Abstract
This study aimed to investigate the occurrence and age-related changes of the first night effect (FNE) in school-age children using both macro (sleep architecture) and micro (frequency analysis) structures to polysomnography (PSG) data. PSG data from two consecutive nights were obtained from 38 healthy children aged 6–15 years. Sleep variables and power spectral analysis were compared between the two nights. The relationship between age and the difference in sleep variables and power values between the two nights was examined using correlation analysis. The first night showed significant reductions in total sleep time, sleep efficiency, N1, N2, and REM sleep, as well as significant increases in sleep onset latency and wake after sleep onset. The decrease in N3 and the increase in N2 due to FNE were positively and negatively correlated with age, respectively. Spectral analysis showed no effect of FNE for most variables, but there was a trend toward an increase in the convergence value of the δ band with age. FNE occurs in school-age children, and its manifestation changes with age. The decrease in N3 and increase in N2 become more pronounced with age, while the enhancement of low-frequency power is consistent across ages. These findings highlight the importance of considering age and specific sleep indicators when interpreting pediatric PSG results and underscore the need for a multi-level approach to understanding sleep changes across development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gold standard for objective evaluation of human sleep is overnight polysomnography (PSG). PSG provides detailed information related to sleep/wake states by measuring electroencephalography (EEG), chin electromyography (EMG), and electrooculography (EOG). However, it requires sleeping in a special environment, such as wearing unfamiliar electrodes, in a laboratory setting with bedding that is not one's own. This may result in a deterioration of sleep quality on the first night of PSG, which is called the First Night Effect (FNE) [1]. FNE is characterized by prolonged sleep onset latency, increased wake after sleep onset, reduced total sleep time, increased light sleep (Stage N1), and decreased REM sleep [1]. To avoid FNE, it is generally required to set an adaptation night when conducting PSG in a laboratory setting.
A meta-analysis targeting all age groups suggested that being a child increases the risk of FNE [2]. The high risk of FNE in children may be related to sleep changes associated with brain development during childhood. Cortical maturation and synaptic pruning are phenomena that are prominently observed during adolescence [3] and are known to affect sleep changes during this period. For example, slow-wave power is high in infancy but decreases during puberty. In addition, the peak frequency of sigma waves increases throughout adolescence [4]. However, most studies on FNE in children have focused on disease-specific characteristics in children with sleep disorders such as sleep apnea syndrome [5,6,7] or evaluation using home PSG [8,9,10]. The characteristics of FNE in healthy children and age-related changes remain largely unknown.
In addition to evaluating sleep architecture based on visual scoring, there is also the approach of frequency analysis targeting EEG. Sleep architecture captures the overall proportion and changes of sleep stages, but the temporal resolution is constrained by the visual scoring time window (e.g., 30 s). On the other hand, frequency analysis can evaluate changes in EEG frequencies in more detail with finer temporal resolution, although it is difficult to detect qualitative characteristics of sleep. Combining these approaches is expected to enable a more multifaceted understanding of FNE. Indeed, several studies have elucidated the association between FNE and EEG frequencies. Tamaki et al. showed that FNE suppressed slow wave activity in visual areas [11]. Mayeli et al. found lower slow wave activity and higher sigma/beta activity depending on the brain area using high-density EEG (hd-EEG) [12]. However, frequency analysis has not been applied to FNE studies in children.
The purpose of this study was to examine the occurrence of FNE in healthy school-aged children, including the onset of puberty, using both macro (sleep architecture) and micro (frequency analysis) structures of PSG data from two nights in a laboratory setting.
Methods
Participants
This study used PSG data from 38 children (F16, M22, mean 10.5 ± 2.6 years, age range 6–15 years) obtained in a previous study. Those with serious mental or physical illnesses, sleep disorders, or who had traveled across time zones within 6 months prior to the experiment were excluded at the enrollment. Written informed consent was obtained from the guardian of each child, and informed assent was obtained from each child. Opt-out was conducted for secondary use of the data. This study was approved by the Ethics Committee of the National Center of Neurology and Psychiatry (NCNP, A2023-016).
Procedures
The data used were PSG data from two nights (420 min each) conducted in a laboratory with cross-over design. Each child came to the laboratory twice for the study. Each child arrived approximately 5 h before their habitual bedtime, and explanations, meals, showers, and sensor attachment were performed. PSG of more than 420 min per night was measured in the laboratory unit and the first 420 min data was used for analyses. Lights Out was set based on each child’ s habitual bedtime in principle, but varied slightly for each night depending on the original experimental purpose. The unit was maintained at 25 ℃ and 50% relative humidity (RH).
Measurements
PSG recordings were made using the Neurofax digital EEG system (EEG-1200, Nihon Kohden, Tokyo, Japan), which included an EEG with a conventional montage (F3, F4, C3, C4, O1, O2) based on the contralateral mastoid (M1, M2), an EOG at the outer canthus of each eye, a chin EMG, and an electrocardiogram (ECG). Upon recording, the EEG, EOG, EMG, and ECG signals were digitized at 200 Hz; the signal was filtered using a high-pass filter with the following time constants: EEG 0.3 s, EOG 0.03 s, chin EMG 0.03 s, and ECG 1.0 s. The signal was filtered using a low-pass filter as follows: EEG 60 Hz, EOG 60 Hz, submental EMG 60 Hz, and ECG 60 Hz. The sleep stage (Stage N1, Stage N2, Stage N3, Stage R, or Stage W) was determined every 30 s according to the American Academy of Sleep Medicine (AASM) Manual for the scoring of sleep and associated events [13].
Data analyses
Total recording time (TRT), total sleep time (TST), sleep efficiency (SE), sleep latency (SL), time spent in each sleep stage, and percentage of each sleep stage in TST were calculated as sleep variables. These sleep variables were compared between Day1 and Day2 using a one-way analysis of covariance, adjusting for the differences in Lights Out.
To examine the association between age and FNE, the difference between the two days for each sleep variable (Δ sleep variable) was calculated, and the correlation with age was evaluated for each. Pearson's product-moment correlation coefficient (r) was calculated for the correlation analysis.
For frequency analysis, power values were calculated for each band of δ (0.5–4 Hz), θ (4–7 Hz), α (8–13 Hz), β (13–30 Hz), and σ (11–16 Hz). Multitaper spectral analysis [14] was performed for the C3 channel. The analysis was performed with a window interval of 5 s and a 2 Hz step. Outlier removal was performed using the Inner Quartile Range for the calculated power values for each band, followed by z-score processing. The z-scored power values were averaged for each NREM cycle [15]. The 6 NREM cycle was only observed on one night (1.3%) out of a total of 76 nights and was, therefore, excluded from the analysis. The averaged power values for each NREM cycle were compared using linear mixed models with fixed factors (CYCLE, DAY) and random intercept (ID).
Furthermore, the z-scored δ power values calculated for NREM epochs were averaged every 10 min and fitted to the following exponential decay curve [16, 17]:
Here, SSO represents the initial value of z-scored δ power at sleep onset, LA is the convergence value of the exponential decay curve, and τd is the time constant of the exponential decay curve. SSO, LA, and τd were compared between Day1 and Day2 using a one-way analysis of variance.
All results are expressed as means ± SD. All analyses were conducted using IBM SPSS v.26 and R 4.3.1 (R Core Team) with the following R packages: anomalize v.0.3.0 (Dancho and Vaughan 2023), emmeans v.1.8.4–90,004 (Lenth 2023), lme4 v.1.1–31 (Bates et al. 2015), lmerTest v.3.1–3(Kuznetsova et al. 2017), nls2 v.0.3.3 (Grothendieck 2022) and performance v. 0.11.0 (Lüdecke 2021). To conduct multitaper spectral analysis, we utilized an R code provided by Prerau et al. (https://github.com/preraulab/multitaper_toolbox). Statistical significance was set to 5%.
Results
FNE on sleep variables
From the two-night PSG data, all 38 participants experienced some form of sleep deterioration due to FNE. Compared to Day 2, the sleep structure on Day 1 showed a significant reduction in TST (Day1: 365.01 ± 43.49, Day2: 391.34 ± 7.06, p = 0.01) and SE (Day1: 87.18 ± 10.53, Day2: 94.72 ± 1.71, p < 0.001), and a significant prolongation of SL (Day1: 12.82 ± 8.77, Day2: 6.46 ± 1.42, p < 0.001). Regarding the amount of sleep stages, Stage W significantly increased (Day1: 38.72 ± 35.54, Day2: 21.43 ± 5.76, p = 0.011) while Stage R and N1 significantly decreased (Stage R Day1: 56.83 ± 19.04, Day2: 71.29 ± 3.09, p < 0.001; Stage N1 Day1: 19.75 ± 9.07, Day2: 25.32 ± 1.47, p = 0.039). Stage N2 showed a trend toward significant decrease (Day1: 161.22 ± 32.27, Day2: 174.24 ± 5.23, p = 0.056). On the other hand, there was no significant difference in Stage N3 between the two nights (p = 0.28) (Table 1).
Relationship between Δ sleep variables and age
When examining the correlation between the two-night difference for each sleep variable (Δ sleep variable) and age, the Δ amount of Stage N3 showed a significant positive correlation with age (r = 0.53, p = 0.001), indicating that the decrease in the Δ amount of Stage N3 due to FNE increased with age in children. On the other hand, the Δ relative amount (%TST) of Stage N2 showed a significant negative correlation with age (r = –0.54, p < 0.001), suggesting that the decrease in Stage N2 due to FNE was more pronounced at younger ages (Fig. 1). For the other Δsleep variables, no significant correlations were found with age (|r|< 0.35, ps > 0.05).
FNE-induced changes in power spectrum
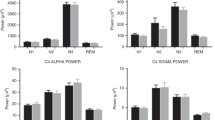
The linear mixed models were employed to explore the impact of DAY and CYCLE on the spectral power within different EEG frequency bands (δ, θ, α, β, and σ) during sleep. The models revealed a robust explanatory power for δ, and θ bands, with conditional R2 of 0.586, and 0.547, respectively, indicating a significant portion of variance explained by both fixed and random effects. The marginal R2 (δ: 0.570, θ: 0.539) suggests that the fixed effects alone also substantially account for the observed variability. In contrast, the α band exhibited lower R2 (conditional: 0.260, marginal: 0.255), and models for the β and σ bands encountered issues with singularity, resulting in incomputable conditional R2 and markedly low marginal R2 (β: 0.0219; σ: 0.0248). Therefore, only δ and θ are reported hereafter. No significant main effect of DAY was detected across the δ, or θ bands, with p-values of 0.489 and 0.351, respectively, indicating that the NREM cycle predominantly influences these measures. Similarly, interactions between DAY and CYCLE were not significant (p-values: 0.670 and 0.481), underscoring the primary role of the NREM cycle (Fig. 2).
The correlation analysis between age and the difference in each band power value for each NREM cycle (Δδ, Δθ, Δα, Δβ, and Δσ) showed that the FNE-induced changes in each band were not significantly correlated with age (|r|< 0.40, ps > 0.15).
Non-linear regression analysis
SSO and τd derived from fitting the exponential decay curve to the z-scored δ power values in NREM epochs showed no significant influence of FNE, but LA tended to be higher on the first night (F (1,56) = 3.297, p = 0.075) (Table 2, Fig. 3).
The correlation between age and ΔSSO, and Δτd was not significant for any of the parameters. However, ΔLA showed a trend toward a negative correlation, suggesting that the δ band tended to be maintained at a higher level with increasing age (r = -0.38, p = 0.089, Fig. 4).
Discussion
This study investigated the occurrence and age-related changes of FNE in healthy school-aged children aged 6–15 years, using both macro (sleep architecture) and micro (frequency analysis) structures. The sleep macrostructure showed that FNE caused a significant reduction in TST, SE, Stage N1, N2, and R, and a significant increase in sleep onset latency and Stage W on the first night. These results are consistent with previous findings indicating that FNE occurs in children [7].
Regarding the age-related changes in sleep macrostructure, this study found a positive correlation between the two-night difference in Stage N3 and age, suggesting that the decrease in Stage N3 due to FNE becomes more pronounced with age. Stage N3 is a sleep stage dominated by slow waves closely related to the development of the central nervous system network and is more prominent in younger children [18 19]and is known to gradually decrease through adolescence [3]. Therefore, Stage N3 in younger children may be robust against FNE, while in older children, it may be more susceptible to the effects of FNE. Additionally, the proportion of Stage N2 (%TST) showed a negative correlation with age. Stage N2 is known to increase through puberty [4], suggesting that it may be more robust against FNE at older ages. These results differ from a previous meta-analysis targeting individuals aged 10 years and older [2]. This meta-analysis reported that the impact of FNE weakens between the ages of 10 and 20, and then increases again with age. This discrepancy may be due to the inclusion of younger children in this study and the comprehensive evaluation of multiple indicators. The relationship between FNE and age may differ depending on the sleep component.
The frequency analysis (multitaper frequency analysis and non-linear regression) targeting the δ, θ, α, β, and σ bands showed no occurrence of FNE in any bands. Also, SSO and τd did not be associated with FNE, but LA, the convergence value of the δ band, had trend toward significance, resulting in the δ band being maintained at a relatively higher level due to FNE. It was assumed that the δ band activity was prolonged until the latter half of sleep, indicating that the sleep homeostasis mechanism was disrupted due to the FNE. Similar changes are found in the insomnia patients [20] and critically ill children [21]. This result is inconsistent with sleep variables that showed no association between FNE and the amount of Stage N3. This discrepancy might occur due to the difference in detecting power for δ band between the two approaches. With the visual inspection, Stage N3 could only be calculated when slow waves predominantly occur in the epoch, while frequency analysis could detect more subtle changes in δ band even in the less dominate epoch. On the other hand, age-related changes in ΔLA tended to be higher with increasing age due to FNE. This is in accordance with the macrostructure of sleep variables.
Although this study provides valuable insights into the age-related changes of FNE in healthy school-aged children, it has several limitations. First, while Lights Out was set based on each child's habitual bedtime in principle, it varied slightly between participants due to differences in the original study protocols. Although the impact of this was adjusted using analysis of covariance, it may not have been fully accounted for. Similarly, the 420-min TRT due to the original study protocols is shorter than the recommended sleep duration for children and may not capture the lower quality of the last periods of sleep, leading to an underestimation of FNE. Second, the age range of the children in this study was limited to 6–15 years, including only elementary and junior high school students. To further investigate the relationship between FNE and brain development, studies targeting a wider age range, including infants and preschool children, are needed. Third, the relatively small sample size may have limited the power to detect differences between age groups, especially in the spectral analysis. Despite these limitations, the results of this study provide important insights into the impact and age-related changes of FNE in healthy school-aged children's sleep. The results highlight the need to consider age and specific sleep variables when interpreting pediatric PSG studies. For example, researchers and clinicians should be aware that the decrease in Stage N3 due to FNE may be more pronounced in older children and adolescents.
In conclusion, this study demonstrates that FNE occurs in healthy school-aged children and that there are age-related changes in its manifestation. Macro-level sleep structure analysis revealed that the decrease in Stage N3 and increase in Stage N2 due to FNE become more pronounced with age. Furthermore, micro-level spectral analysis showed a trend toward an increase in the convergence value of δ band with age. These findings highlight the importance of considering age and specific sleep indicators when interpreting pediatric PSG results. By deepening our understanding of FNE in the pediatric population, we can improve the accuracy and clinical utility of sleep research, ultimately leading to better diagnosis and treatment of sleep disorders in children.
References
Agnew HW Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–6. https://doi.org/10.1111/j.1469-8986.1966.tb02650.x.
Ding L, Chen B, Dai Y, et al. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022;89:159–65. https://doi.org/10.1016/j.sleep.2021.12.007.
Feinberg I, Campbell IG. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. https://doi.org/10.1016/j.bandc.2009.09.008.
Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33(6):801–9.
Busby K, Firestone P, Pivik RT. Sleep patterns in hyperkinetic and normal children. Sleep. 1981;4(4):366–83. https://doi.org/10.1093/sleep/4.4.366.
Li AM, Chan DFY, Fok TF, et al. Childhood obstructive sleep apnoea: an update. Hong Kong Med J. 2004;10(6):406–13.
Verhulst SL, Schrauwen N, De Backer WA, et al. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child. 2006;91(3):233–7. https://doi.org/10.1136/adc.2005.085365.
Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11(3):273–6. https://doi.org/10.1093/sleep/11.3.273.
Palm L, Persson E, Elmqvist D, et al. Sleep and wakefulness in normal preadolescent children. Sleep. 1989;12(4):299–308. https://doi.org/10.1093/sleep/12.4.299.
Pedersen MJ, Leonthin H, Mahler B, et al. Two nights of home polysomnography in healthy 7–14-year-old children – Feasibility and intraindividual variability. Sleep Med. 2023;101:87–92. https://doi.org/10.1016/j.sleep.2022.10.027.
Tamaki M, Bang JW, Watanabe T, et al. The first-night effect suppresses the strength of slow-wave activity originating in the visual areas during sleep. Vision Res. 2014;99:154–61. https://doi.org/10.1016/j.visres.2013.10.023.
Mayeli A, Janssen SA, Sharma K, et al. Examining first night effect on sleep parameters with hd-EEG in healthy individuals. Brain Sci. 2022. https://doi.org/10.3390/brainsci12020233.
Berry RB, Albertario C, Harding S, et al 2018 for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications, Version 2.5. Darien, IL
Prerau MJ, Brown RE, Bianchi MT, et al. Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology. 2017;32(1):60–92. https://doi.org/10.1152/physiol.00062.2015.
Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16(3):283–91. https://doi.org/10.1111/j.1469-8986.1979.tb02991.x.
Robillard R, Massicotte-Marquez J, Kawinska A, et al. Topography of homeostatic sleep pressure dissipation across the night in young and middle-aged men and women: sleep in the middle-aged. J Sleep Res. 2010;19(3):455–65. https://doi.org/10.1111/j.1365-2869.2010.00820.x.
Rusterholz T, Dürr R, Achermann P. Inter-individual differences in the dynamics of sleep homeostasis. Sleep. 2010;33(4):491–8. https://doi.org/10.1093/sleep/33.4.491.
Kurth S, Jenni OG, Riedner BA, et al. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–80.
Buchmann A, Ringli M, Kurth S, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21(3):607–15. https://doi.org/10.1093/cercor/bhq129.
Lunsford-Avery JR, Bidopia T, Jackson L, et al. Behavioral treatment of insomnia and sleep disturbances in school-aged children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30(1):101–16. https://doi.org/10.1016/j.chc.2020.08.006.
Kudchadkar SR, Yaster M, Punjabi AN, et al. Temporal characteristics of the sleep EEG power spectrum in critically ill children. J Clin Sleep Med. 2015;11(12):1449–54. https://doi.org/10.5664/jcsm.5286.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enomoto, M., Eto, T. & Kitamura, S. Investigation of macro and micro sleep structures of first night effect in school-aged children. Sleep Biol. Rhythms 22, 523–529 (2024). https://doi.org/10.1007/s41105-024-00542-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-024-00542-z