Abstract
Geopolymers are emerging construction materials with lower carbon dioxide emissions compared to the conventional cementitious materials. In this paper, an experimental investigation on the strength, durability and microstructural characteristics of GC blended with fly ash (FA), ground granulated blast furnace slag (GGBFS) and sodium-based alkaline solution. It was observed that the addition of 40% GGBFS in FA-based GC increases the 91-day compressive strength by 37% compared to control mix (cement-based concrete). The durability studies are revealed that the addition of FA with GGBFS has shown great impact in the decrement of pore structure. The microstructure investigation of the GC using SEM, and XRD showed the generation of steady and uniform geopolymeric reaction. The SEM images showed a closely packed and dense geopolymer matrix, which explains the reason behind the strength attainment of FA-GGBFS based GC samples. This was confirmed experimentally by the detection of the Ca-rich C-A-S-H gel in the interfacial transition zone. The experimental results showed that the inclusion of GGBFS with FA (Class F) helped achieve enhanced compressive strength to those of ordinary Portland cement. It was concluded that the fly ash and GGBFS combination did tend to form successful stable geopolymer concrete with high mechanical durability properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Geopolymer technology has shows potential move towards the sustainable use of industrial wastes into cement-less construction materials. The geopolymers are produced by the reaction of an dissolved alkaline solutions (sodium/potassium based) with a alumino-silicate rich industrial waste materials [1, 2]. The utilization of industrial wastes such as fly ash (FA), slag and rice husk ash are rich in alumino-silicate have been identified constructive value in the making of geopolymer concrete (GC) which has become a substitute for ordinary Portland cement (OPC) concrete with its superior mechanical properties, greater bond strength, higher durability and denser matrix [3, 4]. However, GC has been considered as a low carbon footprint material compared to ordinary Portland cement (OPC) concrete [5]. Among all the industrial wastes, FA (class-F) and ground granulated blast furnace slag (GGBFS) have a greater impact in the production of geopolymers because these combinations are mainly reported by various researchers. Annually the production of these industrial waste materials are increasing abundantly as well as the comparatively steady geopolymeric reactions were observed [6, 7]. The geopolymeric reaction is a complex matrix that the researchers are still annoying to recognize the formation of geopolymer compounds. However, it is essential to understand that published reports has showed that the strength performance of GC is subjective by the chemical composition of source materials, curing type, alkaline liquid ratios and the molarity of NaOH [8,9,10]. The existing literature on the FA-GGBFS based GC has concentrated on the mechanical, bond strength, durability, and microstructural characteristics [10,11,12,13].
Bellum et al. [14] reported on the strength characteristics of GC on addition of GGBFS. In the production of GC, five dissimilar percentages of FA was replaced with GGBFS. It was found that, higher the amount of GGBFS in the geopolymer concrete, higher the strength was observed. However, addition of higher percentages of GGBFS based GC mix attained 90% of compressive strength under seven days of open atmospheric curing. In contrast, 20% of strength loss was noticed when the GC samples were exposed to 500 °C of elevated temperature.
Hardjito et al. [15] and Venu and Gunneswara Rao [16] researched on the replacement of GGBFS in the development of strength and durability properties of FA-based GC. The authors are claimed that the presence of higher quantities of CaO is helpful in the development of C-A-S-H gel and other geopolymer products, particularly in the early ages. However, the additional properties that influenced the appropriateness of high calcium FA to be a GGBFS for the development of GC are the particle size, amorphous content, as well as morphology and the origin of fly ash and GGBFS.
On the other hand, most of the researchers reported that the presence of higher calcium content in the FA is more suitable for geopolymers as compared to lower calcium FA [17, 18]. The formation of C-A-S-H gel is more predominant in the high calcium based geopolymers. The greater quantities calcium in the FA-based GC is lead to formation of additional geopolymeric products which will provide better mechanical properties. Besides, it has been reported that, for GC containing higher calcium, the calcium enters into the geopolymer matrix, relatively develops an charge-balancing cation [19, 20]. In recent times, Karthik et al. [21] reported the influence of GGBFS on the strength and microstructural performance of FA-based GC samples under different environmental conditions. The experimental results are revealed that the addition of GGBFS has great impact on the strength characteristics of FA-based GC. However, the FA-GGBFS based GC samples have showed better morphology in the formation of geopolymeric end products without any oven curing.
Karthik et al. [21] studied the strength behavior and hydration products of fly ash/slag pastes and found that the compressive strength reached more than 50 MPa at 28 days by the mix having a high fly ash/slag ratio of 1.0, activated with 10 M NaOH solution and cured at the temperature of 25 °C. Bellum et al. [14] concluded that the early-age strength of fly ash/slag mixtures activated by NaOH and sodium silicate can be increased significantly by adding a small amount of hydrated lime. In regards to the durability properties, slag blended fly ash geopolymer showed good resistance to permeation [22], elevated temperature and fire [23] and sodium sulfate attack [24], but suffered deterioration in magnesium sulfate attack and showed increased shrinkage [25]. The rate of deterioration was reported to decrease with the increase of slag in the fly ash-slag blend.
The main objective of this paper is to investigate the mechanical, durability and Microstructural properties of FA-GGBFS based GC samples under ambient curing. The most favorable combination of FA and GGBFS was established based on the strength properties found from the GC mixes with various replacements of FA with GGBFS. The mechanical properties are like compressive, splitting tensile and flexural strengths were tested for 7, 14, 28, 60 and 91 days of ambient curing and compared with OPC (controlled mix). On the other hand, the durability studies are conducted on FA-GGBFS based GC samples such as porosity, water absorption, sorptivity, rapid chloride permeability test (RCPT) and acceleratory corrosion test (ACT). To analyze the micro-level reactions the SEM and XRD studies were also conducted on GC samples after 28 days of curing period.
2 Materials
In this study, locally available industrial by-products such as FA (class-F) and GGBFS are used as binders according to ASTM C 618-19 [26], ASTM C 989-2018 [27]. Fly ash is obtained from NTPC located at Vijayawada, while the GGBFS is purchased from JSW cements limited located at Guntur, Andhra Pradesh, India. Table 1 presents the chemical composition of FA and GGBFS. The specific gravity of FA and GGBFS are 2.30 and 2.81, while fineness modulus values are 478, 670 m2/kg, respectively. The sodium-based chemicals such as sodium silicate (Na2SiO3) and sodium hydroxide (NaOH) are used to prepare alkaline solution; there are obtained from Kiran Global solutions limited, Hyderabad, India. The Na2SiO3 solution contained 27.8% SiO2, 8% Na2O and 64.5% water, while more than 98% purity of NaOH pellets was used. The 8 M NaOH solution is used for all the geopolymer mixes. The sodium silicate to sodium hydroxide ratio was considered as 2.5:1, this ratio is considered as a constant for all the geopolymer mixes because of better results observed in previous literature [4, 11]. However, an alkaline solution to binder ratio (S/B ratio) was taken as 0.4 for all geopolymer mixes. Nearby available sand and compressed granite particles were utilized as aggregates in the production of GC.
The SEM analysis was conducted to identify the micro-tracts in raw FA and GGBFS. Figure 1 depicts that the FA particles are in spherical shape while the GGBFS particles are irregular in shape [28]. Figure 2 shows the XRD patterns of raw FA and GGBFS. The amorphous peaks like quartz and mullite phases are detected in FA. The major peaks quartz and mullite were identified at a 2-theta angle of 26.72° (2θ ≈ 26/72°, d ≈ 3.34 [Å]) and 16.87° (2θ ≈ 16/87°, d ≈ 5.72 [Å]), respectively. Similarly, the major peaks detected in GGBFS are calcite and quartz at a 2-theta angle of 27.09° (2θ ≈ 27/09°, d ≈ 4.91 [Å]) and 21.24° (2θ ≈ 21/24°, d ≈ 5.72 [Å]).
3 Mix proportions
The FA-GGBFS based GC mixes were prepared with respect to the previous literature [20, 21, 28]. Table 2 presents the mix proportions of materials used in the production of geopolymer concrete. The mix M1 (OPC) represents the control mix prepared with cement. The mixes M2–M6 are prepared with 8 M NaOH solution while the mixes M7–M11 are prepared with 10 M NaOH solution (Fig. 3).
4 Methods
The Aimil-AIM-246 slump cone was used to determine the workability of fresh GC according to ASTM C143-15 [29]. The mechanical properties such as compressive, splitting tensile and flexural strength tests (150 mm cube, 150 × 300 mm cylinder and 100 × 100 × 500 mm prism moulds are used respectively) are performed as per BS EN 12390-3-09 [30], ASTM C496/C496 M-17 [31] and ASTM C78/C78M-18 [32], respectively. After testing of GC samples the broken pieces are used to find the microstructural properties such as SEM, EDS and XRD. All the GC samples strength properties were tested after 7, 14, 28, 60 and 91 days of open air curing in the laboratory at a relative humidity of 52–58%.
ARF-2568 RCPT equipment was used to determine the rapid chloride ion penetration into GC samples according to ASTM C1202-07 [33]. The size of the cylindrical mould used for this test is 100 × 50 mm disk. The RCPT test was performed by placing GC sample in a cell enclosed by 0.3 N NaOH solution (one side) and 3% of NaCl solution (another side). The following Eq. 1 can be used to find the charge passed through each test sample. Figure 4 shows the setup for RCPT test.
where, Q = Charge passed (Coulombs).
I0, I30, I60, …, I330, I360 = current at 0, 30, 60, …, 330, 360 min.
CRF ACT-8-Cell ACT test apparatus was considered to calculate the struggle of GC samples in corrosion test. The test samples were prepared with a cylindrical mould of size 100 × 200 mm. The test samples were placed a chamber contain 3% NaCl solution. After that a constant current was applied to find the NaCl ions penetration into the GC samples up to the failure. The failure of the samples identified through the crack formation on the GC samples as well as the sudden drop in the current passage. Figure 5 presents the ACT test setup to find the corrosion resistance of GC samples.
To estimate the percentage of air and water voids of FA-GGBFS blended GC samples the porosity and water absorption experiments were performed as per ASTM C642-13 [34]. The following Eqs. 2 and 3 were used to find the average porosity and water absorption percentages of the GC samples after 28 days immersion in the water.
where, Wa = saturated sample wt (g), Wd = dry sample wt (g), and Ww = saturated sample wt in water (g).
The sorptivity test was conducted to estimate water absorption of GC samples as per ASTM C 1585-13 [35]. The cumulative water absorption values were used to estimate the sorptivity of the concrete samples. This test was performed by placing the GC samples in water by covering all the surfaces except bottom surface of the sample. The following Eq. 4 can be used to estimate the sorptivity of the GC samples.
where, t = time in minute and i = cumulative water absorption.
5 Research methodology
The flow chart related to methodology followed to carry out this research work is presented in Fig. 6.
6 Result and discussions
6.1 Workability
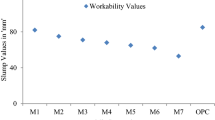
Figure 7 illustrates the workability properties of different molarities of NaOH solution based fresh GC samples. From the slump cone that it was clear that the increase in the GGBFS quantity in the GC mixes the workability was decreased. Compared to GC samples conventional concrete has shown better workability. However, it was noticed that the increase in the molarity of NaOH solution the workability of GC was decreased. The GC mixes prepared with 100% FA has shown good slump values as compared to all other geopolymer mixes. The 8 M NaOH solution based GC mixes F100-8M, F90G10-8M, F80G20-8M, F70G30-8M, and F60G40-8M slump values were 91, 83, 77, 72, and 68 mm respectively. Similarly, the 10 M GC samples F100-10M, F90G10-10M, F80G20-10M, F70G30-10M, and F60G40-10M workability values were 85, 74, 67, 61, and 55 mm respectively. These values are indicating that the increases in the slag quantity the workability of the GC samples were lowered (observed in both 8 and 10 M GC samples). On the other hand, the GC mixes produced with 40% GGBFS and 60% FA has shown lower slump values in both 8 and 10 M samples. The available literature was indicating that the replacement of FA with GGBFS in the GC samples affects the workability in a greater extent because of the finer particles in GGBFS [36,37,38]. While the increase in the molarity leads to effective thickening of the alkaline solution (leads to early hardening of binder) by reducing the water content in the alkaline solution, because of this reason the increase in the molarity of NaOH solution from 8 to 10 the workability values were gradually decreased [39]. However, the presence of 10 M NaOH solution and greater amount of GGBFS based GC samples were shown lower slump values. But, compared to all the GC mixes the OPC concrete mix has shown the good workability.
6.2 Compressive strength
The compressive strength of 8 and 10 M NaOH solution based GC samples under ambient curing for 7, 14, 28, 60 and 91 days were presented in Fig. 8. The experimental data revealed that the increase in the molarity of NaOH solution from 8 to 10 the compressive strength was also increased. It was also observed that the molarity of NaOH solution plays a major role in the early strength attainment. In contrast, it has been found that the enhancement in the GGBFS quantity in the FA-based GC mixes the compressive strength was increased. The increase in the GGBFS was also helpful in the early age strength attainments of the GC samples under ambient curing. The obtained results are compared with the control mix (OPC) it was clear that the presence of GGBFS has shown better impact in the strength of GC. However, as compared the 100% FA based GC samples the OPC concrete has shown little higher compressive strength in both 8 and 10 M samples. In contrast, the strength attainment of pure FA blended GC samples were very low at ambient temperature (25–29 °C). It was also one of the reasons for lower strength attainments of the FA-based GC samples. Nevertheless, the incorporation of GGBFS in the GC mix with a 10 M NaOH solution the strength was enhanced considerably from the early age of 7 days. At 91 days, GC mixes with 8 M NaOH solution having 10, 20, 30 and 40% of GGBFS has attained 19.6, 27.76, 40.17, and 57.23% superior strength as compared to the pure FA-based GC sample (F100-8M) respectively. Similarly, the 91 days strength of GC produced with 10 M NaOH solution having 10, 20, 30 and 40% of GGBFS based samples attained 26.67, 37.12, 52.44, and 64.84% higher as compared to 100% FA based sample (F100-10M) respectively. From these results it was clear that the 91-days compressive strength was enhanced about 5–10 MPa for every 10% augmentation of GGBFS quantity in the FA-based GC mixtures. On the other hand, it can be noted that the strength was gradually increasing after 28 days also, because of the reaction of FA particles in the later ages. From the microstructure of the geopolymer samples it was clear that the most of the FA particles are reacted and achieved maximum strength at the end of 91 days of ambient curing.
6.3 Splitting tensile strength
The influence of GGBFS on indirect tensile characteristics of FA-based GC samples after 7, 14, 28, 60 and 91 days of curing (25–29 °C). Figure 9 illustrates the experimental splitting tensile strength results of different molarities based GC samples. Increasing the molarity of NaOH in the alkaline solution, the splitting tensile strength was improved in both early and later ages. However, not only the molarity of NaOH solution but also the increase in the GGBFS content the splitting tensile strength values are increased. On the other hand, the addition of GGBFS in the FA-based GC samples with a 10 M NaOH solution the strength was improved greatly even in the early ages also i.e. 7 days. At 91 days, GC mixes with 8 M NaOH solution having 10, 20, 30 and 40% of GGBFS has attained 7.76, 22.41, 33.84, and 69.40% superior strength as compared to the pure FA-based GC sample (F100-8M) respectively. Similarly, the 91 days strength of GC produced with 10 M NaOH solution having 10, 20, 30 and 40% of GGBFS blended mixes attained 17.59, 32.57, 54.93, and 78.75% greater splitting tensile strength as compared to 100% FA based sample (F100-10M), respectively. From experimental data it was clear that the 91-days splitting tensile strength was improved about 2–6 MPa for every 10% increase of GGBFS quantity in the FA-based GC samples. However, as compared to control mix (OPC) geopolymer samples attained superior tensile properties in all curing ages with the combination of FA and GGBFS. But the FA-based GC samples were taken more than 48 h to harden and also strength gaining was very low compared to OPC samples as well FA-GGBFS based GC samples.
6.4 Flexural strength
Figure 10 represents the effect of GGBFS on flexural strength of GC after 7, 14, 28, 60 and 91 D of curing. From the experimental results it was observed that the increase in the molarity of NaOH solution the flexural strength was improved in both early and later ages. However, not only the molarity of NaOH solution but also the increase in the GGBFS content the flexural strength values were enhanced. However, the inclusion of GGBFS in the FA-based GC mixes with a 10 M NaOH solution the flexural strength was enhanced significantly even in the early ages also i.e. 7 days. The GC samples with 8 M NaOH solution with 10, 20, 30 and 40% of GGBFS mixes has attained 8.65, 24.71, 35.28, and 70.85% higher strength as compared to the 100% FA-based GC sample (F100-8M) respectively. Similarly, the 91 days strength of GC produced with 10 M NaOH solution having 10, 20, 30 and 40% of GGBFS blended mixes attained 18.72, 33.61, 55.18, and 80.69% greater flexural strength as compared to FA based GC sample (F100-10M), respectively. On the other hand, as compared to OPC mix, geopolymer samples attained superior flexural strength in all curing ages with the combination of FA and GGBFS.
6.5 Water absorption and porosity
Figure 11 shows the water absorption and porosity results of FA-GGBFS based GC samples in order to access the percentage of pores. The experimental results were revealed that the enhancement in the amount of GGBFS from 0 to 40% the water absorption and porosity percentages were reduced considerably. On the other hand, raise in the NaOH solution molarity the water absorption and porosity values were also decreased. It was indication that the increase in the molarity as well as GGBFS in geopolymer samples the pore percentages was decreased. The GC samples with 8 M NaOH solution with 10, 20, 30 and 40% of GGBFS mixes has shown 2.8, 2.5, 2.1 and 1.4% of water absorption, these values are much lesser as compared to the mix F100-8M (3.1%) respectively. However, for 10 M NaOH solution having 10, 20, 30 and 40% of GGBFS blended mixes shown 2.2, 1.8, 1.3, and 1% of water absorption as compared to F100-10M (i.e. 2.7%), respectively. Similarly, the porosity values were decreased with the increase in GGBFS quantity and molarities of NaOH solution. It was understand that the higher NaOH solution molarity based GC samples lower void percentage. Moreover, there was a significantly better correlation between water absorption, porosity and strength characteristics of GC specimens. With the enhancement in the GGBFS amount in the GC samples has shown denser structure that was the major reason for the lower void ratios [40,41,42].
6.6 Sorptivity
Figure 12 presents the sorptivity values of FA-GGBFS based GC samples cured under ambient temperature. The results are indicated that the GC samples prepared with 10 M were shown better sorptivity values compared to 8 M based samples. Moreover, the presence of higher quantities of GGBFS has also shown lower porosity values compared to 100% FA-blended GC samples. This evident reality indicated that GC is good resilient in provisions of water entrée compared to OPC samples. The GC mixes with 8 M NaOH solution with 10, 20, 30 and 40% of GGBFS samples has shown 0.11, 0.09, 0.08 and 0.05 mm/min0.5 of sorptivity, these values are much lesser as compared to the mix F100-8M (0.13 mm/min0.5), respectively. However, for 10 M NaOH solution having 10, 20, 30 and 40% of GGBFS blended mixes shown 0.09, 0.078, 0.05, and 0.03 mm/min0.5 of sorptivity as compared to F100-10M (i.e. 0.11 mm/min0.5), respectively. On the other hand, it has been recognized that the inclusion of slag to FA, improved the characteristics of GC in terms of sorptivity.
6.7 Rapid chloride permeability test (RCPT)
RCPT was performed to identify the chloride ion permeability of GC by the addition of GGBFS from 0 to 40% (increment of 10%). The RCPT was performed according to ASTM C 1202-07 and the standard values are presented in Table 3. Table 4 illustrates the RCPT test results of geopolymer and OPC concrete samples. The results are depicted that the substitute of FA with slag has shown greater impact in the decrement of chloride ion permeability through GC samples. However, higher chloride ion penetration was observed for 100% FA-based GC samples in terms of charge passed i.e. 2876 coulombs for 8 M NaOH solution based samples while it was 2575 for 10 M NaOH solution based samples. This difference indicates that the molarity of NaOH solution was played an important role in the decrement of chloride ion permeability.
On the other hand, the GC samples prepared with 100% FA were indicated reduced resistance aligned with chloride ion entrance. The current accesses into the GC mixes like F100-8M and F100-10M are 2879 and 2573 C, correspondingly. It has been understand that GC produced with the combination of FA and GGBFS exhibited excellent chloride ion battle as related to FA-based mixes.
6.8 Acceleratory corrosion test (ACT)
The charge entered (mA) through the OPC and GC specimens with point in time in number of days are presented in Fig. 13. The experimental data shows that the current passage was study at the starting days, this phenomenon was due to the development of an inert layer by the GC sample, which results in the decrement of corrosion and corrosion initiation time. After some days the sudden increase in the current passage was noticed this indicates the failure of the samples. The sudden raise of charge was found because to the increase in the penetration of ‘Cl’ ions with moment and gives in deactivation. The crack beginning time for the sample F100-8M was observed at the end of 17th day and failure was found after 20 days. Similarly, the crack initiation time for the sample F100-10M was found at the starting of 21st day and failure of the sample was observed after 23 days. The difference in number days taken for the failure of the sample was indicates that the molarity of the NaOH solution plays an important role in the crack initiation due to corrosion. It was found that the increase in the NaOH molarity the crack initiation time was also increased.
On the other hand, substitute of FA with GGBFS in GC mixes has attained enhanced resistance against corrosion attack. The specific surface area of GGBFS is very high compared to FA; this difference has shown the greater impact in the chloride ion penetration in case of FA-GGBFS based GC samples. The GC samples produced with 40% GGBFS and 60% FA with 10 M NaOH solution were shown better resistance to chloride ions and the current passage was low. The GGBFS particles are filled the gapes in between the FA particles and forms a denser structure, this was the major reason for lower chloride ion permeability into GC samples with the combination of FA and GGBFS.
6.9 X-ray diffraction (XRD)
The mechanical changes were considered to analyze the XRD mineral peak identification of geopolymer samples. Figure 14 represents the identification of mineral peaks of GC samples under ambient curing for 28 days. The first major peak was identified in all the GC samples was Quartz, while the second major peak identified in FA-GGBFS based GC samples was Calcite. However, it was noticed that the increase in the addition of GGBFS the calcite peak was very sharp and study. This indicates the increase in the strength characteristics of GC samples under the addition of GGBFS to the FA-based GC samples. It was clear that there was a good correlation found in between the XRD analysis and mechanical properties of GC mixes. Quartz and calcite are recognized at an angle of 2θ = 26.193 with 2.778 [Å] (d-spacing) and 2θ = 29.169 with 2.780 [Å] (d-spacing), respectively. The ‘Al’, ‘Ca’ and ‘Si’ in the FA and GGBFS respond with a geopolymeric liquid and generating an C-A-S-H gel initiations. In the meantime, ‘Si’, ‘Al’, ‘Ca’ was detected in GC samples; these elements are mainly accountable for the enhancement of various properties. On the other hand, the mullite is detected at 2-theta angle of 16 with a 3.395357 [Å] (d-spacing).
The geopolymerization matrix improves with the development in the addition of GGBFS in FA-blended GC. The majority of the sharp crystalline peaks were detected in between 16° and 50° and these are the apparent confirmation of occurrence of strong C-A-S-H gel. Geopolymerization products were clearly identified in the mix M11, but in the 100% FA-based specimens the concentration has dropped due to absence of GGBFS. This difference was the main reason for the enhancement of mechanical properties for the specimen M11 as related to all other mixes of GC. The increase in the molarity of NaOH solution from 8 to 10 M was also one of the reasons for strength enhancement of GC samples.
6.9.1 Scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS)
Figure 15 illustrates the SEM images of geopolymer samples under 28 days of ambient curing. From the SEM images it was clear that the increase in the GGBFS content in the GC mixes the formation of geopolymeric products are increased. This increase in the geopolymeric reactions may lead to the development of additional strength properties to GC with the inclusion of GGBFS with FA. However, the increases in the molarity of NaOH solution lead to the generation of extra C-A-S-H gel. Figure 15d is evident that the formation of C-A-S-H gel is higher as compared to other SEM images of geopolymer samples. The formation of extra C-A-S-H gel was directly involved in the enhancement of mechanical properties of FA-GGBFS based GC samples. On the other hand, most of the FA particles are unreacted at the initial 28 days of ambient curing incase of 100% FA-based GC samples, observed in Fig. 15a, c. Moreover, the increase in the molarity of NaOH solution from 8 to 10 M the unreacted FA particles were decreased (ref. Figure 15c). Because of the unreacted and semi-reacted FA particles observed in case of pure FA-based GC samples the strength attainment was low in the initial days of the curing. Furthermore, in the formation of denser structure towards the enhancement of mechanical properties the GGBFS and molarity of NaOH solution were played an important role. The denser microstructure was observed at a 40% replacement of FA with GGBFS along with 10 M NaOH solution. It was clear that the pores were will by GGBFS particles and make the structure strong and steady in order to formation of better geopolymeric structure [43]. This important role was filled by addition of GGBFS and it’s also leads to enrich the strength at early ages.
7 Conclusions
In the present study, the effect of GGBFS and molarity of NaOH on mechanical, durability and microstructural properties of FA-blended GC was thoroughly examined. The FA and GGBFS were blended at dissimilar proportions (0, 10, 20, 30 and 40%) to manufacture GC. The mechanical properties of FA-GGBFS blended GC was studied along with durability characteristics such as RCPT and ACT. In order to access the micro level properties the XRD and SEM tests were conducted for geopolymer samples. The experimental results on geopolymer concrete samples revealed the following conclusions,
-
The enhancement in the addition of GGBFS, the workability of GC samples was decreased about 12–37%, while the increase in the molarity of NaOH solution was also played an significant role in the decrement of slump value.
-
The mechanical properties of GC is increased with the increase in the replacement of 40% GGBFS. From these results it was clear that the 91-days compressive strength was enhanced about 5–10 MPa for every 10% augmentation of GGBFS quantity in the FA-based GC mixtures.
-
The increase in the durability properties (such as porosity, water absorption, RCPT and ACT) of GC was noticed with the greater GGBFS content with 10 M NaOH solution.
-
The presence of calcite in GGBFS holds the geopolymeric reaction in the pore structure, which is necessary to improve the structure of Ca2+, Si4+, and Al3+ ions these enhance the generation of geopolymer products. This leads in the development of microstructural properties of GC samples at a 10 M NaOH solution with the generation of enhanced C-A-S-H gels.
-
The denser microstructure was observed at a 40% replacement of GGBFS with FA along with 10 M NaOH solution. It was clear that the pores were will by GGBFS particles and make the structure strong and steady in order to formation of better geopolymeric structure.
8 Future recommendations
This research paper spotlights a way forward for further research and development of GC in the construction field by considering four key role points. First, the use of GC will lead to sustainable utilization of industrial by-products, and it can be used as a novel option of cementitious material, which has a lower CO2 emission than conventional concrete. Second, more research is required on the fly ash-GGBFS based geopolymer concrete to produce bulk quantity of GC and to apply it to all ready-mix plants. Third, it minimizes the use of cement-based concretes in all areas of civil engineering applications. Four, from the results, it is recommended to use GGBFS in the fly ash-based GC, in order to achieve better mechanical and durability properties.
References
Shi C, Roy D, Krivenko P (2006) Alkali-activated cements and concretes. CRC Press, Boca Raton
Davidovits J (1991) Geopolymers. J Therm Anal Calorim 37:1633–1656
Provis JL, Bernal SA (2014) Geopolymers and related alkali-activated materials. Annu Rev Mater Res 44:299–327
Bellum RR, Muniraj K, Madduru SRC (2020) Influence of slag on mechanical and durability properties of fly ash-based geopolymer concrete. J Korean Ceram Soc 57(5):530–545. https://doi.org/10.1007/s43207-02000056-7
Habert G, Ouellet-Plamondon C (2016) Recent update on the environmental impact of geopolymers. RILEM Tech Lett 1:17–23
Van Deventer J, Provis J, Duxson P, Lukey G (2007) Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J Hazard Mater 139:506–513
Duxson P, Provis JL, Lukey GC, van Deventer JSJ (2007) The role of inorganic polymer technology in the development of ‘green concrete.’ Cem Concr Res 37:1590–1597
Saloma H, Elysandi DO, Meykan DG (2017) Effect of Na2SiO3/NaOH on mechanical properties and microstructure of geopolymer mortar using fly ash and rice husk ash as precursor. AIP Conf Proc. https://doi.org/10.1063/1.5011552
Hadi MNS, Zhang H, Parkinson S (2019) Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J Build Eng 23:301–313
Bellum RR, Muniraj K, Madduru SRC (2020) Exploration of mechanical and durability characteristics of fly ash-GGBFS based green geopolymer concrete. SN Appl Sci. https://doi.org/10.1007/s42452-020-2720-5
Puertas F, Fernández-Jiménez A (2003) Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem Concr Compos 25:287–292
Lee NK, Lee HK (2015) Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr Build Mater 81:303–312
Puligilla S, Mondal P (2013) Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem Concr Res 43:70–80
Bellum RR, Muniraj K, Madduru SRC (2020) Characteristic evaluation of geopolymer concrete for the development of road network. Sustain Infrastruct Innov Infrastruct Solut 5:91. https://doi.org/10.1016/s41062-020-00344-5
Hardjito D, Rangan BV, Sumajouw DMJ, Wallah SE (2004) On the development of fly ash-based geopolymer concrete. ACI Mater J 101(6):467–472
Venu M, Gunneswara Rao TD (2017) Tie-confinement aspects of fly ash-GGBS based geopolymer concrete short columns. Constr Build Mater 151:28–35
Chindaprasirt P, Chareerat T, Sirivivatnanon V (2007) Workability and strength of coarse high calcium fly ash geopolymer. Cem Concr Compos 29(3):224
Rattanasak U, Pankhet K, Chindaprasirt P (2011) Effect of chemical admixtures on properties of high-calcium fly ash geopolymer. Int J Miner Metall Mater 18(3):364
Somna K, Jaturapitakkul C, Kajitvichyanukul P, Chindaprasirt P (2011) NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 90(6):2118
Bellum RR, Muniraj K, Madduru SRC (2019) Empirical relationships on mechanical properties of class-F fly ash and GGBS based geopolymer concrete. Annales de Chimie Science des Matériaux 43:189–197. https://doi.org/10.18280/ascm430
Karthik A, Sudalaimani K, Vijaya Kumar CT (2017) Investigation on mechanical properties of fly ash-ground granulated blast furnace slag based self-curing bio-geopolymer concrete. Constr Build Mater 149:338–349
Aydin E, Arel HŞ (2017) Characterization of high-volume fly-ash cement pastes for sustainable construction applications. Constr Build Mater 157:96–107
Singh GB, Subramaniam KVL (2019) Production and characterization of low-energy Portland composite cement from post-industrial waste. J Clean Prod 239:118024
E Aydın (2009) Utilization of high volume fly ash cement paste in civil engineering construction sites. In: Fifth international conference on construction in the 21st century (CITC-V), Collaboration and Integration in Engineering, Management and Technology, May 20–22, Istanbul, Turkey, p 1526–1535
Arel HŞ, Aydin E (2018) Use of industrial and agricultural wastes in construction concrete. ACI Mater J 115(1):55–64. https://doi.org/10.14359/51700991
ASTM C 618-17 (2017) Standard specification for Coal FA and raw or calcined natural pozzolan for use in concrete. ASTM International, West Conshohocken, PA
ASTM C989/C989M-18a (2018) Standard specification for slag cement for use in concrete and mortars. ASTM International, West Conshohocken, PA. www.astm.org
Bellum RR, Chava V, Madduru SRC (2021) Influence of red mud on performance enhancement of fly ash based geopolymer concrete. Innov Infrastruct Solut 6:215. https://doi.org/10.1007/s41062-021-00578-x
ASTM C143/C143M (1998) Standard test method for slump of hydraulic-cement concrete. ASTM International, West Conshohocken, PA, USA
BS 12390-3 (2009) Testing hardened concrete, compressive strength of test specimens, BSI, London, UK
ASTM C496/C496M-17. Standard test method for splitting tensile strength of cylindrical concrete specimens. American Society for Testing and Materials
ASTM C78/C78M-18. Standard test method for flexural strength of concrete (using simple beam with third-point loading), American Society for Testing and Materials
ASTM C1202-07 (2007) Standard test method for electrical indication of concrete's ability to resist chloride ion penetration. ASTM International, West Conshohocken, PA
ASTM C642-13: Standard test method for density, absorption, and voids in hardened concrete. American Society for Testing and Materials
ASTM C 1585:2013. Standard test method for measurement of rate of absorption of water by hydraulic-cement concretes.
Nath P, Sarker PK (2014) Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr Build Mater 66:163–171
Deb PS, Nath P, Sarker PK (2014) The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater Des 62:32–39
Hadi MNS, Farhan NA, Sheikh MN (2017) Design of geopolymer concrete with GGBFS at ambient curing condition using Taguchi method. Constr Build Mater 140:424–431
Leong HY, Ong DEL, Sanjayan JG, Nazari A (2016) The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr Build Mater 106:500–511
Astutiningsih S, Liu Y (2005) Geopolymersation of Australian slag with effective dissolution by the alkaline. In: Proceedings of the world congress of geopolymer 2005, St Quentin, p 69
Dutta D, Thokchom S, Ghosh P, Ghosh S (2010) Effect of silica fume additions on porosity of FA geopolymers. J Eng Appl Sci 5(10):74
García Lodeiro I, Fernández-Jimenez A, Palomo A, Macphee DE (2010) Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem Concr Res 40(1):27
Bellum RR, Muniraj K, Madduru SRC (2020) Influence of activator solution on microstructural and mechanical properties of geopolymer concrete. Materialia 10:100659. https://doi.org/10.1016/j.mtla.2020.100659
Acknowledgements
The authors thank AEC (autonomous) for financial support to carry out this study in a systematic approach.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellum, R.R., Al Khazaleh, M., Pilla, R.K. et al. Effect of slag on strength, durability and microstructural characteristics of fly ash-based geopolymer concrete. J Build Rehabil 7, 25 (2022). https://doi.org/10.1007/s41024-022-00163-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41024-022-00163-4