. 2005; Simões et al. 2015;Adamczuk et al. 2015). They are usually considered to be a good indicator of environmental changes (Paganelli et al. 2014). The abundance and biomass levels of zooplankton are largely regulated by changes in resources at the base of the food web (Canfield and Jones 1996) and also by the direct effect of predators (Shapiro and Wright 1984; McQueen 1990).The species richness and composition of zooplankton groups are known to be affected not only by physical and chemical factors but also by other parameters, such as habitat type, lake depth (Keller and Conlon 1994), nutrients (Jeppesen et al. 2000; Xiong et al. 2019), predation, and competition (Shurin 2000; Larson et al. 2009). The environmental factors responsible for community variation in lakes (natural or dam lake) are varied. The various human perturbations in lake watersheds have an important effect on changing these factors. Therefore, it is important to identify the lake-specific environmental factors that have affected spatial and temporal succession dynamics of zooplankton communities.
In Algeria, dam lakes are the main major source that meets human water needs, but the intensity of agricultural activities along these dams and in the wadis banks (a valley with a very irregular hydrological regime) which flow into these dams increases the risk of their water being contaminated, changing their physical and chemical characteristics (Hamil et al. 2018). The Ghrib Dam was planted in the Cheliff Wadi; it is the longest wadi (700 km) and vulnerable to anthropic activities in Algeria. Therefore, the water quality of the Ghrib Dam has been deteriorating since 2005; high nutrient concentrations were detected (Harkat et al. 2011). Hamil et al. (2018) indicated that the parameters responsible for water quality variation in the Ghrib Dam are mainly related to domestic and agricultural non-point sources runoff.
In the present study, we investigated zooplankton species composition, dominant species, zooplankton abundance variation, species diversity, and environmental parameter variation from June 2013 to May 2015 in Ghrib Dam. The main environmental factors affecting the community structure of each group of zooplankton (Rotifera, Cladocera, and Copepoda) and the identification of the species, which are most sensitive to changes in the quality of water, were analysed in this study.
Materials and methods
Study area
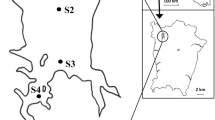
The Ghrib Dam is located in the northwestern part of Algeria approximately 30 km far from Medea City and 150 km from the Algerian capital Algiers. The dam is at an altitude of approximately 435 m and is between 36° 12′ 36° 16′ N and 2° 55′ 2° 60′ E (Fig. 1). The reservoir receives water from Cheliff Wadi (the Cheliff River). The dammed water is used for the irrigation both the Mitidja Plain in the east and the upper and lower Cheliff Plains to the west. This eutrophic water body is 14 km2 and has a maximum depth of 40 m (Hamil et al. 2018).
Field sampling and laboratory analyses
The sampling was carried out monthly between 10.00 and 13.00 GMT + 1, from June 2013 to May 2015, at six stations and the surface layers ranged from 10 to 50 cm of the upper surface (Fig. 1).
The water samples for nutrient analysis were preserved in pre-rinse 1-L polypropylene bottle at 4 °C in darkness and analysed within 24 h. Water temperature (WT), pH, electrical conductivity (EC), and dissolved oxygen (DO) were measured in situ using a multiparameter analyser (the Multi 340i metre from WTW). Water transparency was measured using a Secchi disc. Other chemical substances (nitrate NO3−, nitrite NO2−, and phosphate PO4−) were measured by the spectrophotometric method (Rodier 2004).The obtained data are compared with the Algerian quality standards adopted by the national agency of water resources (NAWR 2009).
Zooplankton samples were collected from each site by the filtration of 5 l of surface water through a 50-μm mesh net. Samples were stored in 60-ml plastic bottles containing 5% formaldehyde before being transported to the laboratory and processed for identification and counting. The taxonomic identification of zooplankton was carried out according to Dussart (1967, 1969), Voigt and Koste (1978), and Błędzki and Rybak (2016). The juvenile copepods and nauplii were included in the density calculations. However, they were excluded from the diversity indices.
To measure changes in biodiversity, we used the Shannon–Wiener index (H′) (Shannon and Weaver 1963) and Margalef index (Dmg) (Margalef 1958):
where ni = the average density of species (individuals per L), N = the average density of individuals in the sample, and S = number of species.
Statistical analysis
Monthly variations of physicochemical parameters and abundances of the zooplankton assemblages were tested using one-way ANOVA (at P < 0.05). The relationships between the zooplankton species density for each group and the environmental parameters were assessed using the canonical correspondence analysis (CCA). The Monte Carlo permutation test (999 permutations) was applied to determine the significance (P < 0.05) of the variables. The data were log-transformed prior to all analyses. Additionally, all statistical analyses were applied to the average values of the six stations. Pearson’s correlation was used to test the relationships among the environmental factors and zooplankton. All steps of this method were computed using the R programme (R version 3.4.4; R Development Core Team 2018).
Results
Environmental parameters
The primary physical and chemical parameters of water in the six stations over time are presented in Fig. 2. Generally, the WT, DO, pH, NO2−, and EC values of the six studied stations showed a small difference in these parameters which is not significant (ANOVA, P > 0.05), while a significant spatial difference of Secchi disc transparency, phosphorus, and nitrate was observed during the study period (ANOVA, P < 0.05; Fig. 2). As illustrated in Fig. 2, the average of WT showed a similar seasonal pattern in the whole water bodies, varying from 6 °C to 28 °C. The WT revealed an upward trend from December to August, while DO concentration showed a reversed trend varying from 3.1 to 15.9 mg. L−1 (Fig. 2). The maximum DO content (15.9 mg. L−1) occurred in May 2014 at S3, while the minimum value (3.1 mg. L−1) was recorded in October 2013 at S5. The pH values fluctuated from 7.1 to 9.2 and the EC values ranged from 1617 to 4150 μs cm−1; the highest value was observed in November 2013 at S4 and the lowest values were recorded in February 2015 at S1.
Temporal variation of variables water quality in different sampling sites in Ghrib Dam; WT, water temperature (°C); pH; DO, dissolved oxygen (mg L−1); PO4−(mg. L−1); NO3− (mg L−1); NO2− (mg L−1); EC, electrical conductivity (μs cm−1); Secchi; Secchi disc transparency (cm). Colours signification: S1 (black), S2 (red), S3 (green), S4 (blue), S5 (cyan), S6 (purple)
In the Ghrib Dam, nutrient concentrations showed considerable values in S1 (Fig. 2). The phosphate concentration varied between 0.002 and 0.44 mg L−1 with the highest concentrations in spring season, including some peaks of concentration at the end of the summer. Generally, the highest concentrations of PO4− were detected at S1. During the study period, NO3− ranged between 0.622 and 9.029 mg L−1 with a mean value of 2.829 mg L−1. According to Algerian standards, the water quality of Ghrib is good to polluted for both parameters.
As shown in Fig. 2, the Secchi disc transparency (Secchi) of S1 was lower than that of the other five stations in the Ghrib Dam (average value of 39.7 cm at S1), while the high value of water transparency (368 cm) was recorded in May 2014 at S6.
The Pearson’s rank correlation coefficients revealed significant correlations between the dissolved oxygen and water temperature (F DO/WT = − 0.61, P = 0.001), transparency (F DO/Transp = − 0.45, P = 0.026), conductivity (F DO/EC = − 0.48, P = 0.015), and NO3 (F DO/NO3 = 0.42, P = 0.037). On the other hand, the pH was positively correlated with conductivity (F pH/EC = 0.46, P = 0.022) and water temperature (F pH/WT = 0.44, P = 0.030).
Zooplankton community composition
Sixty-one species of zooplankton were recorded in the Ghrib Dam (Table 1). Copepod crustaceans had the lowest species diversity, with 3 taxa; 13 taxa were identified among the cladocerans, and rotifers had the greatest zooplankton diversity (45 taxa).
Three species were identified in Algeria’s freshwater for the first time: Encentrum saundersiae, Hexarthra oxyuris, and Notholca acuminate. The three frequent species, Diaphanosoma brachyurum (Cladocera), Arctodiaptomus salinus (Copepoda), and Keratella quadrata (Rotifera), were found at the sampling stations; these counted for 95.18%, 85.79% and 65.23% of the species abundance, respectively (Table 1). In terms of abundance, Rotifera was more important than other both taxonomic groups (58.3% of the average total density).
Zooplankton variations in abundance and diversity
There were distinct temporal changes in zooplankton abundance in the dam lake (ANOVA one-way; P value < 0.01). The average abundance was 392.64 ind. L−1, ranging from 4 to 3363 ind. L−1. The minimum abundance was found in December 2014 and was made up of 15.55% Rotifera species. The maximum abundance was observed in April 2014, with 66.66% cladoceran species and 8.88% rotifer species, with the dominance of Keratella quadrata. Seasonally, the total zooplankton abundance decreased after the summer period, remained low during the autumn and winter and increased at the beginning of spring (Fig. 3). Spatially, the variation in the average abundance of zooplankton between the sampling sites was very small, varying from 192.39 (S4) to 549.04 ind. L−1 (S5). The analysis of the evolution in the abundance of the three groups (rotifers, cladocerans, and copepods) by the ANOVA one-way with a factor demonstrated a significant temporal variation (p value < 0.01) with the absence of the variation spatial (p value = 0.8; 0.56; 0.71, respectively), which means that the population of zooplankton in the Ghrib water body changes only as a function of time.
Zooplankton diversity of Ghrib Dam in each month at six stations was illustrated in Fig. 4. The spatial difference in the values of the Shannon–Wiener index and the specific richness index of the six stations was not significant (ANOVA, F = 0.99, F = 1.92 respectively, P > 0.05), while a significant spatial difference of species number was observed during the study period (ANOVA, F = 4.17, P < 0.001; Fig. 3). The Shannon–Wiener index (H′) of zooplankton diversity in Ghrib Dam varied from H′ = 0.41 to 3.77 during the 2-year study period (Fig. 4). The highest diversity index was registered at sampling site S1 in January 2015, followed by the increase of specific richness (Ni = 27, Dmg = 5.75). The H′ was lowest (0.41) in March 2014 at S3, when the dominance value of K. quadrata was 0.941.
Generally, the variation of specific richness index by Margalef (Dmg) follows the changes in taxonomic richness (Ni) and varied between 1.33 and 5.75.
The relationship between zooplankton species and environmental factors
The results of the canonical correspondence analysis (CCA) identified the environmental factors that are responsible for seasonal variability in the species composition of zooplankton assemblages of the dam.
The CCA of the distribution of rotifers showed that all the environmental variables in the first two axes explained 54.5% of the total variance. The Monte Carlo permutation test showed the significance of the water temperature (λ = 0.21; F = 8.60; P = 0.001), dissolved oxygen (λ = 0.13; F = 2.94; P = 0.005), pH (λ = 0.17; F = 5.30; P = 0.001), conductivity (λ = 0.13; F = 3.18; P = 0.015), and PO4 (λ = 0.09; F = 2.03; P = 0.043) values in the variability of the rotifers.
On the CCA biplot, samples are divided into three groups (Fig. 5a). The species commonly observed in the summer (Hexarthra fennica, Hexarthra oxyuris, Collotheca pelagica, Collotheca sp., and Keratella tropica) correspond to gradient in the water temperature. The species observed in the spring (Ascomorpha sp., Brachionus quadridentatus var. cluniobicularis, Brachionus urceolaris, Filinia passa, Keratella tecta, Notholca acuminata, and Polyarthra dolichoptera) were associated with an increase in PO4 concentration, while both species Filinia longiseta and Keratella quadrata corresponded to the dissolved oxygen gradient.
Canonical correspondence analysis (CCA) biplot for (a) Rotifera, (b) Cladocera, (c) Copepoda assemblages showing species and environmental variables; Naup, Nauplius; Copt, Copepods juveniles; WT, water temperature (°C); T, air temperature (°C); pH; DO, dissolved oxygen (mg. L−1); PO4−(mg. L−1); NO3− (mg. L−1); NO2− (mg. L−1); EC, electrical conductivity (μs cm−1); (Secchi) Secchi disc transparency (cm)
For cladocerans, all environmental variables together explained 67.36% of the total variance. The Monte Carlo permutation test indicated the significance of the water temperature (λ = 0.164; F = 3.91; P = 0.006), dissolved oxygen (λ = 0.12; F = 3.14; P = 0.012), and NO3 (λ = 0.13; F = 2.97; P = 0.017) values for the cladocerans. On the CCA biplot, Bosmina longirostris and Diaphanosoma brachyurum correspond with the water temperature (Fig. 5b); NO3 was found to be significantly correlated with the abundance of Daphnia longispina and Oxyurella tenuicaudis. Only Macrothrix hirsuticornis was associated with the dissolved oxygen. For copepods, all environmental variables together explained 87.86% of the total variance (Fig. 5c). The abundance of Cyclops strenuus strenuus showed a strong relationship with the increasing dissolved oxygen gradient (λ = 0.16; F = 3.58; P = 0.005) (Fig. 5c).
Discussion
Zooplankton community composition
Zooplankton is one of the most diverse fauna groups; the structure of this community is mainly formed according to the physical and chemical characteristics of the environment, as well as biological interactions (Dodson et al. 2005; Adamczuk et al. 2015). The present study assessed the structure, the composition, and the dynamics of zooplankton in the Ghrib Dam. A total of 61 species were identified. The rotifers had a higher number of species and abundance than cladocerans and copepods. The taxonomic dominance of rotifers has been attributed in several Algerian water bodies (Samraoui et al. 1998; Cherbi et al. 2008; Bidi-Akli et al. 2014), in North Africans (Sellami et al. 2009, 2011; Badsi et al. 2010), and in the Mediterranean region (León et al. 2010).
The population abundance of zooplankton is controlled by various factors (abiotic and biotic) that are variable and some of them are interdependent on each other in natural conditions (Sodré and Bozelli 2019; Hamil 2019; Ju et al. 2019). In general, significant temporal variations were observed in the zooplankton communities in the lake under study. The high abundances of zooplankton were attributed to the dominance of some rotifers species such as Keratella quadrata, Hexarthra fennica, and Hexarthra oxyuris. A rapid development of small-bodied rotifers takes place in a rapidly changing environment and their domination is a common phenomenon, especially when high nutrient concentrations and high density of cyprinids occur (Rosińska et al. 2019). Furthermore, our study showed that the rotifers were abundant in number and in species at the site S1 which receive more pollution loads in the form of sewage outfalls (Hamil et al. 2018), clearly indicating that this group is more tolerant to pollution (Gannon and Stemberger 1978).
The CCA ordination diagram suggests that most physicochemical variables influenced the abundance and occurrence of rotifers (Fig. 4a). Water temperature was found to have the strongest influence, followed by pH, DO, and PO4 concentrations. In fact, some studies have shown that water temperature is the most important factor regulating the occurrence of rotifers (Sellami et al. 2009; Ji et al. 2013). Among these species, Hexarthra fennica and Hexarthra oxyuris, both warm stenotherm species were recorded in summer. pH might be the main influence on the occurrence of many genera, such as Notholca, Cephalodella, Encentrum, Epiphanus, and Polyarthra, which were collected in winter, when water pH was relatively low (pH = 7.2). The distribution of Filinia longiseta and Keratella quadrata was related with dissolved oxygen level variations in water. A similar trend was observed in eutrophic lakes by Wang et al. (2016) and Doukhandji and Arab (2017) who reported that the distribution and the dominance of Keratella quadrata were affected by high oxygen concentrations.
Both crustaceans, cladocerans, and copepods are the most useful and nutritive groups for fish in their food chains. In the present study, the most abundant cladoceran species were Diaphanosoma brachyurum, which were found in summer, when the water temperature levels were relatively high. Similar results were observed in Boukourdane Lake (Cherbi et al. 2008) and the Kasseb Reservoir (Sellami et al. 2011). As a stenothermal hot-water species, these species develop in the warm period at a temperature range of 20–25 °C. Like others, we observed the development of Daphnia in spring followed by their disappearance in summer; this is probably the result of grazing by planktivorous fish. Many studies have established that planktivorous fish deplete the abundances of large cladocerans such as Daphnia through direct grazing (Siegfried and Kopache 1984; Pont et al. 1991; Badsi et al. 2010). We also observed the genera Leydigia, Alona, Chydorus, and Macrothrix with very low abundances; they may be more abundant in the littoral zone. Rocha and Güntzel (1999) concluded that species of the families Chydoridae and Macrothrycidae are predominant in littoral zones.
The copepods that were found during the present investigation were mainly comprised of three perennial species, among which Cyclops strenuus strenuus presented significant development in the spring (95 ind. L−1). Copidodiaptomus numidicus, an herbivorous species characteristic of the rainy and endemic zone of west of the Mediterranean, also presented significant springtime development. These species have previously been recorded in eastern Numidia (Algeria) (Samraoui 2002).
The variations in the density of Cladocera and Copepoda could have also been due to their abilities to take advantage of resources. In the present study, both the Copepoda and Cladocera populations show a positive correlation with some environmental factors. The CCA for cladocerans and copepods (Fig. 5b and c, respectively) showed that some species were indicative of changing environmental conditions. The distributions of Bosmina longirostris and Diaphanosoma brachyurum were associated with water temperature. The occurrence of Macrothrix hirsuticornis and Cyclops strenuus strenuus coincided with high dissolved oxygen concentrations. The presence of Daphnia longispina and Oxyurella tenuicaudis in Ghrib Dam was correlated with high NO3− concentrations.
Diversity indices can serve as a good indicator of the overall quality of water (Arab et al. 2019). A high diversity index (H′) indicates a healthy ecosystem, while a low value denotes a less healthy or degraded ecosystem, while a fall in the value of the Margalef index shows the rise in the level of pollution. During the study period, the average values of H′ and Dmg in Ghrib Dam were low (2.56 and 2.52, respectively), indicating a moderate pollution. The low values of the biodiversity indices during some months may be related to several factors, such as a decrease in dissolved oxygen and nutrients and an increase in alkalinity and salinity of water (Dodson et al. 2000; Jeppesen et al. 2000; Brönmark and Hansson 2005). Some significant correlation was observed in our study between diversity index and PO4− (r H′/PO4 = 0.43), pH (r H′/pH = −0.68), and electrical conductivity EC (r H′/EC = −0.41). Generally, phosphate was considered the limiting nutrient for freshwater ecosystem; P bioavailability will boost primary production and thus will enhance the secondary production including zooplankton, affecting the composition and abundance of species.
Conclusions
The results obtained in our study showed significant changes in the species richness, density, and temporal distribution of zooplankton assemblages. Among the three major zooplankton groups in the study area, rotifers were the most dominant groups, followed by cladocerans and copepods. The average abundance of zooplankton in lake was 4 and 3363 ind. L−1. The zooplankton community of the Ghrib Dam changes significantly over time (the study period), but their spatial variation between the sampling sites was very low. The Shannon–Wiener diversity index varied from 0.41 to 3.77, with an average of 2.54, which indicates that this water body was in moderate pollution. Canonical correspondence analysis (CCA) showed that the changes in species densities and their temporal distributions were connected to changes in the environmental variables. Rotifers were more sensitive to the environmental changes; their distribution in the Ghrib Dam was associated with water temperature gradient, followed by the pH, dissolved oxygen, electrical conductivity, and PO4−. The variations in the density and distribution of the cyclopoid Cyclops strenuus strenuus were determined by the change in dissolved oxygen. The density and distribution of the stenotherm species Diaphanosoma brachyurum were determined by water temperature variations.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adamczuk M, Mieczan T, Tarkowska-Kukuryk M, Demetraki-Paleolog A (2015) Rotatoria–Cladocera Copepoda relations in the long-term monitoring of water quality in lakes with trophic variation (E. Poland). Environ Earth Sci 73(12):8189–8196. https://doi.org/10.1007/s12665-014-3977-z
Arab S, Hamil S, Rezzaz MA, Chaffai A, Arab A (2019) Seasonal variation of water quality and phytoplankton dynamics and diversity in the surface water of Boukourdane Lake, Algeria. Arab J Geosci 12:29. https://doi.org/10.1007/s12517-018-4164-4
Badsi H, Oulad Ali H, Loudiki M, El Chakli R, Aamiri A (2010) Zooplankton abundance in the Messa Lagoon, southern Morocco: impact of environmental variables. Int J Biodivers Conserv 2(10):289–299 http://www.academicjournals.org/journal/IJBC/article-abstract/9092E7116692
Bidi-Akli S, Arab A, Samraoui B (2014) Variation spatio-temporelle du zooplancton dans le barrage de la réserve de chasse de Zéralda (Algérie). Rev Écol (Terre Vie) 69(3–4):214–224. http://hdl.handle.net/2042/56022
Błędzki LA, Rybak JI (2016) Freshwater crustacean zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) key to species identification, with notes on ecology, distribution, methods and introduction to data analysis. Springer International Publishing, Switzerland
Brönmark C, Hansson LA (2005) The biology of lakes and ponds. Oxford University Press, Second Edition ed.
Canfield TJ, Jones JR (1996) Zooplankton abundance, biomass, and size-distribution in selected Midwestern waterbodies and relation with trophic state. J Freshw Ecol 11(2):171–181. https://doi.org/10.1080/02705060.1996.9663476
Cherbi M, Lek-Ang S, Lek S, Arab A (2008) Distribution du zooplancton dans les lacs à climat méditerranéen. C R Biologies 331(9):692–702. https://doi.org/10.1016/j.crvi.2008.06.003
Development Core Team (2018) R: a language and environment for statistical computing. Version 3.4.4. Vienna: R Foundation for Statistical Computing. Available at https://www.R-project.org/
Dodson SI, Arnott SE, Cottingham KL (2000) The relationship in lake communities between primary productivity and species richness. Ecology. 81(10):2662–2679. https://doi.org/10.1890/0012-9658
Dodson SI, Lillie RA, Will-Wolf S (2005) Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecol Appl 15(4):1191–1198. https://doi.org/10.1890/04-1494
Doukhandji N, Arab A (2017) The spatiotemporal distribution of rotifers in a dam in a Saharan climate region (Foum El Gherza, Algeria). Rev Écol (Terre Vie) 72:168–176 http://hdl.handle.net/2042/62077
Dussart B (1967) Les Copepodes des Eaux Continentales d’ Europe Occidentale, Tome I, Calanoides and Harpacticoides, editions N. Boubee, and cie, Paris
Dussart B (1969) Les Copepodes des Eaux Continentales d’Europe Occidentale Tome II, Cyclopoides and Biyology, editions N. Boubee and cie, Paris
Gannon JE, Stemberger RS (1978) Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans Am Microsc Soc 97(1):16–35
Hamil S (2019) Structure and functioning of aquatic fauna; zooplankton in the Ghrib Dam lake. PhD Dissertation, école normale superieurs de Kouba (in french)
Hamil S, Arab S, Chaffai A, Baha M, Arab A (2018) Assessment of surface water quality using multivariate statistical analysis techniques: a case study from Ghrib Dam, Algeria. Arab J Geosci 11:754. https://doi.org/10.1007/s12517-018-4102-5
Harkat S, Arabi M, Taleb S (2011) Impacts of human activities on water erosion and pollution of surface water in the Algerian Cheliff watershed. Rev Sci Tech 19:56–75
Jeppesen E, Peder Jensen J, Søndergaard M, Lauridsen T, Landkildehus F (2000) Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. BIOL 45(2):201–218. https://doi.org/10.1046/j.1365-2427.2000.00675.x
Ji G, Wang X, Wang L (2013) Planktonic rotifers in a subtropical shallow lake: succession, relationship to environmental factors, and use as bioindicators. Sci World J 2013:14–14. https://doi.org/10.1155/2013/702942
Ju Y, Sun X, Shabani EI, al. (2019) Relationships between zooplankton biomass and environmental factors of Xiaoxingkai Lake in northeastern China. Environ Sci Pollut Res 26:30279–30285. https://doi.org/10.1007/s11356-019-06171-1
Keller W, Conlon M (1994) Crustacean zooplankton communities and lake morphometry in Precambrian Shield lakes. Can J Fish Aquat Sci 51(11):2424–2434. https://doi.org/10.1139/f94-242
Lampert W, Sommer U (2007) Limnoecology: the ecology of lakes and streams, 2nd edn. Press, Oxford University
Larson GL, Hoffman R, Mcintire CD, Lienkaemper G, Samora B (2009) Zooplankton assemblages in montane lakes and ponds of Mount Rainier National Park, Washington State, USA. J Plankton Res 31(3):273–285. https://doi.org/10.1093/plankt/fbn125
León D, Peñalver P, Casas J, Juan M, Fuentes F, Gallego I, Toja J (2010) Zooplankton richness in farm ponds of Andalusia (southern Spain) a comparison with natural wetlands. Limnética. 29(1):153–162 http://hdl.handle.net/11441/60723
Margalef R (1958) Temporal succession and spatial heterogeneity in phytoplankton. In: Buzzati-Treverso AA (ed) Perspectives in Marine Biology, Berkeley, pp 323-349
McQueen DJ (1990) Manipulating lake community structure: where do we go from here? Freshwat. Biol. 23:613–620. https://doi.org/10.1111/j.1365-2427.1990.tb00299.x
N.A.W.R. National Agency for Water Resources Alger (2009). Annu Hydrol de l’Algérie (Alger, Hydrological Yearbook of Algeria). Water Resources Ministry
Paganelli D, Saltarelli M, Sconfietti R (2014) Seasonal evolution of the zooplankton community in two riverine wetlands of the Ticino River (Lombardy, northern Italy). Ann Limnol Int J Lim 50:241–247. https://doi.org/10.1051/limn/2014017
Pont D, Crivelli AJ, Guillot F (1991) The impact of 3-spined sticklebacks on the zooplankton of a previously fish-free pool. Freshw Biol 26:149–163
Rocha O, Güntzel A (1999) Crustáceos Branchiopodos. In: Ismael D, Valenti WC, Matsumura-Tundisi T, Rocha O (eds) Biodiversidade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX. FAPESP, São Paulo, pp 107–120
Rodier J (2004) L’analyse de l’eau: Eaux naturelles, Eaux résiduaires, Eaux de mer, Dunod, Paris
Rosińska J, Romanowicz-Brzozowska W, Kozak A, Gołdyn R (2019) Zooplankton changes during bottom-up and top-down control due to sustainable restoration in a shallow urban lake. Environ Sci Pollut Res 26(19):19575–19587. https://doi.org/10.1007/s11356-019-05107-z
Samraoui B (2002) Branchiopoda (Ctenopoda and Anomopoda) and Copepoda from eastern Numidia, Algeria. Hydrobiologia 470:173–179. https://doi.org/10.1023/A:1015640525662
Samraoui B, Segers H, Maas S, Baribwegure D, Dumont HJ (1998) Rotifera, Cladocera, Copepoda, and Ostracoda from coastal wetlands in Northeast Algeria. Hydrobiologia. 386:183–193. https://doi.org/10.1023/A:1003538730152
Sellami I, Hamza A, Alaoui Mhamdi M, Aleya L, Bouain A, Ayadi H (2009) Abundance and biomass of rotifers in relation to the environmental factors in geothermal waters in southern Tunisia. J Therm Biol 34:267–275
Sellami I, Elloumi J, Hamza A, Alaoui Mhamdi M, Ayadi H (2011) Local and regional factors influencing zooplankton communities in the connected Kasseb Reservoir, Tunisia. Water Research Commission 37:201–212. https://doi.org/10.1016/j.jtherbio.2009.03.003
Shannon CE, Weaver W (1963) The mathematical theory of communication. Press, University of Illinois
Shapiro J, Wright DI (1984) Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshwat Biol 14:371–383. https://doi.org/10.1111/j.1365-2427.1984.tb00161.x
Shurin JB (2000) Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology 81:3074–3086. https://doi.org/10.1890/0012-9658(2000)081[3074:DLIRAT]2.0.CO;2
Siegfried CA, Kopache ME (1984) Zooplankton dynamics in a high mountain reservoir in Southern California. Calif Fish Game:70/18–70/38
Simões NR, Nunes AH, Dias JD, Lansac-Tôha FA, Velho LFM, Bonecker CC (2015) Impact of reservoirs on zooplankton diversity and implications for the conservation of natural aquatic environments. Hydrobiologia 758:3–17. https://doi.org/10.1007/s10750-015-2260-y
Sodré EO, Bozelli RL (2019) How planktonic microcrustaceans respond to environment and affect ecosystem: a functional trait perspective. Int Aquat Res 11:207–223. https://doi.org/10.1007/s40071-019-0233-x
Voigt M, Koste W (1978) Rotatoria : die Rädertiere Mitteleuropas : ein Bestimmungswerk : Überordnung Monogononta, Borntraeger, Berlin
Wang C, Wang L, Deng D, Zhou Z (2016) Temporal and spatial variations in rotifer correlations with environmental factors in Shengjin Lake, China. Environ Sci Pollut Res 23:8076–8084. https://doi.org/10.1007/s11356-015-6009-y
Xiong W, Ni P, Chen Y, Gao Y, Li S, Zhan A (2019) Biological consequences of environmental pollution in running water ecosystems: a case study in zooplankton. Environ Pollut 252(Pt B):1483–1490. https://doi.org/10.1016/j.envpol.2019.06.055
Acknowledgements
I would like to thank and express my very great appreciation to Dr. Willem De Smet from the Department of Biology University of Antwerpen, for his valuable contribution in the determination and confirmation of some zooplankton species (Encentrum saundersiae, Hexarthra oxyuris) and his help, advice, and encouragement.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Somia Hamil, Abdeslem Arab, Monia Baha, Djaouida Bouchelouche, Siham Arab, and Malika Alili. The first draft of the manuscript was written by Hamil Somia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Somia Hamil: Conceptualization; formal analysis; methodology; investigation; writing the original draft; writing, review, and editing; visualization.
Djaouida Bouchelouche: Formal analysis, methodology.
Siham Arab: Investigation, writing the original draft, writing, review, and editing.
Malika Alili: Writing, review, and editing.
Mounia Baha: Formal analysis, resources writing, review and editing, writing the original draft.
Abdeslem Arab: Supervision, formal analysis, resources, writing the original draft, project administration, visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamil, S., Bouchelouche, D., Arab, S. et al. The relationship between zooplankton community and environmental factors of Ghrib Dam in Algeria. Environ Sci Pollut Res 28, 46592–46602 (2021). https://doi.org/10.1007/s11356-020-10844-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10844-7