Abstract
The pelagic mollusk Clione is a naked pteropod with a sympatric two-species distribution in the southern Okhotsk Sea, Japan, consisting of the morphologically and genetically distinct C. elegantissima and C. okhotensis. Clione elegantissima appears in both winter and spring, and body length differs between the winter coastal population (WCP, January to March, 10–20 mm) and the spring offshore population (SOP, April to July; up to 30 mm). This body size difference and temporal-spatial separation of the populations suggests that the SOP is either a cryptic species or C. limacine drifted from the Subarctic Atlantic Ocean or an interspecies of C. elegantissima resulting from reproductive isolation. We investigated the taxonomic positions of both populations using morphological and genetic analyses and identified both as C. elegantissima with very high genetic similarity. We explain the occurrence of spatio-temporal isolated populations using the water mass dynamics in the Okhotsk Sea. Warm water entering the southern Okhotsk Sea around Japan through the Soya Straits is divided into the Soya Warm Water (SWW: June to November) and the Forerunner of the SWW (FSWW: March to May); cold water entering the Okhotsk Sea around Japan, east of Sakhalin Island, is divided into the East Sakhalin Current Water (ESCW: November to April). The Cold Water Belt (CWB) is frequently formed off the SWW during summer and autumn and comprises upwelling cold water originating from either subsurface water of the Japan Sea off Sakhalin or Okhotsk Sea bottom water. We present the temporal-spatial isolation mechanism of WCP and SOP per the SWW, FSWW, ESCW, and CWB dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Okhotsk Sea, located in the northwestern corner of the North Pacific Ocean, is one of the largest semi-enclosed seas in the world (Fig. 1). Whereas sea ice is typically present in the Antarctic and Arctic waters during the winter when it covers an area of up to 7% of the earth’s surface (Comiso et al. 1990), in the North Pacific Ocean sea ice occurs only during a part of the winter (Ruth and Patrick 1998). From spring to autumn, Clione elegantissima (Dall 1871) lives at a depth of approximately 200 m in the Okhotsk Sea, whereas in winter this species migrates toward the coast of the southern Okhotsk Sea with drift ice (Hamaoka 2002).
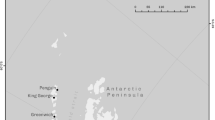
a Distribution of the southern limit of Clione elegantissima, b Distribution of the winter coastal population (WCP) and spring offshore population (SOP) and sampling location. WCP areas were based on Yamazaki and Kuwahara (2017) and the collected record points are shown as ○. SOP areas based on Suzuki et al. (2005) and Kunihiro (1995). Lake Saroma (N44°13, E143°97) and K.Y.B (N44°46, E144°23) points indicated with ● are sampling locations. Abbreviation: TWW, Tsushima Warm Water; SWW, Soya Warm Water; ESCW, East Sakhalin Current Water; CWB, Cold Water Belt; O.M, Oarai Marina; Me, Menashidomari; Es, Esashi; Sa, Sawaki; Om, Omusaro; Mo, Monbetsu; L.S, Lake Saroma; Ab, Abashiri; Ut, Utoro; Ra, Rausu; Ne, Nemuro; K.Y.B, Kitami-Yamato Bank
Despite being important members of zooplankton communities, the Gymnosomata are poorly understood (Lalli and Gilmer 1989; Sromek et al. 2015). Although their anatomy and ecology have been well studied (Martens 1675; Wanger 1885; Smith 1902; Massy 1932; Morton 1958; Mileikovsky 1970; Van Der Spoel 1976; Lalli and Conover 1976; Lalli and Gilmer 1989; MClintock and Janssen 1990; Gilmer and Lalli 1990; Richter and Seapy 1999; Van Der Spoel and Dadon 1999; Suprenand et al. 2015), the genetics of these pteropods have received little attention (Jennings et al. 2010; Sromek et al. 2015; Yamazaki and Kuwahara 2017). The characteristics of the family Clionidae include a thin integument and the presence of anterior tentacles, which are mostly transparent and typically very small. The defining characteristic of the genus Clione is three pairs of buccal cones (Van Der Spoel 1976; Lalli and Gilmer 1989). Members of the genus have been called “sea angels” owing to their characteristic swimming behavior (Wrobel and Mills 1998). Clione species are an important component of pelagic food webs in temperate and polar waters, providing a food source for baleen whales, salmon, and other fish species (Martens 1675; Phipps 1774; Lalli and Gilmer 1989; Azuma 1995; Davis et al. 2009; Haigh et al. 2015).
The genus Clione consists of four known species with a bipolar distribution: the Northern and Southern Hemispheres have three and one species, respectively (Yamazaki and Kuwahara 2017). The genus is distributed in Japan and the adjacent waters of the southern limit, Oarai Marina (Fig. 1a, O.M), Ibaraki prefecture in the North Pacific Ocean, (Inaba and Okutani 1999), and Rudnaya Bay Japan Sea (Chichvarkhin 2016) (Fig. 1a).
The Northern Hemisphere C. limacina (Phipps 1774) and South Hemisphere C. antarctica (Smith 1902) are considered as synonyms or subspecies (Gilmer and Lalli 1990). Sromek et al. (2015) suggested that the two species are genetically distinct. The taxonomical complexes of the northern and southern populations could be distinguished using morphological and genetic approaches.
The Northern Hemisphere C. limacina consists of two known populations: the North Pacific and the Arctic to North Atlantic Ocean populations. The two populations differ morphologically in body size and swimming speed (Gilmer and Lalli 1990), and Yamazaki and Kuwahara (2017) suggested they are genetically distinct. The taxonomy of the Northern Hemisphere Clione species is organized into the Subarctic Atlantic Ocean population and North Pacific Ocean population, which were identified respectively as C. limacina and C. elegantissima (Dall 1871) by Yamazaki and Kuwahara (2017). Yamazaki and Kuwahara (2017) described a new species, C. okhotensis, from the southern Okhotsk Sea with evidence of its morphological and genetic features.
The southern Okhotsk Sea has two sympatric Clione species, C. elegantissima and C. okhotensis (Yamazaki and Kuwahara 2017). C. elegantissima appears in two seasons: winter (January to March) in coastal waters, and spring (April to July) in the offshore area. There are clear differences in the body lengths of the winter coastal population (WCP) and spring offshore population (SOP), which are 10–20 mm and up to 30 mm, respectively (Suzuki et al. 2005). The maximum adult body length of Clione is known to be 70–85 mm in C. limacina (Conover and Lalli 1972; Lalli and Wells 1978; Gilmer and Lalli 1990), whereas it is <30 mm in C. elegantissima (Agersborg 1923; McGowan 1963; Gilmer and Lalli 1990). The spatio-temporal isolation and different body lengths suggest that a cryptic species or C. limacina could have drifted from the Subarctic Atlantic Ocean to the southern Okhotsk Sea and without being damaged by the sea ice because of their medium size. In this study, the taxonomical positions of both the WCP and SOP were investigated morphologically and genetically. Additionally, isolation mechanisms were discussed based on the water dynamics of adjacent waters.
Materials and Methods
Sampling
For the sampling, the WCP was collected from January 30 to February 3, 2017 during the spring tide time from shallow waters at a depth range of 0–50 cm at the entrance of Lake Saloma (N44°13, E143°97), in the southern Okhotsk Sea (Fig. 1, L.S, ●WCP). In addition, the SOP was collected from May to June 2017 from night-time shallow waters at a depth range of 0–50 cm at Kitami-Yamato Bank (N44°13, E143°97), from breaktime fishermen fishing Kichiji rockfish (Fig. 1, K.Y.B, ●SOP). The individual specimens were placed and observed in 150 mm × 150 mm × 20 mm viewing aquariums and preserved in 99.9% ethanol as DNA samples.
Morphology
The external morphology of the live animals was observed in situ in the aquarium and under a stereomicroscope (Nikon SMZ18). The radula of three specimens of WCP and SOP was extracted from the buccal mass and cleaned in 1 N sodium hydroxide (NaOH) solution in a 50 °C water bath for 30 min. Sputter coating was performed for 5 min at 20 mA (JEOL JEC-3000FC; EC-30020RTS) and samples were observed using a stereomicroscope (Nikon SMZ18) and a scanning electron microscope (SEM, JEOL JCM-6000Plus NeoScope) at an acceleration voltage of 5 kV.
Molecular Sequence Analysis
Eleven specimens were used for DNA extraction, amplification, and sequencing, consisting of six and five specimens of the WCP and SOP. The DNA was extracted using the QIAGEN DNeasy blood and tissue kit (Qiagen). The cytochrome c oxidase subunit 1 (COI) gene was amplified using the universal DNA primers LCO-1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO-2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. 1994). Polymerase chain reactions (PCRs) were performed using the GeneAmp PCR System 9600 (Applied Biosystems). Reactions were run using the following schedule: initial denaturation, 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 50 °C for 45 s, 72 °C for 1 min; and final extension, 72 °C for 5 min (Jennings et al. 2010; Yamazaki and Kuwahara 2017). PCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega). Sequencing reactions were performed using a BigDye Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems) using a GeneAmp PCR System 9600 (Applied Biosystems). Reaction products were purified using ethylenediaminetetraacetic acid (EDTA), sodium acetate, and ethanol precipitation, and then electrophoresed using an ABI PRISM 3100 genetic analyzer (Applied Biosystems). Sequences were aligned and edited using the MEGA7 program (Kumar et al. 2016). There were 569 positions in the final dataset.
New sequences have been deposited in the DNA Data Bank of Japan (accession numbers: LC341683-LC341693). One and six COI sequences of C. elegantissima and C. limacine, respectively, which were already available in GenBank were included in the calculation of the genetic distance. Two COI sequences for pseudothecosome pteropods were downloaded from GenBank and used as an outgroup to root the tree (see Fig. 4 for accession numbers).
The molecular phylogenetic analysis was conducted using the maximum likelihood (ML) method based on the Kimura 2-parameter (K2P) model (Kimura 1980). Initial trees for the heuristic search were obtained by applying the Neighbor-Ioining and BioNJ algorithms to a matrix of pairwise distances estimated using the PhyML ML, and then selecting the topology with superior log likelihood values. A bootstrap consensus tree was calculated from 10,000 replicates and branches found in <50% of the bootstrap replicates were collapsed. The analysis involved 28 nucleotide sequences and the evolutionary analyses were conducted using MEGA 7 (Kumar et al. 2016).
Results
Morphology
The total materials were collected for up to 1000 individuals and up to 2000 individuals for both the WCP and SOP, respectively. The WCP live materials had a length of 8 to 23 mm, whereas SOP materials had a length of up to 45 mm (Fig. 2); the specimens shrank by approximately two-thirds, but almost no shrinkage of the viscera occurred in 99.9% of the ethanol-preserved specimens. The radula morphology of WCP was approximately 500 μm long, the central teeth lacked approximately 9 lines of the anterior rows, the posterior to anterior consisted of approximately 30 lines (Fig. 3a), and a crescent-like morphology was observed (Fig. 3b). The lateral teeth consisted of 11 lines (Fig. 3c) and an L-like morphology, whereas the old part of the anterior showed desquamative central teeth and was spread out in a fan-like form outside (Fig. 3a). In contrast, SOP was approximately 600 μm long, the central teeth lacked approximately 10 lines of the anterior rows, the posterior to anterior consisted of approximately 40 lines (Fig. 3d), and a crescent-like morphology was observed (Fig. 3e). The lateral teeth consisted of 11 lines (Fig. 3f) and an L-like morphology (Fig. 3d).
Back side of the radula of Clione elegantissima winter coastal population (WCP; a–c) and spring offshore population (SOP; D-F): (a, d) Overall, (b, e) central teeth, and (c, f) lateral teeth. Arrows indicate lateral teeth inner to outer on one line. Scale 50 μm (a) and 10 μm (b and c), 200 μm (d), 20 μm (e and f)
Molecular Analysis
The K2P distances observed within and between the two populations were 0.005–0.014 within WCP, 0.007–0.016 within SOP, and 0.005–0.019 between WCP and SOP (Table 1).
The PhyML software generated a tree with a topology very similar to that of the bootstrap consensus tree obtained from MEGA (Fig. 4). The monophyly of the ingroup (genus Clione) and the sister relationship between the WCP (Fig. 4○) and SOP (Fig. 4◎) each formed an unsupported clade (Fig. 4).
Discussion
Morphology
The SOP specimens showed the longest body length (up to 45 mm), which has been the focus of considerable attention from the news media such as in the coverage of the “Giant Clione” at the habitat museum (Fig. 2 below individual).
In the North Pacific Ocean, the C. elegantissima population has an average body size of 28 mm according to its original description. Subsequently, Gilmer and Lalli (1990) reported a size of <30 mm in the North Pacific regions.
SOP was collected at night, when it appears at the sea surface at depths of 0–50 cm, using nets. This suggests that they inhabit the areas of the East Sakhalin cold current (at a depth range of 0 to 200 m; see next paragraph), in the Soya warm current (Takizawa 1982). Clione might exhibit diel vertical migration in the East Sakhalin current and appear at the sea surface at night. Migration is supported by the swimming ability based on the body size. In the Arctic to the North Atlantic Ocean, C. limacina species has a body size of 70 mm and swims at a speed of 100 mm/s (Gilmer and Lalli 1990). However, the vertical distribution and swimming ability of both populations are unknown in the North Pacific Ocean and from the Arctic to North Atlantic Ocean.
The radula is a common morphological feature of the genus Clione that consists of the central and L-like lateral teeth (Fig. 3). The radula tooth distribution allows the distinction of C. antarctica from other species of the genus (Smith 1902). However, the numbers of lateral teeth show usually intraspecific variation (Gilmer and Lalli 1990). In this study, WCP and SOP showed similar variation in radula traits, supporting a conspecific status.
Population Genetics
The K2P distances indicate that the differences observed between populations are of the same magnitude as those observed within populations, and therefore, they show that the genetic differentiation between the two populations is almost nil. Moreover, the COI sequences from the two populations, WCP and SOP, appear in the same clade and exhibit a maximum K2P distance of 1.9% (Table 1). This distance is at the intraspecies level in both populations (Jennings et al. 2010). Therefore, the two populations are conspecific based on the mitochondrial genetic analysis. Therefore, the two populations are intraspecies based on the genetic analysis. However, mtDNA data alone do not provide a full demonstration that the two populations are conspecific, as occasional hybridization can lead to the presence of the same mtDNA in two species that otherwise differ at the nDNA level (e.g., Azuma et al. 2011). An additional study of nuclear DNA sequences or microsatellite marker analysis should be conducted to fully confirm the conspecificity of the WCP and SOP populations.
Emergence of Spatio-Temporal Separated Populations
Because WCP and SOP are genetically similar, a mechanism that allows the separation of the two populations in time and space should be sought. Here, we propose a mechanism based on the pattern of water circulation in the region.
The warm water flowing into the Okhotsk Sea through the Soya Straits is divided into two parts: the Soya Warm Water (SWW: June to November) and the Forerunner of the Soya Warm Water (FSWW: March to May) (Aota 1975; Matsuyama et al. 1999). At the end of May, the SWW flows into the subsurface layer (approximately 200–400 m deep) of the Abashiri Bay. Furthermore, it flows northeastward as a subsurface current just off the coast of the Kuril Islands and reaches the region northwest of Etorofu Island (Takizawa 1982). In addition, the cold water flowing into the Okhotsk Sea around Japan along the east of Sakhalin Island is divided into the East Sakhalin Current Water (ESCW: November to April) (Ohshima et al. 2002). Additionally, the Cold-Water Belt (Fig. 1b, CWB) is frequently formed off the SWW during summer and autumn. The CWB is upwelling cold water that originates from either subsurface water of the Japan Sea off Sakhalin or bottom water of the Okhotsk Sea (Kuma et al. 2014). During the seasonal change, both populations were found based on the pattern of FSWW, SWW, WSCW and CWB. In conclusion, the WCP fades in the SWW, whereas the SOP is protected by the sunken warm current. Therefore, the SOP population is found in the spring. The mechanisms of isolation of the two populations are related to the water currents of both warm and cold temperatures.
Summary of the Results and Conclusions
Two types of Clione with different body lengths were found in the southern Okhotsk Sea, which was the emergence of temporal-spatial separated populations. The small type appeared in winter in coastal areas, whereas the large type appeared in spring in offshore areas. The large type of Clione was not recorded in North Pacific areas, which suggests cryptic species or drift of middle-sized C. limacine from Arctic Atlantic Ocean. mtDNA COI barcodes were applied at the intraspecies level in both populations, and they identified C. elegantissima. We found that the isolation mechanism involved specific dynamics of the adjacent waters. However, mtDNA data alone do not provide a full demonstration that the two populations are conspecific, as occasional hybridization can lead to the presence of the same mtDNA in two species, which otherwise differ at the nDNA.
References
Agersborg HPK (1923) Gymnosomatous Pteropoda from Friday Harbor, Washington. Ann Sci Nat 10:391–402
Aota M (1975) Studies on the soya warm current. Low Temp Sci A33:151–172
Azuma N, Yamazaki T, Chiba S (2011) Mitochondrial and nuclear DNA analysis revealed a cryptic species and genetic introgression in Littorina sitkana (Mollusca, Gastropoda). Genetica 139:1399–1408
Azuma T (1995) Biological mechanisms enabling sympatry between salmonids with special reference to sockeye and chum salmon in oceanic waters. Fisheries Research 24 (4):291-300
Chichvarkhin A (2016) Shallow water sea slugs (Gastropoda: Heterobranchia) from the northwestern coast of the Sea of Japan, north of Peter the Great Bay, Russia. Peer J 4:e2774. https://doi.org/10.7717/peerj2774
Comiso JC, Maymard NG, Smith WO, Sullivan CW (1990) Satellite ocean color studies of Antarctic ice edges in summer and autumn. J Geophys Res Oceans 95:9481–9496. https://doi.org/10.1029/JC095iC06p09481
Conover RJ, Lalli CM (1972) Feeding and growth in Clione limacina (Phipps), a pteropod mollusc. J Exp Mar Biol Ecol 9:279–302
Dall WH (1871) Descriptions of sixty new forms of mollusks from the west coast of North America and the North Pacific Ocean, with notes on others already described. Am J Conchol 7:93–160 pls. 13–16
Davis ND, Volkov AV, Efimkin AY, Kuznetsova NA, Armstrong JL, Sakai O (2009) Review of basic salmon food habits studies. North Pacific Anadromous Fish Commission Bulletin 5:197–208
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299
Gilmer R, Lalli C (1990) Bipolar variation in Clione, a gymnosomatous pteropod. Am Malacol Bull 8:67–75
Haigh R, Ianson D, Holt CA, Neate HE, Edwards AM (2015) Effects of ocean acidification on temperate coastal marine ecosystems and fisheries in the Northeast Pacific. PLoS One e0117533:10. https://doi.org/10.1371/journal.pone.0117533
Hamaoka S (2002) Seasonal change and breeding season of Clione limacina in coastal Okhotsk Sea. Gekkan Kaiyo 30:172–177
Inaba Y, Okutani T (1999) Clione limacina occurred in Oarai Marine at 36°20′N. Newsl Malacol Soc Jpn 29:47–49
Jennings RM, Bucklin A, Ossenbrugger H, Hopcroft RR (2010) Species diversity of planktonic gastropods (Pteropoda and Heteropoda) from six ocean regions based on DNA barcode analysis. Deep Sea Res Part Top Stud Oceanogr 57:2199–2210. https://doi.org/10.1016/j.dsr2.2010.09.022
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111–120
Kuma K, Sasayama R, Hioki N, Morita Y, Isoda Y, Hirawake T, Imai K, Aramaki T, Nakamura T, Nishioka J, Ebuchi N (2014) Chemical evidence for the origin of the cold water belt along the northeastern coast of Hokkaido. J Oceanogr 70:377–387
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870–1874
Kunihiro Y (1995) Behaviour and fisher of Kichiji rock fish, in Okhotsk Sea No 1. Hokusuishi Latter 28:2–8
Lalli CM, Conover RJ (1976) Microstructure of the veliger shells of gymnosomatous pteropods (Gastropoda: Opisthobranchia). Veliger 18:237–240
Lalli CM, Gilmer RW (1989) Pelagic snails. The biology of holoplanktonic gastropod mollusks. Stanford University Press, Stanford
Lalli CM, Wells FE (1978) Reproduction in the genus Limacina (Opisthobranchia: Thecosomata). J Zool 186:95–108
Martens F (1675) Spitzbergische Oder Gröenlandische Reise Beschreibung Gethan im Jahr 1671. Schultze, Hamburg
Massy AL (1932) Mollusca: Gastropoda Thecosomata and Gymnosomata (Pteropoda). Discovery Repts 3:268–296
Matsuyama M, Aota M, Ogasawara I, Matsuyama S (1999) Seasonal variations of soya current. Oceanogr Jpn 8:333–338
McGowan JA (1963) Geographical variation in Limacina helicina in the North Pacific. In: Harding JP & Tebble N (eds.) Speciation in the Sea. Systematics Association, London, Publication No. 3, pp 109–128
MClintock JB, Janssen J (1990) Pteropod abduction as a chemical defence in a pelagic Antarctic amphipod. Nature 346:462–464. https://doi.org/10.1038/346462a0
Mileikovsky SA (1970) Breeding and larval distribution of the pteropod Clione limacina in the North Atlantic, Subarctic and North Pacific Oceans. Mar Biol 6:317–334
Morton JE (1958) Observations on the gymnosomatous pteropod Clione limacina (Phipps). J Mar Biol Assoc UK 37:287–297. https://doi.org/10.1017/S002531540002368
Ohshima KI, Wakatsuchi M, Fukamachi Y, Mizuta G (2002) Near-surface circulation and tidal currents of the Okhotsk Sea observed with satellite-tracked drifters. J Geophys Res 107:3195. https://doi.org/10.1029/2001JC001005
Phipps CJ (1774) A voyage towards the North Pole undertaken by HM Command 1773. London
Richter G, Seapy RR (1999) Heteropoda. In: South Atlantic zooplankton, vol. 1 (D. Boltovskoy, ed.), Backhuys, Leiden, pp. 621–647
Ruth HP, Patrick JH (1998) Oceanography of the sea of Okhotsk and the Japan/East Sea. In: The sea, Vol. 11 (RR Allan and HB Kenneth, ed.), pp. 429–481. Harvard University Press, Cambridge, MS.
Smith EA (1902) Mollusca In: Report on the collections of natural history made in Antarctic regions during the voyage of the "Southern Cross" (I. Franklin, ed.), British Museum (Natural History), London, pp. 201–213, pls. 24–25
Sromek L, Lasota R, Szymelfenig M, Wolowicz M (2015) Genetic evidence for the existence of two species of the “bipolar” pelagic mollusk Clione limacinae. Am Malacol Bull 33:118–120
Suprenand PM, Jones DL, Torres JJ (2015) Distribution of gymnosomatous pteropods in western Antarctic Peninsula shelf waters: influences of Southern Ocean water masses. Polar Rec 51:58–71. https://doi.org/10.1017/S003224741300065X
Suzuki A, Miyano Y, Mawatari K (2005) Biological features of Clione limacina Phipps (Gymnosomata: Clionidae) collected in Abashiri, Hokkaido. Newsl Malacol Soc Jpn 36:12–17
Takizawa J (1982) Characteristics of the soya warm current in the Okhotsk Sea. J Oceanogr Soc Jpn 38:281–292. https://doi.org/10.1007/BF02114532
Van Der Spoel S (1976) Pseudothecosomata, Gymnosomata and Heteropoda (Gastropoda). Bohn, Scheltema, and Holkema, Utrecht
Van Der Spoel S, Dadon JR (1999) Pteropoda. In: South Atlantic zooplankton (D. Boltovskoy, ed.), Backhuys, Leiden, pp. 649–706
Wanger N (1885) Die Wirbellosen des Weissen Meeres. Vol. 1. Zoologische Forschungen an der Küste des Solowetzkischen Meerbusens in den Sommermonaten der Jahre 1877, 1878, 1879 und 1882. W. Engelmann, Leipzig
Wrobel D, Mills C (1998) Pacific coast pelagic invertebrates: a guide to the common gelatinous animals. Sea Challengers, Monterey Bay Aquarium, Monterey, CA
Yamazaki T, Kuwahara T (2017) A new species of Clione distinguished from sympatric C. limacina (Gastropoda: Gymnosomata) in the southern Okhotsk Sea, Japan, with remarks on the taxonomy of the genus. J Molluscan Stud 83:19–26. https://doi.org/10.1093/mollus/eyw032
Acknowledgements
This work was supported by research grants to the Uminomanabi Museum Support from the Museum of Maritime Science (the Nippon Foundation), and National Institute of Polar Research (NIPR) through Project Research no. 29-39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamazaki, T., Kuwahara, T. & Takahashi, K.T. Genetic Differences in Spatially and Temporally Isolated Populations: Winter and Spring Populations of Pelagic Mollusk Clione (Mollusk: Gymnosomata), Southern Okhotsk Sea, Japan. Thalassas 34, 447–458 (2018). https://doi.org/10.1007/s41208-018-0092-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-018-0092-z