Abstract
Mining is an important activity for many countries, especially some in development, such as Chile, where it is a pillar of its economy. However, it generates large impacts that are undesirable for the population such as the generation of polluting solid and effluents with a high content of heavy metals and metalloids, which are traditionally accumulated in deposits. In recent years, bionanomining emerged as a cutting-edge scientific-technological development associated with the application of micro- and macro-organisms to generate nanotechnological products by using mining and industrial wastes and wastewaters. Biomass of many species of bacteria, plants, algae and fungi have the ability to reduce or oxidise cations, which can physically be deposited as nanometric materials such as the nanoparticles. Nanoparticles are materials that are increasingly used, and therefore, their demand increase, based on the high surface area characteristics to improve thermal, electrical and optical properties of materials, and metallic ones have also antimicrobial activity. This review addresses the biosynthesis of metal nanoparticles, focusing on mining waste recovery strategies, which is an emerging reality in mining countries. Transformation of potentially hazardous waste into a valuable product through techniques that are eco-friendly is an opportunity to develop sustainably depressed or polluted sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mining is an important activity that provides critical raw materials for the human development, and also, it is an economic driver for several countries, especially for some developing (Moreno et al. 2014). Within the most exploited mineral resources are the metals, which are extracted with different magnitudes, but whose global production trend has been increasing, as shown in Table 1. The leading metal producers for the metals that concentrate the exploration activities are China, Australia, Chile, South Africa and Indonesia (Brown et al. 2019; US Geological Survey (USGS) 2018). In the case of Chile, it is well known that it supports much of its economy in the mining industry, representing 13% of its GDP. In addition to copper, other minerals in which Chile occupies a leading position are lithium, iodine, silver and molybdenum. In the case of copper, 31% of the metal traded in the world comes from Chile (5,700,000 Mt) (COCHILCO 2013; Moreno et al. 2017; US Geological Survey (USGS) 2018).
There are two main processes for base metal extraction: hydrometallurgical and pyrometallurgical methods (Fig. 1). The first use aqueous solutions to dissolve soluble species, and on the other side, the second one works with very high temperature to melt materials. In the hydrometallurgy, the dissolution of the oxidised ores is performed by using acid or alkali at high or low temperature and pressure, or even can be bacterially assisted (bioleaching). The conditions of processing are basically defined according to the composition of the ore. The obtained solution is subsequently concentrated and purified by solvent extraction and electro-winning. Pyrometallurgical operations join melting, refining, casting and alloying processes. However, due to the concentration of metal in the ore are typically low, the direct smelting treatment is not economically feasible. For this reason, minerals must be physically concentrated before passing, in the case of copper, below 1 to about 30%. The concentration is conducted by intensive comminution and froth flotation, where the copper-containing minerals are separated from the rest of the ore.
Despite the important benefits that mining brings to the country and surrounding communities in which it operates, it also generates large impacts that are undesirable for the population and that require treatment or mitigation strategies, such as intensive use of soil and water, emission of particulate material, generation of polluting effluents with a high content of heavy metals and metalloids, among others (Adiansyah et al. 2015). It is estimated that globally, there are about 3500 active deposits of mining waste around the world, which consist in rock and tailing dumps, with a generation rate that rounds 100,000 million tonnes per year (Starke 2002; Lébre and Corder 2015; Rankin 2015). Waste rocks contain coarse or crushed material that can produce acid mine drainage (AMD) by the oxidation of sulphide minerals. The increasing demand for essential metals, the increasingly diminution of ore grades and the extraction of complex ore bodies have resulted in the generation of greater quantities of tailings that require strategies to handle in order to diminish the risks to environment (Chryss et al. 2012; Jeldres et al. 2018).
On the other hand, nanotechnology is a promising discipline in modern technology processes and material construction, due to that it has as main basis the ability to work at a molecular level in order to create large structures with a new molecular organisation. Today, nanotechnology progresses as cutting-edge technology with numerous interdisciplinary applications, including physics, chemistry, biology, engineering and medicine (Mansoori 2017). The properties of nanomaterials, such as physical, chemical, optoelectrical, mechanical, magnetic and thermal, are very different from their macro-materials. These differences mainly provoked two effects: surface (large surface area of the atom, large surface energy and reduced imperfections) and quantum (discontinuous behaviour due to spatial confinement in materials with delocalised electrons) (Buzea et al. 2007; Narayanan and Sakthivel 2010).
In terms of the size, materials with sizes less than 100 nm are termed as nanomaterials and can be classified in different ways, depending on their composition, origin and dimensions. By composition, nanomaterials are carbon-, inorganic-, organic- and composite-based. The classification by origin considers the source used to obtain the nanomaterials, which can be synthetic or natural. On the other hand, the dimension of nanomaterial is very dependent of the level of electron movement and the movement sense, so nanomaterials are categorized as 0D, 1D, 2D and 3D (Jeevanandam et al. 2018); within the last are included the nanoparticles. The concepts of nanoparticle, nanorod and nanoplate are differentiated among them through the relation between their dimensions.
Synthesis of nanoparticles and the incursion of biotechnology
Traditional methods of synthesis
Nanoparticles (NPs) are traditionally synthesized using top-down or bottom-up methods (Fig. 2). The top-down methods gradually break down bulk materials to nanometre-sized materials. It is usually applied in the electronics industry. However, it has some drawbacks, as it is challenging to generate uniform nanoparticles; introduce internal stresses, structural defects, and contaminants; and consume a large quantity of energy (Su and Chang 2018). On the other hand, in the bottom-up method, atoms or molecules are combined to form molecular structures in the nanometre-sized range using chemical or physical forces (Rotello 2004). It is possible to control the size and shape of the nanoparticle depending on the final application through variations in the precursor concentrations and reaction conditions such as temperature and pH (Virkutyte and Varma 2013). Whilst physical and chemical treatments of bottom-up are extensive in the traditional synthesis of nanoparticles, this method has the disadvantage that the usage of toxic chemicals limits its application in sensitive fields, such as medicine (Ahmed et al. 2016; Duan et al. 2016).
Biosynthesis of nanoparticles
In the search for synthesis processes that mean lower environmental and economic costs, the biosynthesis, which uses biological sources, emerged. Although biosynthesis is a kind of bottom-up process, it is generally handled as a third route since it has noted differences in the scope and requirements than conventional bottom up, i.e. natural synthesis and absence of intensive chemical and energy requirements (Singh et al. 2010). The nanoparticles produced by biological ways have demonstrated better biocompatibility due to the capping or encapsulation activity on the metallic cores with non-toxic and biodegradable materials. For this reason, the biosynthesis of metallic nanoparticles (as part of the bionanotechnology) have gained increasingly attention, facing the challenges to develop non-toxic, reliable, eco-friendly, easy scaling up, and well-defined nanostructures (Whitesides 2003).
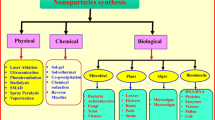
Bionanotechnology uses plants, algae and microorganisms such as bacteria, fungi and yeast, to biosynthesise nanoparticles with different types, forms and distribution in a broad spectrum of pH, temperature and use of precursors (Gan and Li 2012; Vena et al. 2016; Wadhwani et al. 2018). Metallic nanoparticles such as gold, silver, platinum, aluminium, zinc, titanium, palladium, iron and copper have been biogenically synthesised (Hulkoti and Taranath 2014). Additionally, oxide and sulphide nanoparticles have been studied because they have important applications as semiconductors, reagents for cancer treatments and additives for the improvement of the thermochemical properties of phase change materials in energy storage (Sanghi and Verma 2009; Ramanathan et al. 2011).
The biosynthesis mediated by microorganisms can be intra and extracellular, being the latter more practical due to the recovery of nanoparticles that does not require additional processing. In both cases, the reduction of the metals is catalysed by enzymes and other organic substances that can be secreted in the cell wall or outside the cell. Although current studies have not yet completely deciphered the mechanisms by which biological sources synthesise metal nanoparticles, there is a consensus that the main pathways involve enzymatic oxidation, reduction, adsorption and chelation with peptides or polysaccharides of cell walls.
The biosynthesis of NPs could be classified as a bottom-up approach because NPs are formed through oxidation or reduction reactions between ions and chemical derivatives from the cell. The uptake of metal ions by biomass and the subsequent transformation to their elemental form are dynamic processes. In the intracellular biosynthesis, the ions are incorporated into the cell, for which, the process is controlled by the cell wall and plasma membrane activity; carrier proteins, reductive enzymes and cofactors are involved (Li et al. 2011; Salunke et al. 2016). In the cytoplasm, the ions are reduced as a consequence of the metabolic reactions with enzymes, e.g. nitrate reductase. On the other side, the extracellular biosynthesis can be performed by the union of metal ions with functional chemical groups on the cell wall (biosorption process) and/or with secreted metabolites (Fig. 3). The intracellular NP biosynthesis is frequently metabolism dependent because it is required that the cell has the conditions for growth and for developing biological activity; for this reason, the microorganisms must be alive. This has operational considerations like control of temperature, pressure, salinity, culture medium, contamination and concentration of metals during the biosynthesis. One of the advantages of this type of pathway is the possibility to obtain metal oxides and metal sulphides NPs.
Mechanisms of NP biosynthesis. There are two recognized mechanisms by which microorganisms synthesise nanoparticles. Intracellular biosynthesis is carried out by means of proteins, enzymes and cofactors. Extracellular biosynthesis can be mediated by secreted biomolecules and by the interaction with functional groups on the cell wall (Salunke et al. 2016)
On the other hand, in the extracellular biosynthesis, it is not necessary that cells are alive during biosynthesis, which from an industrial perspective, seems to be a better option for the bioreduction of metals, because the operational conditions and control are simpler, e.g. dead cells have less toxicity, readily recovery of biosorbed metals and the biomass can be regenerated and reused (Bishnoi and Garima 2005). As mentioned above, obtaining metal nanoparticles from biological sources has characteristics that make it convenient to face the challenge of generating highly specialised products through cleaner and less expensive techniques.
Biosynthesis develops in simpler systems than chemical or physical synthesis, given that the operational conditions since biosynthesis can be carried out using live or dead biomass and generally works at ambient (and near) ambient temperatures and pressure. If biosynthesis requires the use of live biomass, for example, for the generation of NPs of metal oxides (metabolically active cells that produce oxidase enzymes are needed), greater attention in cell culture conditions must be provided to achieve growth and biosynthesis (Ghorbani et al. 2015; Singh 2015). When biosynthesis occurs inside cells (depending on metabolism), the recovery of NPs is done by cell lysis (Lee et al. 2004; Gahlawat and Choudhury 2019). If, on the other hand, biosynthesis is performed under extracellular mechanisms, obtaining NPs is facilitated and reduced to solid-liquid separation operations such as centrifugation and filtration (Salvadori et al. 2014). The availability of a growing literature has demonstrated the feasibility of synthesising many types of metal nanoparticles (metals, metal oxides and sulphides), varying the type of metal salt and the microorganism, demonstrating the versatility of the method. A key aspect for the application of nanoparticles has to do with their stabilisation since due to their small size, they tend to agglomerate, which affects their performance. In the case of biosynthesis from microorganisms or plants (and their extracts), nanoparticles are generated accompanied by stabilising and covering agents, which prevent the grouping of NPs (David et al. 2019; Rufus et al. 2019). In the case of traditional synthesis, the use of stabilizers such as BSA (bovine serum albumin) (Chakraborty and Parak 2019; Wang et al. 2019) and glycerol (Parveen et al. 2019) is added.
Simpler operational conditions, lower demands on pressure, temperature and use of chemical agents have an effect on the synthesis costs being considerably lower (Fig. 4). Additionally, another advantage of nanoparticle biosynthesis is that it generates very few toxic residues. The main waste of the process corresponds to biomass, which in many cases can be reused for new cycles of biosynthesis. Among the main disadvantages of working with biosynthesis is the low volume of nanoparticles generated and the high synthesis time, compared with conventional techniques. The use of experimental designs to optimise operating conditions, such as the amount of biomass, the volume of solution and the concentration of metal in the solution, is useful for achieving the best conditions. Thus, the use of not only residual solids as bioreductive agents (agroindustrial waste, stabilised sludge, etc.) but also industrial effluents as a metallic source for the biosynthesis of nanoparticles (mining tailings, industrial waters, etc.).
The use of toxic chemicals in the process of synthesis via the bottom-up methodology generally leaves toxic residues on the surface of the nanoparticles and non-polar solvents, and this limits its applications, especially in the medical and biotechnological areas. Because of this, there has an increase in the use of biomass for the synthesis of nanoparticles as it corresponds to a clean, friendly environment and economical method. The microorganisms have proved to be highly efficient biofactories for the generation of NPs of different compositions, shapes and sizes. Although chemical processes are capable of producing large amounts of NPs, the nanoparticles produced by a biogenic enzymatic process are much superior, in various ways, since almost any metallic element, metal oxides and metal sulphides can be produced (Tiquia-Arashiro and Rodrigues 2016).
Current scientific and technological development about the biosynthesis of metal NPs
From a bibliometric approach, the research associated with nanoparticle biosynthesis dates back to the beginning of 2000, resulting in the production of 981 articles, distributed in 382 indexed journals (Fig. 5). However, the rate of publications on nanoparticle biosynthesis has experienced remarkable and sustained growth since 2009. This is how it has happened since the 225 articles published between the 2009 and 2013 period and the 615 articles between 2014 and 2018. So far in 2019, the number of articles amounts to 124, which should be projected as the year of the greatest amount of scientific contributions in the field. Publications related to the biosynthesis of nanoparticles bring together the generation of nanomaterials not only metallic but also those based on organic compounds, such as nanopolymers. Focusing only on the context of the biosynthesis of metal nanoparticles, these correspond to one-third of the total publications, and the metals that concentrate the biogenesis studies are with distance silver (79%) and gold (59%), due to the growing new applications that have been discovered for their use in medical therapies. The countries that lead the generation of basic and applied knowledge in the field of biosynthesis of metal nanoparticles are India, Iran and the USA.
Undoubtedly, the concepts that appear most in the titles of the publications are “nanoparticle” (which also considers its plural version) and “synthesis” and “biosynthesis”. It is interesting to note that there is a tendency of the authors to build the title of their work with the concept of “synthesis” and not to put the prefix bio. It is also noted that the most studied metal for biosynthesis corresponds to silver and then gold. The studies tend to closely relate biosynthesis with the concepts of eco-friendly and green since its protocols dispense with the use of contaminating chemical reagents and the use of energy-intensive techniques. The most reported biological agents to produce metal nanoparticles correspond to plants (and their extracts), bacteria and fungi. The activities that are most frequently carried out in the works are the characterisation and analysis of their properties as catalysts and as antibacterial (or antimicrobial) agents (Fig. 6).
The impact of the works, from the point of view of citations, goes hand in hand with the growing trend of publication, obtaining average increases in the citation rate of 425 citations/year. The accelerated growth in the volume of knowledge related to the biosynthesis of metal nanoparticles has not had the same impact on technological transfer, which can be seen in the less number of invention patents related to nanoparticle biosynthesis processes, in total 66 patents since 2003 (Fig. 5). The various patent search engines frequently issue a greater number of patents, but they include other aspects such as the application of nanoparticles to achieve biological activity effects, which is not in line with the study of biosynthesis. In this work, an exhaustive filter in the scope of patents was made. The amount of invention patents on biosynthesis demonstrates that the scientific development of this technology is still, for the most part, in an experimental validation stage. Despite the foregoing, in recent years, it has been observed that the trend of intellectual protection has achieved promising rates since only in the last 3 years, 42% of the patents awarded on metal nanoparticle biosynthesis have been concentrated. USA and China are the countries whose patent offices have granted the highest number of patents.
Bionanomining = mining + bionanotechnology
The application of biotechnologies in mining processes has been carried out for years, through, for example, bioleaching, where the solubilisation of metals from minerals is sought from the catalytic activity that some microorganisms exert, mainly bacteria and archaea. Multiple studies about the physiology of microorganisms, the operational conditions and the influence of the mineral processing reagents on the growth and oxidation activity have been performed (Watling 2006; Jafari et al. 2019). On the other side, the commercial application of copper bioleaching has had an important review, due to the ability to treat secondary minerals of low grade by bacterial leaching in heaps. Recently, Yin et al. (2018) compiled studies about the heap bioleaching of copper mines, including the isolation and identification of bacterial strains, the improvement of mechanisms and interface reactions, multistage percolation, and also the projection to use bioleaching microorganisms in the recovery of metals from electronic waste, which is a novel application with increasing demand.
Beyond the development of bioleaching as the most common of the biotechnological strategies that are related to mining, there are other more recent, and therefore, less developed applications that seek the recovery or removal of metallic species from solutions produced by mining, which usually correspond to waste streams or effluents. Several methods for metal immobilisation that are used in the treatment of mining and industrial effluents are biosorption and bioaccumulation. In that line, microorganisms with biosorbent potential for the removal of heavy metals have been studied, determining the sequestration mechanisms of metals and biosorption capabilities (Ayangbenro and Babalola 2017). It is frequent that the strains used in the biosorption experiments have been isolated from the same places where the mines operate due to that they have proved tolerance to metals present at high concentrations. Cadmium and zinc have been bioaccumulated by bacteria isolated from a zinc mine, locating the metal species on their cell walls (Limcharoensuk et al. 2015).
On the other hand, the biosynthesis of metal NPs can be studied and even applied under different approaches. After lab level researches focusing the biogenic reduction of metals and the conditions that are optimal for the natural reactions between biomass and ions, the second derivative is the use of residual biomasses like agro-waste solids and effluents as reducing agents (Adelere and Lateef 2016). In other level, the use of biomass obtained by microbiological procedures is used to bioreduce metals that are contained in industrial or mining effluents, the latter being related to the concept of bionanomining, i.e. obtaining biologically synthesised nanomaterials from mining resources or wastes.
The main challenge in the valorisation of mining or industrial wastes is that the metal species are frequently present in low concentration. The recovery of metal species from mining effluents has been traditionally addressed by expensive or pollutant methods, such as precipitation (sulphate and hydroxide), adsorption, membrane filtration, ion exchange, solvent extraction and electrochemical processes (He and Kappler 2017; Isosaari and Sillanpää 2017). In this context, the emergence of biotechnological processes and the experience gathered in the various research on bioremediation provide fundamental information about the natural interaction between biomass and metals and give the opportunity to develop low-cost and eco-friendly techniques to treat extractively industrial effluents (Gadd 2009; Bharagava 2017).
In most studies, the experimental biosynthesis of nanoparticles uses a synthetic metal source prepared in the laboratory at known concentration and chemically designed according to pre-obtained optimal conditions. Few scientific contributions apply biomass to deal with actual and multi-species solutions. The idea to obtain metal nanoparticles from mining wastes is, securely, a challenging task, due to the complex composition of these types of effluents. In terms of the treatment of mining waste, tailings and leached solutions generally have low pH and many ionic species (Table 2).
The abiotic synthesis of nanoparticles from mining waste was firstly reported by Kumar et al. (2015), who synthesise magnetite NPs from iron ore tailings that were previously leached with hydrochloric acid to dissolve the iron fraction. The magnetite NPs were used as carrier to support silver coating that has proved antibacterial properties. The use of nanostructured magnetite particles allows improving the extraction process once NPs is applied in the treatment of bacterial diseases, avoiding silver toxification problems in the host organism.
On the other hand, approaching the valorisation of industrial waste by NP biosynthesis, more recent researches have also focused on the synthesis mediated by microorganisms. In order to protect metals from corrosion, materials are frequently electroplated with chromium. However, this process also generates effluents charged in heavy metals and chromium, which are very toxic to humans. In this context, several authors have treated electroplating wastewater and produced Cr(III) NPs by using the Cr-resistant bacteria Bacillus subtilis (Kanakalakshmi et al. 2017). The cytotoxicity and antibacterial properties of the obtained NPs were evaluated and satisfactorily described.
A poor handling of dump heaps and tailing deposits or the abandonment of metal mines can lead to the generation of acid mine drainage (AMD), which is one of the most severe environmental problems of the mining industry (Akcil and Koldas 2006). The reaction between sulphide minerals and the water in aerobic conditions produces the releasing of sulphuric acid (as sulphate) that acidifies natural watercourses and groundwater and increase the level of metals by their leaching, becoming a potential source of pollution and toxicity for inhabitants, animal and plants (Favas et al. 2011). One of the biotechnological ways to treat these undesired reactions is by bioremediation using sulphate-reducing bacteria (SRB). These bacteria use sulphate as terminal electron acceptor and finally induce the precipitation of metal sulphides. Authors have used the continuous process of bioreduction of sulphate with SRB and carefully control the treatment, obtaining metal sulphur nanocomposites, as ZnS and TiO2 (Vitor et al. 2015, 2016). This work is the first attempt to biosynthesise NPs and nanocomposites coupled with a bioremediation process of actual effluents in a columnar system. The produced nanomaterials have industrial application as semiconductors or photocatalysts. It is remarkable that this work states that from bioremediation, not only environmental benefits but also economic benefits are obtained. In the same way, other authors have used the AMD effluents coming from an abandoned gold mine to produce gold nanoparticles by using the SRB-mediated bioremediation process (the main bacteria is Desulfovibrio desulfuricans) (Assunção et al. 2016). They also prove that the type of metal precursor (or solution) has a critical influence on the type of product obtained after bioreduction; thus, if Au (III) solution is used, the bacteria produced AuS NPs, whilst when AMD is used, AuNPs are obtained. The AuNPs have a high economic value and wide range of applications, such as medicine, optics and catalysts.
In the treatment of AMD, one of the recent approaches for the removal of metals and metalloids released as a result of the acid leaching produced by this solution is the application of metal nanoparticles, which act as nucleating agents. This technique has been used for the significant removal of Al, Mg, Mn and Zn from AMD using magnetite and Co ferrite NPs (Kefeni et al. 2017). NPs also are applied for soil remediation, e.g. Fe and goethite NPs are recently described as good fixation agents for arsenic, reducing the phytotoxicity and improving the quality of soils (Baragaño et al. 2020).
In other approach, several industries like mining, electroplating, pigments, fertilizers and batteries produce effluents charged with cadmium, a metal that is accumulated in the food chain and that its exposition causes cancer. Traditionally, there are numerous methods reported for the removal of cadmium and other heavy metals, such as chemical reduction, electrochemical treatment, ion exchange and precipitation. There is an emergent approach to treat these Cd effluents and produce economically valued products chained to biorestoration or remediation processes. Vena et al. (2016) describe several experimental efforts that use microorganisms to biosynthesise nanocrystals of Cd chalcogenides from toxic effluents. Cd chalcogenides (CdS, CdSe and CdTe) are a group that in nanostructured form are also named as quantum dots (QDs), which have high technological demand in bioimaging and as sensors and electronic devices. The works reviewed by Vena et al. (2016) are initial laboratory attempts that support the idea of industrial effluent recovery, but definitely, there are still elements to touch for leading higher level of development.
Recently, bioreduction of copper nanoparticles from treated tailings was reported by using the bacterial biomass of Pseudomonas stutzeri (DSM 5190), which is a strain previously described in the biosynthesis of silver nanoparticles. Characterisation of biogenic CuNPs revealed that they were spherical with an average size of 60 nm. The study showed also that the bioreduction occurs extracellularly, since CuNPs are attached on the cell wall or in the solution. The oxidation state of nanoparticles was determined by X-ray photoelectron spectroscopy (XPS), detecting the coexistence of the three oxidation states of copper, indicating that the main nanomaterial obtained was Cu(0), but also Cu(I) and Cu (II), which may be related with oxidised or sulphurised nanoparticles (Ordóñez and Wong 2019; Wong et al. 2019).
Other mine waste that is environmentally important is the coal tailings, containing sulphur species that eventually lead to metal leaching of metals. In a recent investigation, coal tailings were treated with bacteria Rhodococcus erythropolis ATCC 4277 to biosynthesise Fe NPs as part of the strategy to improve the environment with added value (Maass et al. 2019). Although the conversion obtained is relatively low, about 19%, it corresponds to the first bionanotechnological initiative for this type of waste, which represents a starting point to get new methodologies for adding coal mining waste value.
The pyrometallurgical route for mineral beneficiation produces high volume of particulate matter that generally is composed by multiple heavy metals and metalloids in large concentrations and that are spread by wind (Yasipourtehrani et al. 2020). The solid by-product obtained from smelters is the slag. Experimental techniques have been evaluated to recover selectively copper from the metallurgical sludge using sugarcane bagasse as biosorbent, obtaining a solution with Cu concentration of 95.4% (Xie et al. 2018). Although biosorption tests have not led to bioreduction activity, it is a contribution to the recovery of valuable/polluting species from very complex matrices, due to the high presence of metals, which is a challenging issue for almost all mining wastes.
The knowledge about the biovalorisation of actual effluents by using bacteria or other higher organism is still in the early stages of research. More investigations in a multidisciplinary way are required to propose competitive and sustainable methods for biosynthesis of nanoparticles; however, the trend in this research field is growing and increasingly intense in the environmental-extractive applications.
Conclusions
There is a growing interest in having nanomaterials that allow to solve increasingly specific problems or substantially improve new applications in high-tech industries and medical treatments. On the other hand, various intensive industrial activities, such as mining, generate a large amount of waste that, if not treated or confined, can generate unwanted impacts for the population and the environment. Thus, under a new concept of circular economy applied to these wastes considering them as resources, and given the increasing evidence of the capacity of certain prokaryotic and eukaryotic biomasses to bioreduce metallic species in nanomaterials, the concept of bionanomining emerges. The thematic of this work is novel due to although the biosynthesis of nanoparticles is increasingly studied, the most part of the research is focused on a fundamental approach by performing lab experiments and or using synthetic solutions.
In this sense, the challenges and projections of the bionanomining, especially in developing countries that based their economy in the exploitation of mineral resources, are very important in the diversification of productive matrix, even more considering that the nanomaterials are expensive products with high demand, e.g. 1 kg of bulk copper is internationally traded at 6 US$ whilst the same mass of copper nanoparticle is valued in 500 US$, depending of its homogeneity and particle size. In addition, bionanoming offers cheap synthesis processes that are more environmentally friendly than traditional top-down and bottom-up methods for production of nanomaterials.
Notwithstanding the multiple-approach advances that are in progress, it is necessary that certain aspects reach a higher level of development, in order to achieve an industrial application. It is expected that by addressing the challenges, in the future, the number of invention patents associated with nanotechnological products produced by biotechnology, and particularly by bionanomining, will increase. Some of the challenging issues are
Extraction and purification processes: This is undoubtedly one of the main aspects that have been less studied and that becomes critical for the potential industrial application of biosynthesis processes. Whilst the separation of NPs from cells becomes simpler when extracellular separation repeatedly used centrifugation, which has a potential binding of NPs. On the other hand, in intracellular biosynthesis, cell lysis can cause aggregation, precipitation and sedimentation of NPs, together with an increase in costs if the enzymatic breakdown is evaluated.
Stabilization: The stabilization of biosynthesised nanoparticles must also be improved to take advantage of the natural property of biosynthesis processes to generate more stabilized NPs than physicochemical processes. The duration of biosynthesised NPs has not yet been widely determined with precision.
Scaling-up from the laboratory to industrial plants: Although many types of bacteria used to produce various types of NPs are recognized, what will happen in biosynthesis tests carried out under less controlled conditions (laboratory) at pilot level remains to be validated and compared if biosynthesis processes could efficiently replace traditional synthesis processes.
Optimization in the conditions of cell growth and bioreduction: Biosynthesis will in the future points to the use of enzymes and nano synthetic proteins that avoid using microorganisms. However, for this, together with the determination of the various organic substances involved, the conditions that allow for optimum reaction yields must be established. At present, the identification of strains resistant to high levels of heavy metals and with high growth rates has been evaluated for biosynthesis.
References
Adelere IA, Lateef A (2016) A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev 5:567–587. https://doi.org/10.1515/ntrev-2016-0024
Adiansyah JS, Rosano M, Vink S, Keir G (2015) A framework for a sustainable approach to mine tailings management: disposal strategies. J Clean Prod 108:1050–1062. https://doi.org/10.1016/j.jclepro.2015.07.139
Ahmed S, Annu IS, Yudha S (2016) Biosynthesis of gold nanoparticles: a green approach. J Photochem Photobiol B Biol 161:141–153. https://doi.org/10.1016/j.jphotobiol.2016.04.034
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment and case studies. J Clean Prod 14:1139–1145. https://doi.org/10.1016/j.jclepro.2004.09.006
Antonijević MM, Dimitrijević MD, Stevanović ZO, Serbula SM, Bogdanovic GD (2008) Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J Hazard Mater 158:23–34. https://doi.org/10.1016/j.jhazmat.2008.01.063
Assunção A, Vieira B, Lourenço JP, Costa MC (2016) Recovery of gold(0) nanoparticles from aqueous solutions using effluents from a bioremediation process. RSC Adv 6:112784–112794. https://doi.org/10.1039/C6RA24503J
Ayangbenro A, Babalola O (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94–109. https://doi.org/10.3390/ijerph14010094
Baragaño D, Alonso J, Gallego JR, Lobo MC, Gil-Díaz M (2020) Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils Chemosphere 238:. https://doi.org/10.1016/j.chemosphere.2019.124624
Bharagava RN (2017) Environmental pollutants and their bioremediation approaches. CRC Press, Boca Raton, Florida
Bishnoi NR, Garima A (2005) Fungus-an alternative for bioremediation of heavy metal containing wastewater: a review. J Sci Ind Res (India) 64:93–100
Brown TJ, Idoine NE, Raycraft ER, Hobbs SF, Shaw RA, Everett P, Kresse C, Deady EA, Bide T (2019) World mineral production 2013–17. Keyworth, Nottingham
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71. https://doi.org/10.1116/1.2815690
Chakraborty I, Parak WJ (2019) Protein-induced shape control of noble metal nanoparticles. 1801407:1–7. https://doi.org/10.1002/admi.201801407
Chryss A, Fourie AB, Mönch A, Nairn D, Seddon KD (2012) Towards an integrated approach to tailings management. J South African Inst Min Metall 112:965–969
COCHILCO (2013) Inversión, Exploración e Insumos estratégicos para la. Minería, Santiago
David SA, Veeraputhiran V, Vedhi C (2019) Biosynthesis of cobalt oxide nanoparticles-a short review. J Nanosci Technol 5:734–737. https://doi.org/10.30799/jnst.s01.19050308
Duan J, Yu Y, Li Y, Li Y, Liu H, Jing L, Yang M, Ji W, Li C, Sun Z (2016) Low-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryos. Nanotoxicology 10:575–585. https://doi.org/10.3109/17435390.2015.1102981
Favas PJC, Pratas J, Gomes MEP, Cala V (2011) Selective chemical extraction of heavy metals in tailings and soils contaminated by mining activity: environmental implications. J Geochemical Explor 111:160–171. https://doi.org/10.1016/j.gexplo.2011.04.009
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. https://doi.org/10.1002/jctb.1999
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9:12944–12967. https://doi.org/10.1039/c8ra10483b
Gan PP, Li SFY (2012) Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev Environ Sci Bio/Technology 11:169–206. https://doi.org/10.1007/s11157-012-9278-7
Ghorbani HR, Fazeli I, Fallahi AA (2015) Biosynthesis of copper oxide nanoparticles using extract of E.coli. Orient J Chem 31:515–517. https://doi.org/10.13005/ojc/310163
He J, Kappler A (2017) Recovery of precious metals from waste streams. Microb Biotechnol 10:1194–1198. https://doi.org/10.1111/1751-7915.12759
Hulkoti NI, Taranath TC (2014) Biosynthesis of nanoparticles using microbes-a review. Colloids Surfaces B Biointerfaces 121:474–483. https://doi.org/10.1016/j.colsurfb.2014.05.027
Isosaari P, Sillanpää M (2017) Use of sulfate-reducing and bioelectrochemical reactors for metal recovery from mine water. Sep Purif Rev 46:1–20. https://doi.org/10.1080/15422119.2016.1156548
Jafari M, Abdollahi H, Shafaei SZ, Gharabaghi M, Jafari H, Akcil A, Panda S (2019) Acidophilic bioleaching: a review on the process and effect of organic–inorganic reagents and materials on its efficiency. Miner Process Extr Metall Rev 40:87–107. https://doi.org/10.1080/08827508.2018.1481063
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Jeldres RI, Piceros EC, Wong L, Leiva WH, Herrera N, Toledo PG (2018) Dynamic moduli of flocculated kaolinite sediments: effect of salinity, flocculant dose, and settling time. Colloid Polym Sci 296:1935–1943. https://doi.org/10.1007/s00396-018-4420-x
Kanakalakshmi A, Janaki V, Shanthi K, Kamala-Kannan S (2017) Biosynthesis of Cr(III) nanoparticles from electroplating wastewater using chromium-resistant Bacillus subtilis and its cytotoxicity and antibacterial activity. Artif Cells, Nanomedicine, Biotechnol 45:1304–1309. https://doi.org/10.1080/21691401.2016.1228660
Kefeni KK, Mamba BB, Msagati TAM (2017) Magnetite and cobalt ferrite nanoparticles used as seeds for acid mine drainage treatment. J Hazard Mater 333:308–318. https://doi.org/10.1016/j.jhazmat.2017.03.054
Kumar R, Sakthivel R, Behura R, Mishra BK, Das D (2015) Synthesis of magnetite nanoparticles from mineral waste. J Alloys Compd 645:398–404. https://doi.org/10.1016/j.jallcom.2015.05.089
Lébre É, Corder G (2015) Integrating industrial ecology thinking into the management of mining waste. Resources 4:765–786. https://doi.org/10.3390/resources4040765
Lee H, Purdon AM, Chu V, Westervelt RM (2004) Controlled assembly of magnetic nanoparticles from magnetotactic bacteria using microelectromagnets arrays. Nano Lett 4:995–998. https://doi.org/10.1021/nl049562x
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011:1–16. https://doi.org/10.1155/2011/270974
Limcharoensuk T, Sooksawat N, Sumarnrote A, Awutpet T, Kruatrachue M, Pokethitiyook P, Auesukaree C (2015) Bioaccumulation and biosorption of Cd2+ and Zn2+ by bacteria isolated from a zinc mine in Thailand. Ecotoxicol Environ Saf 122:322–330. https://doi.org/10.1016/j.ecoenv.2015.08.013
Maass D, de Medeiros MM, Rovaris BC, Bernardin AM, de Oliveira D, Hotza D (2019) Biomining of iron-containing nanoparticles from coal tailings. Appl Microbiol Biotechnol 103:7231–7240. https://doi.org/10.1007/s00253-019-10001-2
Mansoori GA (2017) An introduction to nanoscience and nanotechnology. In: Ghorbanpour M, Manika K, Varma A (eds) Nanoscience and plant–soil systems. Springer, Cham, pp 3–20
Moreno L, Ordóñez JI, Cisternas LA (2017) Uso de salmueras de osmosis inversa para el procesamiento de minerales no metálicos. In: Cisternas LA, Moreno L (eds) Agua de mar Atacama: Oportunidades y avances para el uso sostenible de agua de mar en minería. RIL Editores, Santiago, Chile, pp 39–59
Moreno L, Ordóñez JI, Cisternas LA (2014) La industria salitrera y los recursos hídricos. In: Cisternas LA, Moreno L (eds) El Agua de Mar en la Minería: Fundamentos y Aplicaciones. RIL Editores, Santiago, Chile
Muñoz JA, Dreisinger DB, Cooper WC, Young SK (2007) Silver-catalyzed bioleaching of low-grade copper ores. Hydrometallurgy 88:3–18. https://doi.org/10.1016/j.hydromet.2007.04.004
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156(1–2):1–13. https://doi.org/10.1016/j.cis.2010.02.001
Ordóñez JI, Wong L (2019) Biosynthesis of copper nanoparticles from mine waste. In: 15th International Symposium on Biomineralization. Munich
Parveen R, Ullah S, Sgarbi R, Tremiliosi-filho G (2019) One-pot ligand-free synthesis of gold nanoparticles : the role of glycerol as reducing-cum-stabilizing agent one-pot ligand-free synthesis of gold nanoparticles: the role of glycerol as reducing-cum-stabilizing agent. Colloids Surfaces A 565:162–171. https://doi.org/10.1016/j.colsurfa.2019.01.005
Ramanathan R, Bhargava SK, Bansal V (2011) Biological Synthesis of Copper/Copper Oxide In: Chemca Conference 466
Rankin WJ (2015) Towards zero waste. AusIMM Bull: 32-37
Rotello V (ed) (2004) Nanoparticles: building blocks for nanotechnology. Springer US, Boston, MA
Rufus A, Sreeju N, Philip D (2019) Size tunable biosynthesis and luminescence quenching of nanostructured hematite (α-Fe2O3) for catalytic degradation of organic pollutants. J Phys Chem Solids 124:221–234. https://doi.org/10.1016/j.jpcs.2018.09.026
Salunke BK, Sawant SS, Lee S-I, Kim BS (2016) Microorganisms as efficient biosystem for the synthesis of metal nanoparticles: current scenario and future possibilities. World J Microbiol Biotechnol 32:88. https://doi.org/10.1007/s11274-016-2044-1
Salvadori MR, Ando RA, Oller Do Nascimento CA, Corrêa B (2014) Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0087968
Sanghi R, Verma P (2009) A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus. Chem Eng J 155:886–891. https://doi.org/10.1016/j.cej.2009.08.006
Schlesinger M, King M, Sole K, Davenport W (2011) Extractive metallurgy of copper. Fifth, Elsevier Ltd, Oxford, UK
Sernageomin (2016) Catastro de depósitos de relaves en Chile. Santiago, Chile
Singh A (2015) Biological synthesis of copper oxide nano particles using Escherichia coli. 365–369
Singh M, Manikandan S, Kumaraguru AK (2010) Nanoparticles: a new technology with wide applications. Res J Nanosci Nanotechnol:1–11
Singh S, Sukla LB, Mishra BK (2011) Extraction of copper from Malanjkhand low-grade ore by Bacillus stearothermophilus. Indian J Microbiol 51:477–481. https://doi.org/10.1007/s12088-011-0073-x
Starke L (2002) Breaking new ground: mining, minerals and sustainable development. The report of the MMSD project. Earthscan Publications Ltd, London
Su SS, Chang I (2018) Review of production routes of nanomaterials. In: Pellicer E, Zivic F, Sort J, Dolors Baró M, Grujovic N, Choy K-L (eds) Brabazon D. Springer International Publishing, Commercialization of nanotechnologies-a case study approach, pp 15–29
Tiquia-Arashiro S, Rodrigues DF (2016) Extremophiles: applications in nanotechnology. Springer International Publishing, Cham
Torres CM, Taboada ME, Graber TA, Herreros OO, Ghorbani Y, Watling HR (2015) The effect of seawater based media on copper dissolution from low-grade copper ore. Miner Eng 71:139–145. https://doi.org/10.1016/j.mineng.2014.11.008
US Geological Survey (USGS) (2018) Mineral Commodity Summaries 2019. 1–204
Vena MP, Jobbágy M, Bilmes SA (2016) Microorganism mediated biosynthesis of metal chalcogenides; a powerful tool to transform toxic effluents into functional nanomaterials. Sci Total Environ 565:804–810. https://doi.org/10.1016/j.scitotenv.2016.04.019
Virkutyte J, Varma R (2013) Sustainable preparation of metal nanoparticles: methods and applications. In: Luque R, Varma RS (eds) Sustainable preparation of metal nanoparticles: methods and applications. RSC Publishing, pp 7–33
Vitor G, Palma TC, Vieira B, Lourenço JP, Barros RJ, Costa MC (2015) Start-up, adjustment and long-term performance of a two-stage bioremediation process, treating real acid mine drainage, coupled with biosynthesis of ZnS nanoparticles and ZnS/TiO2 nanocomposites. Miner Eng 75:85–93. https://doi.org/10.1016/j.mineng.2014.12.003
Vitor G, Palma TC, Vieira B, Lourenço JP, Barros RJ, Costa MC (2016) Corrigendum to “Start-up, adjustment and long-term performance of a two-stage bioremediation process, treating real acid mine drainage, coupled with biosynthesis of ZnS nanoparticles and ZnS/TiO2 nanocomposites” [Miner. Eng. 75 (2015) 85–93]. Miner Eng 89:18. https://doi.org/10.1016/j.mineng.2015.12.016
Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA (2018) Biosynthesis of gold and selenium nanoparticles by purified protein from Acinetobacter sp. SW 30. Enzym Microb Technol 111:81–86. https://doi.org/10.1016/j.enzmictec.2017.10.007
Wang J, Zhao J, Ma G (2019) Extremely concentrated silver nanoparticles stabilized in aqueous solution by bovine serum albumin (BSA). Nano-Structures & Nano-Objects 19:100349. https://doi.org/10.1016/j.nanoso.2019.100349
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides-a review. Hydrometallurgy 84:81–108. https://doi.org/10.1016/j.hydromet.2006.05.001
Whitesides GM (2003) The “right” size in nanobiotechnology. Nat Biotechnol 21:1161–1165. https://doi.org/10.1038/nbt872
Wong L, Chong G, Ordóñez JI (2019) Biosynthesis of copper nanoparticles from leaching solutions obtained from mining tailings as raw material. International Conference on Functional Nanomaterials and Nanodevices. Prague, In
Xie Y, Xu Y, Yan L, Yang R (2005) Recovery of nickel, copper and cobalt from low-grade Ni–Cu sulfide tailings. Hydrometallurgy 80:54–58. https://doi.org/10.1016/j.hydromet.2005.07.005
Xie Y, Xiong W, Yu J, Tang JY, Chi R (2018) Recovery of copper from metallurgical sludge by combined method of acid leaching and biosorption. Process Saf Environ Prot 116:340–346. https://doi.org/10.1016/j.psep.2018.02.017
Yasipourtehrani S, Strezov V, Evans T, Anawar HM (2020) Pyrometallurgical process for recycling valuable materials and waste management:valorisation applications of blast furnace slags. In: Anawar HM, Strezov V, Abhilash (eds) Sustainable and Economic Waste Management: Resource Recovery Techniques. New York, p 315
Yin SH, Wu AX, Qiu GZ (2008) Bioleaching of low-grade copper sulphides. Trans Nonferrous Met Soc China (English Ed 18:707–713. https://doi.org/10.1016/S1003-6326(08)60122-3
Yin S, Wang L, Kabwe E, Chen X, Yan R, An K, Zhang L, Wu A (2018) Copper bioleaching in China: review and prospect. Minerals 8. https://doi.org/10.3390/min8020032
Funding
This study was funded by CONICYT (PhD scholarship 21190433, FONDECYT 11170616 and FONDEF IT17M10002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong-Pinto, Ls., Menzies, A. & Ordóñez, J.I. Bionanomining: biotechnological synthesis of metal nanoparticles from mining waste—opportunity for sustainable management of mining environmental liabilities. Appl Microbiol Biotechnol 104, 1859–1869 (2020). https://doi.org/10.1007/s00253-020-10353-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10353-0