Abstract
This study assesses seasonal fluctuations, spatial distribution and fractions of phosphorus (P) in the surface sediment layer of the southern Caspian Sea. Seasonal fluctuations were insignificant (p-value > 0.05) relative to the mean total P (TP) concentrations. Still, the highest levels were in autumn samples (1555 mg kg−1), followed by winter (1405 mg kg−1), spring (1378 mg kg−1), and summer (1130 mg kg−1). These minor temporal fluctuations in P concentrations are explained by seasonal differences in runoff amount and intensity of rivers discharging into the Caspian sea and thereby their sediment loading and physicochemical characteristics. The large riverine influx has led to TP contamination hotspots in the river deltas of Anzali wetland, Babolrood, and Sefidrud, where high loadings of suspended particles are discharged into the sea. The spatial distribution of TP is thus site-specific and uneven. The main P fraction was calcium-bound P (CaP), reflecting the phosphate (PO43−) strong affinity for, and association with, Ca-bearing minerals. Only a minor fraction of P was determined as loosely bound P (LP). The fraction of the mud size particles was the main explanatory factor for the spatial distribution of overall low levels of non-residual (or bioavailable) P forms (i.e., LP and iron- and aluminum- bound P: FeP and AlP, respectively) during spring and summer, while the sand fraction had strongest explanatory value for the distribution of residual (non-bioavailable) P form (CaP) during autumn and winter. This study demonstrates that P bioavailability in sediments is mainly governed by the physicochemical characteristics of the sediment material, which again is steered by seawater chemistry. A low content of bioavailable P fractions could therefore be explained by the relatively low content of fine-grained (< 63 µm, i.e., mud) particles in sediments of the southern Caspian Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Determining bioavailability of elements, including macronutrients in environmental compartments, is a pivotal step in order to evaluate the ecotoxicological risk (Keshavarzi et al. 2015; Mohammadi et al. 2021; Guarino et al. 2022). Phosphorus (P) is generally a limiting macronutrient for primary production in aquatic ecosystems and thus plays a central role in governing primary productivity and phytoplankton growth. However, eutrophication due to excess P influx to aquatic ecosystems results in algae blooms that change the water chemistry and deteriorate aquatic ecosystems (Wu et al. 2017; Liang et al. 2017).
Phosphorus in the environment exists as phosphate (PO43−) both in inorganic forms and in organic matter. In water, P is mainly bound to the dissolved organic matter and adsorbed to colloids (Frankowski et al. 2002; Aydin et al. 2009; Gottselig et al. 2014; Baken et al. 2016). The average P content of the Earth's crust is 1200 mg kg−1, mainly as apatite (Bramha et al. 2014). The major natural source of phosphate is from weathering of soil minerals. Within watersheds, the weathered phosphate is rapidly assimilated into biomass and tightly recycled through mineralization of soil organic matter. The phosphate that is bound to the dissolved organic matter or adsorbed to eroded colloids is nevertheless leached into watercourses. The transport and flux of P from the watershed are mainly governed by different water flowpaths through the soil. The highest P fluxes occur during saturated soil water conditions, allowing for a prevalence of overland flow short-circuiting the absorptive capacity of deeper soil horizons. In addition, there are substantial anthropogenic sources, such as household and industrial sewage, as well as manure, fertilizers, and pesticides from agricultural practices (Zhao et al. 2019). Especially during storm flow, the sewage systems are frequently flooded by overland flow entering the system from paved surfaces. This causes a discharge of untreated wastewater through sewage overflows. Overland flow leads also to the erosion of agricultural soils, causing an influx of soil particles saturated with P. Phosphorus levels and spatiotemporal distribution in coastal marine ecosystems are therefore strongly affected by climatic conditions (Kundzewicz et al. 2008).
Sediments are a central part of coastal and marine biogeochemical cycles, as they act both as a sink and a secondary source of contaminants (Moore et al. 2015; Nematollahi et al. 2021; Somma et al. 2021). Hence, P is temporarily stored in the seabed sediments and then, pending the form of P stored in the sediments and the contemporary environmental conditions, it can be re-released into the overlying seawater (Cao et al. 2019). The pool of P in sediments thus constitutes an internal P source to the seawater due to fluctuations in physicochemical conditions. Therefore, despite efficient abatement action mitigating external P inputs, the leaching of P from sediments can sustain elevated nutrient levels in the overlying water body for a long time (Zhou et al. 2021). The extent and rate of release from sediment to water are dependent on the relative amount of P fractions as well as the particle size fraction on which P is sorbed (Jensen et al. 1998; Schenau and De Lange 2001; Aydin et al. 2018; Barik et al. 2019). The forms of P depend on the original sources of P, as well as conversion between the pools governed by physicochemical properties of the sediment, such as pH, pe, texture (granularity), and organic matter content (Johan et al. 2021; Maslukah et al. 2021).
After entering the marine environment, only a minor fraction of the P flux is assimilated. Generally, 90% of the P is rapidly deposited in coastal seabed sediments through physical and biogeochemical mechanisms (Schlesinger and Bernhardt 2020). Eventually, all the P that enters the Caspian sea ends up in its sediments, acting as a permanent sink. Sediments are hence also valuable archives of contaminant levels that may be used to locate their hotspots, determine transport pathways, and ultimately identify contamination sources.
Phosphorous in sediments is mainly in its inorganic forms, including loosely bound P (LP), aluminum-bound P (AlP), iron-bound P (FeP), and calcium-bound P (CaP). These P fractions vary in bioavailability and have very different responses to changes in environmental conditions. Hence, assessing the fractionation of P in sediments is a prerequisite in order to assess the risk of P release from sediments, which causes eutrophication in aquatic and marine ecosystems. A large number of studies have investigated P contamination and fractionation in aquatic and marine ecosystems (e.g., Cheng et al. 2018; Liao et al. 2020; Maslukah et al. 2021; Shen et al. 2021). However, there are a few studies on P pools in the southern Caspian Sea sediments (Samadi-Maybodi et al. 2013; Nasrollahzadeh Saravi et al. 2015; Niyazi et al. 2016; Bastami et al. 2018), limited to either a small number of sediment samples or sampling from shallow sediments and/or non-seasonal sampling. In this article, seasonal fluctuation and distribution of different forms of P, and their empirical and conceptual link to sediment physicochemical characteristics in shallow and deep waters, are studied. The main objectives of this article are to assess seasonal and spatial distribution, as well as to document the chemical forms and bioavailability of P in the surface-seabed sediments of the southern Caspian Sea. This is used to unravel the governing factors for the temporal fluctuation and spatial differences of P pools by studying their empirical interrelations to the physicochemical characteristics of sediments.
2 Materials and Methods
2.1 Study Area
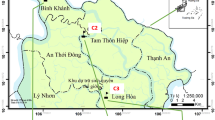
The Caspian Sea with a mean length, width, and surface area of 1200 km, 330 km, and 396,000 km2, respectively, is the largest inland water body in the world. Littoral countries of Iran, Turkmenistan, Kazakhstan, Russia, and Azerbaijan surround the sea. The Iranian Caspian coast (Fig. 1) has a total length of 905 km. The mean depth of water in the southern Caspian Sea is 180 m (Aladin and Plotnikov 2004), with a mean pH of 8.5 and a salinity varying between 12 and 13 % (Nematollahi et al. 2020). The Caspian Sea is yearly recharged (300 km3 water) by 130 surface streams, of which 61 are from Iran (CEP 2002; Alizadeh et al. 2018). The main rivers entering the southern Caspian Sea are Kura in Azerbaijan and Sefidrud in Iran. In addition, there are a large number of anthropogenic surface runoff outlets and drainage channels. The influxes from these discharge points have a significant impact on the coastal sediments by carrying high loadings of suspended matter from domestic and industrial sewage and wastewater treatment plants, as well as agricultural runoff, to the sea (Nematollahi et al. 2020, 2021).
2.2 Sampling, Sample Treatment and Analysis
A total of 64 samples of the surface seabed sediment (0–10 cm), comprising 16 samples from each of the four seasons, were collected at 10 m and 30 m water depths from eight locations, i.e., Astara (As10 and As30), Anzali (An10 and An30), Sefidrud (Sr10 and Sr30), Ramsar (Rs10 and Rs30), Nowshahr (Ns10 and Ns30), Babolsar (Bs10 and Bs30), Amirabad (Aa10 and Aa30), and Turkman (Tm10 and Tm30) (Fig. 1). Detailed information regarding location of the sampling sites and their coding is provided in Table 1. Surface sediments were sampled using a stainless steel Van Veen grab. The samples from the autumn and winter seasons were collected in 2018, while those from the spring and summer seasons are from 2019. A kg of composite wet sediment sample was collected at each sampling site. The sample was composed of a combination of four subsamples taken within an area of 1 km2. Upon collection, samples were placed in pre-labeled plastic bags that were sealed and placed on ice packs in a polyethylene container and transferred to the Environmental Chemistry laboratory at Caspian Sea Ecology Research Centre (CSERC). At the laboratory, the sealed samples were stored in the dark at 4 °C until further treatments.
Wet sediment samples were dispersed out on plastic plates and air-dried at room temperature. After removing visible impurities, the samples were gently ground in a ceramic pestle using a mortar and sieved through a 2-mm stainless steel sieve. Total organic matter (TOM) was determined based on loss on ignition (Heiri et al. 2001) using a high-temperature furnace (Carbolite company, Sheffield, UK). The hydrometry method (Bouyoucos 1962) was used to determine the relative particle size distribution of the mud (i.e., < 63 µm) and sand (between 63 µm and 2 mm) fractions.
Total P (TP) concentrations were determined in 5 g of sieved sediments that had been prepared and kept in pre-labeled plastic bags for further analysis. Phosphorus in the sediments was fractionated using a four-step sequential procedure described by Psenner et al. (1984). In this method, P is divided into four fractions including loosely bound P (LP), reductant soluble P (FeP), metallic oxide-bound P (AlP), and calcium carbonate (CaCO3)-bound P (CaP). LP is the most bioavailable P fraction, comprising labile, exchangeable, water-soluble, carbonate-associated, and hydrolyzed phosphorus. FeP and AlP are predominantly bound to ferric iron (Fe) and aluminum (Al), respectively. Under the prevailing environmental conditions (i.e., reducing and alkaline) these fractions are also conceived as bioavailable phosphorus (non-residual fractions), while CaP is the only residual fraction in this matrix of mainly Ca-bearing minerals. Detailed information on the sequential extraction scheme is outlined in Table S1. The phosphorous concentration in the TP, LP, FeP, AlP, and CaP extracts was analyzed by the molybdate blue method (APHA 1998), measuring the adsorption at λ885 nm using a UV–Vis Cecil1020 spectrophotometer. The sum of LP, FeP, AlP, and CaP constitutes the inorganic phosphorus (IP) pool. Organic phosphorus (OP) concentration was calculated by subtracting IP from TP (Sanyal and De Dutta 1991; dos Reis Oliveira et al. 2019).
High quality of analytical data was achieved by adhering to standard laboratory quality control (QC) protocols. Prior to conducting the analysis, laboratory glassware was washed three times using phosphate-free soap and deionized water. Working solutions and reagents were prepared from standard Merck chemicals. Precision of measured parameters was determined using replicate analyses (n = 6) on duplicate samples. Blank samples (n = 8) and an internal standard were used to check the accuracy of the methods. Concentrations of blank samples were below limit of detection of the instruments. Recovery of total phosphorous was determined to be 91 to 96% (see Table S2) by adding 125 mg P kg−1.dw to sediment samples (Bs, Ns, and Aa) and determining TP concentrations in the replicate samples.
2.3 Data Analysis
2.3.1 Statistical Analyses
Arc-GIS 10.3 was employed to plot maps and demonstrate spatial distribution of TP. Statistical tests were conducted using SPSS 22. Normality of the data was tested using Shapiro–Wilk (S–W) test, while seasonal means were compared through one-way analysis of variance (ANOVA). Levene statistic test was used to check homogeneity of variances. Since equality of variances could not be assumed (p-value < 0.05), Tamhane’s T2 method was implemented to conduct ANOVA. The robust Welch test was used to check equality of means and verify their significant differences based on p-values < 0.05. Empirical relations between variables were also assessed using the Spearman correlation and two-dimensional principal component analysis (PCA). For PCA, we applied XLSTAT 2016 with eigenvalues > 1 and the Varimax rotation method. Kaiser normalization method was applied to obtain optimum component numbers. In addition, Kaiser–Meyer–Olkin (KMO) method was used to check sample adequacy.
2.3.2 Contamination Factor
Contamination factor (CF) is a practical geochemical index to evaluate the degree of contamination of an element in an environmental compartment relative to the corresponding value of the element in a reference medium. The CF for phosphorous in seabed sediment samples was calculated using Eq. (1) (Hakanson 1980):
where Cs is the total phosphorus (TP) concentration and Cr is the reference phosphorus value in the upper continental crust, being 654.63 mg kg−1 (Holland and Turekian 2010).
3 Results and Discussion
3.1 Sediment Characteristics and Levels of Phosphorus Pools
Descriptive statistics of the relative content of TOM and TOC, and absolute levels of P pools in the seasonal sediment samples, as well as their physicochemical characteristics, are given in Table 2. The relative content of TOM is highest during spring and summer. The mean relative content of sand fraction is greatest during autumn and declines as follows: autumn > summer > spring > winter. This trend is inherently opposite to the mud fraction. The sand content is highest at 10 m depth (60%), though drops to 20% at 30 m water depth (see Table S3).
Sediment characteristics are normally distributed (p-value > 0.05). Due to the predominant CaP fraction, the IP and TP are higher at 10 m depth compared to 30 m depth, although the bioavailable P pools (i.e., LP, FeP, and AlP) at 10 m water depth are lower than those of 30 m depth (Table S3). There were no significant differences (p-value > 0.05) in mean TP concentrations between seasons (Table 2), except between the autumn and summer seasons. The TP in summer was close to being significantly (p-value = 0.09) lower than all the other seasons. The mean pools of both IP and OP are highest during autumn. Seasonal mean TP and IP concentrations follow the order of autumn > winter > spring > summer, while the sequence for OP is autumn > spring > winter > summer. Therefore, autumn sediments have the highest spatial variation in levels of TP and IP, with large CV values (i.e., > 30%). Concentrations of OP during all seasons are non-normally distributed, regarding the high dissimilarity between their mean and median concentrations. Based on skewness and kurtosis, IP in autumn and OP during all seasons lie outside the normal distribution range of − 1 to + 1 and − 3 to + 3, respectively (Nematollahi et al. 2020). In addition, the Shapiro–Wilk test indicates non-normality of OP in all seasons and of TOM and TOC during the spring season (p-value < 0.05).
Autumn and summer are considered the opposing governing factors on the seasonal TP distribution. As an explanatory driver, season represents a set of hydrological and biogeochemical governing pressures (e.g., precipitation) that changes from one to the other. There is a seasonal co-variation between the amount of precipitation and the mean TP concentration in the surface sediments (Fig. 2), decreasing from the levels encountered during the rather wet fall toward the dryer summer. On the other hand, ANOVA reveals that season is likely not the best explanatory factor for the temporal distribution of TP concentrations in the sediments. Other co-variable factors (e.g., seasonal fluctuations in the source of TP and sediment physicochemical characteristics) may play a stronger role in explaining temporal differences in the TP pools (Table 3).
Seasonal spatial distribution maps of TP (Fig. 3a-d) show elevated concentrations at sites in the middle of the study area, where population density and anthropogenic activities are relatively higher. The seasonal spatial distribution of sediment physicochemical characteristics (Fig. 3e-l) is very site-specific and shows an uneven spatial trend. However, the sand content is relatively low in the easternmost sites.
The seasonal pollution index (PI) values for TP at water depths of 10 and 30 m are depicted in Fig. 4. The highest PI values (3 < PI < 6), indicating a high degree of contamination, were found during autumn at 10 m seawater depth in the delta of Babolrood, Anzali wetland, and Sefidrud (Bs10, An10, and Sr10, respectively). These three sites are deltas of large rivers where higher content of suspended particles is discharged into the sea. The remaining sites have moderate P contamination with PI values ranging from 1 to 3.
3.2 Correlations Between P Fractions and Sediment Characteristics
Seasonal correlation coefficients between P fractions and sediment physicochemical characteristics are listed in Table 4. TP is inherently strongly correlated (p-value < 0.01) with CaP in all seasons since TP is predominantly (89%) constituted by CaP. More interesting is that the AlP fraction is significantly and strongly correlated to the mud size fraction during the spring and summer seasons. On the other hand, TP and CaP are significantly and strongly negatively correlated to mud (thus positively correlated to the sand fraction) during autumn and positively correlated to TOM in the spring. This simply reflects that the higher concentrations of CaP, and therefore TP, are governed by the higher flux of sand to the sea during the relatively wetter autumn and winter seasons, whereas the higher levels of AlP are governed by sedimentation of mud and TOM fractions during the drier spring and summer seasons. This link between the large CaP and sand fraction is also seen in the differences between 10 and 30 m water depth as described above. AlP is also curiously significantly correlated to LP during autumn and winter. The absence of correlation between TOM and OP reflects that the OP is not governed by the very low organic matter content in the sediments.
The overall PCA of water depth, TOM, sand fraction and P fractions (LP, FeP, AlP, CaP, and OP) gives a PC1 and PC2 explaining 36.5% and 14.1% of the variance in the data, respectively. The LP, FeP, and AlP are clustered together with the water depth, as the main explanatory variable for this main PC, and have all a strong positive loading on PC1, while OP has a strong negative loading along the PC1. The CaP is clustered with the sand fraction, as the main explanatory variable for this secondary PC, having a positive loading on PC2 and a negative loading on PC1, while TOM has positive PC1 and negative PC2 loadings. This PCA mainly simply reflects that the bioavailable P fractions are mainly found at 30 m depth, where there is a predominance of mud, while the dominant CaP fraction is strongly associated with the dominant sand fraction that prevails in the shallower water (10 m depth).
Seasonal empirical interrelations of P fractions and sediment physicochemical characteristics within the southern Caspian surface seabed sediments are depicted as seasonal PCA bi-plots in Fig. 5. PC1 and PC2 accounted for a total of 72.3, 59.8, 68.7, and 62.3% of total variances in the autumn, winter, spring, and summer data, respectively. The PCA results differ between seasons, especially between autumn and winter (Fig. 5a, b), and spring and summer (Fig. 5c, d). During autumn and winter, the CaP and thus the TP and IP form a cluster with the sand fraction (that could be considered on the opposite direction of the mud eigenvector) that has a high positive loading along PC1. These variables have factor loadings > 0.5 and their eigenvectors make small angles together, implying an inherent strong positive correlation. This reflects that the large proportion of TP during autumn and winter, characterized by high discharge, is comprised of CaP which is greatly influenced by the high percentage of sand fraction in sediments, especially at sites Sr10, Bs30, Ns30, Bs10, and Rs30 in autumn and Rs10, Ns30, Rs30, and Bs30 in winter. Eigenvectors of AlP, FeP, and LP are loaded in the same direction along the PC2, reflecting positive correlations, especially at sites An30, Sr30, and As10 in autumn, and An30, Sr30, and An10 in winter. In the spring and summer, the variation rotates between PC1 and PC2, though the clusters with factor loadings > 0.5, and their eigenvectors that make very small angles, remain. This is pointing to a strong positive correlation, especially at sites Aa10, Bs10, and Rs10. Eigenvectors of LP, FeP, and AlP have opposite loading to the sand fraction. Therefore, mud fraction is an important parameter controlling available (non-residual) fractions of P, particularly at sites Bs30, Sr10, Sr30, and Ns30. In summer (Fig. 5d), both PC1 and PC2 show strong positive correlations with CaP, TP, and IP, especially at sites Bs30, Sr10, Sr30, and Tm10. PC1 comprises strong positive loadings with the mud fraction, LP, FeP, and AlP, with eigenvectors that make narrow angles. This demonstrates that the mud fraction is a key parameter controlling distribution of non-residual (bioavailable) forms of P, in particular at sites An30, Tm30, An10, and Bs10.
Overall, the PCA results deconvolute the impact of mud fraction as an explanatory factor for LP, FeP, and AlP from the sand content, which has a strong explanatory value for CaP. In addition, the mud fraction is the main explanatory factor for distribution of non-residual (bioavailable) forms of P during spring and summer with relatively low river discharge (Fig. 5c, d), while the sand fraction is the main explanatory factor for distribution of residual (non-bioavailable) forms of P during the autumn and winter with relatively high river discharge (Fig. 5a, b).
3.3 Factors Governing the Phosphorus Fractionation
The seasonal relative composition of P fractions in seabed sediments of the southern Caspian Sea is shown in Fig. 6. The form in which P exists in the sediments (i.e., the phosphorus fractions) determines the levels and fluxes of dissolved P between sediment and seawater. Hence, knowledge of the distribution of different P forms is a prerequisite in order to evaluate the potential release of PO43− to the water column. The natural background flux of CaP is mainly from two types of Ca-associated phosphorus minerals: (1) fluorapatite which is found in metamorphic and igneous rocks in the watershed; and (2) carbonate fluorapatite which is either eroded from sedimentary bedrock or formed from the biogenic drizzle of apatite skeletal debris in the seawater (Ruttenberg 1992). Makhloukh et al. (2020) found that more than 80% of the phytoplankton in the southern Caspian Sea are Basilariophytes phyla, which are calcareous phytoplankton. Moreover, calcite, being readily precipitated upon entering the high saline waters of the Caspian sea, acts as a P sink by adsorbing dissolved P species (Coelho et al. 2004). Similarly, P adsorption to the surface of ferric oxyhydroxides is found to occur in regions influenced by freshwater (e.g., coastal water) (Zwolsman 1994; Coelho et al. 2004).
The by far dominant P fraction of TP is CaP (87.9% on average, Fig. 6) in the surface sediments of the south Caspian sea, especially in the sediments with high TP concentration (R = 0.726, p-value = 0.000). This is in line with previous studies in coastal and marine areas (Andrieux-Loyer and Aminot 2001; Diaz et al. 2006; Aydin et al. 2010; Bramha et al. 2014; Mohanty et al. 2018). AlP and FeP accounted for only 5.2 and 3.8% of the remaining IP in the sediments, respectively, leaving only 0.43% as LP. Similar results were also obtained by Rose et al. (2010) and Aydin et al. (2010). The predominance of CaP in the sediments is partly because the deposited FeP is readily dissolved since the ferric iron (Fe3+) is reduced to ferrous iron (Fe2+) under the prevailing reducing conditions in the sediments. Moreover, the Fe2+ may in turn be strongly fixated to sulfide, formed from the subsequent reduction of sulfate. Regardless, the much more soluble ferrous phosphate leads to increased levels of free aqueous phosphate (PO43−) in the sediment pore solution. Likewise, the Al3+ in the sedimented AlP is dissolved as aluminon (Al(OH)4−) in the alkaline sea pH. TOM in the sediments is similarly subject to microbial mineralization, releasing the OP as PO43−. This dissolved and mineralized PO43− from FeP, AlP, and OP may eventually diffuse to the sediment surface and disperse into the seawater. FeP and AlP, along with LP and OP, are thus pools of PO43− potentially available to algae (Kaiserli et al. 2002; Wang et al. 2006). Still, much of the released PO43− may instead react with the abundant calcite in the sediments forming CaP, which may further age into authigenic fluorapatite (Katsaounos et al. 2007; Hou et al. 2009; Xu et al. 2014; Bańkowska-Sobczak et al. 2020). This authigenic CaP is found to be stable and nearly insoluble under the prevailing hydro-geochemical (i.e., low redox and high pH) conditions in seawater sediments and thus tends to accumulate (Williams et al. 1980; Gonsiorczyk et al. 1998; Jin et al. 2006). Hence, Samadi-Maybodi et al. (2013) found that the authigenic P is the most abundant P form (89%) in the southern Caspian Sea. There are thus several biogeochemical processes that lead to the low content of available P forms in the seabed sediments of the southern Caspian Sea.
The available P forms are usually more associated with fine-grained silt and clay particles (Delgado-Baquerizo et al. 2013, Hou et al. 2018), due to their large surface area (Gérard 2016). This is also found in Table 4 by a positive correlation between available P forms (LP, FeP, and AlP) and the mud fraction, but this article reveals that phosphorus adsorption to the surface of minerals (e.g., clays and Fe–Mn oxy-hydroxides) is not significant. Hence, an overall low content of silt and clay (i.e., mud, 57 w/w % on average) in the sediments could be a contributing reason for the relatively low quantity of available P forms in the southern Caspian seabed sediments.
4 Conclusions
Seasonal and spatial distribution of different pools of P in the southern Caspian seabed sediments were assessed in order to gain an improved insight into the P contamination and the associated ecological risk. Seasonal fluctuations in the distribution of P pools in the surface sediments were mainly explained by the seasonal precipitation patterns and governing river discharge fluxes, while the spatial variation in P pools was mainly explained by differences in sediment physicochemical characteristics.
The levels of different P pools in the top layer of the sediments were not found to differ significantly between seasons, though the higher rainfall during the autumn had led to the higher sediment TP concentration compared to the summer. By far, the dominant P pool in the sediment is CaP. This is partly reflecting the influx of calcium phosphate minerals (from mineral weathering and soil erosion in the watershed) by the main rivers, specifically Babolrood and Sefidrud and Anzali wetland. In addition, there is also a significant conversion of labile LP, FeP, and AlP pools producing the insoluble authigenic CaP in the sediments. Minor remains of bioavailable P forms (LP) and potentially bioavailable forms (FeP and AlP) were correlated with the content of mud fraction, especially during the spring and summer seasons. In the autumn and winter seasons, the by far dominating CaP was correlated with the sand fraction. The generally low content of mud in the sediments has thus resulted in rather small pools of bioavailable P. On the contrary, the high content of sand fraction is reflected in the large pool of non-bioavailable P (CaP). Sediment physicochemical characteristics may therefore serve as good proxies for the relative distribution of P pools in sediments. The low levels of LP, FeP, and AlP in the sediments of the southern Caspian Sea imply that there is a low risk of internal loading of P, causing eutrophication of the seawater.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Aladin N, Plotnikov I (2004) The Caspian Sea. Lake Basin Management Initiative Thematic Paper
Alizadeh H, Naderi Beni A, Tavakoli V (2018) Heavy metals in coastal sediments of South Caspian Sea: natural or anthropogenic source? Caspian J Environ Sci 16(1):45–61
Andrieux-Loyer F, Aminot A (2001) Phosphorus forms related to sediment grain size and geochemical characteristics in french coastal areas. Estuar Coast Shelf S 52(5):617–629. https://doi.org/10.1006/ecss.2001.0766
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington. DC. https://doi.org/10.1006/ecss.2001.0766
Aydin I, Aydin F, Saydut A, Hamamci C (2009) A sequential extraction to determine the distribution of phosphorus in the seawater and marine surface sediment. J Hazard Mater 168(2–3):664–669. https://doi.org/10.1016/j.jhazmat.2009.02.095
Aydin I, Aydin F, Hamamci C (2010) Phosphorus speciation in the surface sediment and river water from the Orontes (Asi) River. Turkey Water Environ Res 82(11):2265–2271. https://doi.org/10.2175/106143010X12609736967206
Aydin I, Temel Z, Gunduz B, Aydin F (2018) Comparative determination of phosphorus fractions in coastal surface sediment (NE Mediterranean Sea) by ICP-OES and UV/VIS Spectrometry. Atom Spectrosc 12:193. https://doi.org/10.46770/AS.2018.05.003
Baken S, Moens C, van der Grift B, Smolders E (2016) Phosphate binding by natural iron-rich colloids in streams. Water Res 98:326–333. https://doi.org/10.1016/j.watres.2016.04.032
Bańkowska-Sobczak A, Blazejczyk A, Eiche E, Fischer U, Popek Z (2020) Phosphorus inactivation in lake sediments using calcite materials and controlled resuspension—mechanism and efficiency. Minerals-Basel 10(3):223. https://doi.org/10.3390/min10030223
Barik SK, Bramha S, Bastia TK, Behera D, Mohanty PK, Rath P (2019) Distribution of geochemical fractions of phosphorus and its ecological risk in sediment cores of a largest brackish water lake. South Asia Int J Sediment Res 34(3):251–261. https://doi.org/10.1016/j.ijsrc.2018.11.004
Bastami KD, Neyestani MR, Raeisi H, Shafeian E, Baniamam M, Shirzadi A, Esmaeilzadeh M, Mozaffari Sh, Shahrokhi B (2018) Bioavailability and geochemical speciation of phosphorus in surface sediments of the Southern Caspian Sea. Mar Pollut Bull 126:51–57. https://doi.org/10.1016/j.marpolbul.2017.10.095
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54(5):464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Bramha SN, Mohanty AK, Padhi RK, Panigrahi SN, Satpathy KK (2014) Phosphorus speciation in the marine sediment of Kalpakkam coast, southeast coast of India. Environ Monit Assess 186(10):6003–6015. https://doi.org/10.1007/s10661-014-3836-0
Cao X, Zhu J, Lu M, Ge C, Zhou L, Yang G (2019) Phosphorus sorption behavior on sediments in Sanggou Bay related with their compositions by sequential fractionation. Ecotox Environ Safe 169:144–149. https://doi.org/10.1016/j.ecoenv.2018.11.007
CEP (2002) Diagnostic Transboundary analysis for the Caspian Sea, vol2. The Caspian Environment Program, Baku. https://ceic-portal.net/index.php/az/node/3060
Cheng X, Huang Y, Pu X, An R, Huang W, Li J, Wang W, Li R (2018) Spatial and seasonal distribution and transportation of different forms of phosphorus in the middle reaches of the Yarlung Zangbo River. Water-Sui 10(12):1858. https://doi.org/10.3390/w10121858
Coelho JP, Flindt MR, Jensen HS, Lillebø AI, Pardal MA (2004) Phosphorus speciation and availability in intertidal sediments of a temperate estuary: relation to eutrophication and annual P-fluxes. Estuar Coast Shelf S 61(4):583–590. https://doi.org/10.1016/j.ecss.2004.07.001
Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502(7473):672–676. https://doi.org/10.1038/nature12670
Diaz OA, Daroub SH, Stuck JD, Clark MW, Lang TA, Reddy KR (2006) Sediment inventory and phosphorus fractions for water conservation area canals in the Everglades. Soil Sci Soc Am J 70(3):863–871. https://doi.org/10.2136/sssaj2005.0059
dos Reis Oliveira PC, van der Geest HG, Kraak MH, Verdonschot PF (2019) Land use affects lowland stream ecosystems through dissolved oxygen regimes. Sci Rep-UK 9(1):1–10. https://doi.org/10.1038/s41598-019-56046-1
Frankowski L, Bolałek J, Szostek A (2002) Phosphorus in bottom sediments of pomeranian bay (Southern Baltic–Poland). Estuar Coast Shelf S 54(6):1027–1038. https://doi.org/10.1006/ecss.2001.0874
Gérard F (2016) Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—a myth revisited. Geoderma 262:213–226. https://doi.org/10.1016/j.geoderma.2015.08.036
Gonsiorczyk T, Casper P, Koschel RH (1998) Phosphorus-binding forms in the sediment of an oligotrophic and an eutrophic hardwater lake of the Baltic Lake District (Germany). Water Sci Technol 37:51–58. https://doi.org/10.1016/S0273-1223(98)00055-9
Gottselig N, Bol R, Nischwitz V, Vereecken H, Amelung W, Klumpp E (2014) Distribution of phosphorus-containing fine colloids and nanoparticles in stream water of a forest catchment. Vadose Zone J 13(7):1–11. https://doi.org/10.2136/vzj2014.01.0005
Guarino A, Albanese S, Cicchella D, Ebrahimi P, Dominech S et al (2022) Factors influencing the bioavailability of some selected elements in the agricultural soil of a geologically varied territory: the Campania region (Italy) case study. Geoderma 428:116207. https://doi.org/10.1016/j.geoderma.2022.116207
Hakanson L (1980) An ecological risk index for aquatic pollution control. A Sedimentological Approach Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25(1):101–110. https://doi.org/10.1023/A:1008119611481
Holland HD, Turekian KK (eds) (2010) Geochemistry of earth surface systems: a derivative of the treatise on geochemistry. Academic Press, London
Hou LJ, Liu M, Yang Y, Ou DN, Lin X, Chen H, Xu SY (2009) Phosphorus speciation and availability in intertidal sediments of the Yangtze Estuary. China Appl Geochem 24(1):120–128. https://doi.org/10.1016/j.apgeochem.2008.11.008
Hou E, Chen C, Luo Y, Zhou G, Kuang Y, Zhang Y, Heenan M, Lu X, Wen D (2018) Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Global Change Biol 24(8):3344–3356. https://doi.org/10.1111/gcb.14093
Jensen HS, McGlathery KJ, Marino R, Howarth RW (1998) Forms and availability of sediment phosphorus in carbonate sand of Bermuda seagrass beds. Limnol Oceanogr 43(5):799–810. https://doi.org/10.4319/lo.1998.43.5.0799
Jin X, Wang S, Pang Y, Chang WF (2006) Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake. China Environ Pollut 139(2):288–295. https://doi.org/10.1016/j.envpol.2005.05.010
Johan PD, Ahmed OH, Omar L, Hasbullah NA (2021) Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 11(10):2010. https://doi.org/10.3390/agronomy11102010
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments—Lakes Volvi and Koronia. N Greece Chemosphere 46(8):1147–1155. https://doi.org/10.1016/S0045-6535(01)00242-9
Katsaounos CZ, Giokas DL, Leonardos JD, Karayanis MI (2007) Speciation of phosphorus fraction in river sediments by explanatory data analysis. Water Res 41:406–418. https://doi.org/10.1016/j.watres.2006.10.028
Keshavarzi B, Ebrahimi P, Moore F (2015) A GIS-based approach for detecting pollution sources and bioavailability of metals in coastal and marine sediments of Chabahar Bay. SE Iran Chem Erde-Geochem 75(2):185–195. https://doi.org/10.1016/j.chemer.2014.11.003
Kundzewicz ZW, Mata LJ, Arnell NW, Döll P, Jimenez B, Miller K, Oki T, Şen Z, Shiklomanov I (2008) The implications of projected climate change for freshwater resources and their management. Hydrolog Sci J 53(1):3–10. https://doi.org/10.1623/hysj.53.1.3
Liang B, Qian X, Liu X, Zhao S, Cui B, Bai J (2017) Quantitative prediction and typical factor effects of phosphorus adsorption on the surface sediments from the intertidal zones of the Yellow River Delta. China Mar Freshwater Res 69(5):648–657. https://doi.org/10.1071/MF17104
Liao H, Pan C, Gan L, Ke Z, Tang H (2020) Distribution of geochemical fractions of phosphorus in surface sediment in Daya Bay. China Int J Environ Res Pu 17(12):4430. https://doi.org/10.3390/ijerph17124430
Makhloukh A, Nasrollahzadeh Saravi H, Roohi A, Aghaei Moghadam AA (2020) Study on changes in phytoplankton structure to infer water quality in the southern basin of the Caspian Sea. J Mar Biol 12(2):1–14 (In Persian)
Maslukah L, Wirasatriya A, Yusuf M, Sari RS, Salma U, Zainuri M (2021) Phosphorous fractionation distribution in surface sediments of the Jobokuto Bay. Molekul 16(2):100–109. https://doi.org/10.20884/1.jm.2021.16.2.572
Mohammadi Z, Claes H, Cappuyns V, Nematollahi MJ, Helser J, Amjadian K, Swennen R (2021) High geogenic arsenic concentrations in travertines and their spring waters: assessment of the leachability and estimation of ecological and health risks. J Hazard Mater 409:124429. https://doi.org/10.1016/j.jhazmat.2020.124429
Mohanty AK, Bramha SN, Satpathy KK, Padhi RK, Panigrahi SN, Samantara MK, Barath Kumar S, Sarkar SK, Prasad MVR (2018) Geochemical distribution of forms of phosphorus in marine sediment of Bay of Bengal, southeast coast of India. Indian J Geo-Mar Sci 47(2018):1132–1141
Moore F, Nematollahi MJ, Keshavarzi B (2015) Heavy metals fractionation in surface sediments of Gowatr bay-Iran. Environ Monit Assess 187(1):4117. https://doi.org/10.1016/j.marpolbul.2018.06.031
Nasrollahzadeh Saravi H, Pouraria A, Nowruzi B (2015) Phosphorus forms of the surface sediment in the Iranian coast of the Southern Caspian Sea. Casp J Environ Sci 13(2):141–151
Nematollahi MJ, Moore F, Keshavarzi B, Vogt RD, Nasrollahzadeh Saravi H, Busquets R (2020) Microplastic particles in sediments and waters, south of Caspian Sea: frequency, distribution, characteristics, and chemical composition. Ecotox Environ Safe 206:111137. https://doi.org/10.1007/s10661-020-08589-4
Nematollahi MJ, Keshavarzi B, Moore F, Vogt RD, Nasrollahzadeh Saravi H (2021) Trace elements in the shoreline and seabed sediments of the southern Caspian Sea: investigation of contamination level, distribution, ecological and human health risks, and elemental partition coefficient. Environ Sci Pollut R. https://doi.org/10.1007/s11356-021-14678-9
Niyazi L, Chaichi MJ, Nasrollahzadeh Saravi H, Najafpour S (2016) Quantification of individual phosphorus forms in surface sediments of the Southern Caspian Sea-Iranian Coast: a sequential extraction procedure. Iran J Fish Sci 15(2):677–686
Psenner RV, Pucsko R, Sager M (1984) Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten-Versuch einer Definition ökologisch wichtiger Fraktionen. Arch Hydrobiol, Suppl 70(1):111–155
Rose TJ, Hardiputra B, Rengel Z (2010) Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 326(1):159–170. https://doi.org/10.1007/s11104-009-9990-4
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37(7):1460–1482. https://doi.org/10.4319/lo.1992.37.7.1460
Samadi-Maybodi A, Saffar HT, Khodadoust S, Nasrollahzadeh Saravi H, Najafpour S (2013) Study on different forms and phosphorus distribution in the coastal surface sediments of Southern Caspian Sea by using UV–Vis spectrophotometery. Spectrochim Acta A 113:67–71. https://doi.org/10.1016/j.saa.2013.04.071
Sanyal SK, De Datta SK (1991) Chemistry of phosphorus transformations in soil. In: Stewart BA (ed) Advances in soil science. Springer, New York, NY, pp 1–120. https://doi.org/10.1007/978-1-4612-3144-8_1
Schenau SJ, De Lange GJ (2001) Phosphorus regeneration vs. burial in sediments of the Arabian Sea. Mar Chem 75:201–217. https://doi.org/10.1016/S0304-4203(01)00037-8
Schlesinger WH, Bernhardt ES (2020) Biogeochemistry: An Analysis of Global Change, 4th edn. Academic Press, London. https://doi.org/10.1016/C2017-0-00311-7
Shen Y, Peng C, Yuan P, Wu X, Jiang L, Chen S, Song X (2021) Seasonal and spatial distribution and pollution assessment of nitrogen and phosphorus in sediments from one of the world’s largest tidal reservoirs. Water-Sui 13(4):395. https://doi.org/10.3390/w13040395
Somma R, Ebrahimi P, Troise C, De Natale G, Guarino A, Cicchella D, Albanese S (2021) The first application of compositional data analysis (CoDA) in a multivariate perspective for detection of pollution source in sea sediments: The Pozzuoli Bay (Italy) case study. Chemosphere 274:129955. https://doi.org/10.1016/j.chemosphere.2021.129955
Wang XJ, Xia SQ, Chen L, Zhao JF, Renault NJ, Chovelon JM (2006) Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochem 41:824–828. https://doi.org/10.1016/j.procbio.2005.10.015
Williams JOH, Shear H, Thomas RL (1980) A vailability to Scenedesmus quadricauda of different forms of phosphorus in sedimentary materials from the Great Lakes. Limnol Oceanogr 25:1–11. https://doi.org/10.4319/lo.1980.25.1.0001
Wu ML, Wang YS, Wang YT, Yin JP, Dong JD, Jiang ZY, Sun FL (2017) Scenarios of nutrient alterations and responses of phytoplankton in a changing Daya Bay, South China Sea. J Marine Syst 165:1–12. https://doi.org/10.1016/j.jmarsys.2016.09.004
Xu N, Chen M, Zhou K, Wang Y, Yin H, Chen Z (2014) Retention of phosphorus on calcite and dolomite: speciation and modeling. RSC Adv 4(66):35205–35214. https://doi.org/10.1039/C4RA05461J
Zhao Y, Zheng B, Jia H, Chen Z (2019) Determination sources of nitrates into the Three Gorges Reservoir using nitrogen and oxygen isotopes. Sci Total Environ 687:128–136. https://doi.org/10.1016/j.scitotenv.2019.06.073
Zhou B, Fu X, Wu B, He J, Vogt RD, Yu D, Yue F, Chai M (2021) Phosphorus release from sediments in a raw water reservoir with reduced allochthonous input. Water-Sui 13(14):1983. https://doi.org/10.3390/w13141983
Zwolsman JJ (1994) Seasonal variability and biogeochemistry of phosphorus in the Scheldt Estuary. South-West Netherlands Estuar Coast Shelf S 39(3):227–248. https://doi.org/10.1006/ecss.1994.1061
Acknowledgements
The authors state their gratitude to the Caspian Sea Ecology Research Center (CSERC) for support to do this study.
Funding
This work was supported by the Caspian Sea Ecology Research Center (CSERC).
Author information
Authors and Affiliations
Contributions
HNS helped in conceptualization, supervision, resources, project administration. MJN contributed to writing—original draft, conceptualization, formal analysis, validation, writing—review & editing. RDV was involved in validation, formal analysis, writing—review & editing. FV and MB performed methodology. PE helped in partial conceptualization, review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasrollahzadeh Saravi, H., Nematollahi, M.J., Vogt, R.D. et al. Seasonal and Spatial Distribution of Phosphorus Fractions in Surface Sediments of the Southern Caspian Sea. Iran J Sci 47, 411–425 (2023). https://doi.org/10.1007/s40995-023-01426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-023-01426-6