Abstract
In this study, total phosphorus (P) and P released forms were measured in core sediments from the areas affected by human settlement and shrimp farming activities and the core zone of the Can Gio Biosphere Reserve, a coastal district in south Vietnam. Furthermore, ecological risk assessment and parameters controlling P release from sediments were investigated, including pH, major elements (Al-Ca-Mg-Fe), and fine fraction. The average total amount of P in the sediments varied from 287 to 669 mg/kg, with significantly lower values being observed in the mangrove biosphere reserve area. According to the results of the correlation analysis, organic matter was the primary source of P in the sediments, but the majority of the P released was inorganic. Positive correlations were found between Fe and non-apatite inorganic P (NAIP) and apatite P (AP), as well as intercorrelations between P fractions (r = 0.40–0.79, p < 0.05), suggesting that Fe might be the controlling factor of P release in the investigated sediments. The rank orders of concentrations of P forms were significantly different between the areas. The range of P forms was AP (35–248 mg/kg) > NAIP (63–201 mg/kg) > LP (labile P) (4–25 mg/kg) in the human settlement and aquaculture areas and NAIP (84–99 mg/kg) > AP (20–38 mg/kg) > LP (7–12 mg/kg) in the mangrove biosphere reserve area. Risk assessment based on the total concentration of P and the availability of P from a single extraction suggested a relatively low risk of P from sediment as an internal load in the studied areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments in the coastal zones as well as in the mangrove forest are interesting to study because of their complex physical and chemical properties (Alongi, 2002). Nutrients can be retained in mangrove systems in which phosphorus (P) is immobilized in inorganic phases such as aluminum, iron, calcium, and sulfides (Alongi, 2018). However, residence times of the available P pools are short with the generally rapid rates of nutrient recycling (Kristensen et al., 2000). Although mangroves play a crucial role in protecting coastal areas, they have been affected by human disturbances such as the conversion of mangroves to areas for household settlement, the aquaculture and agricultural activities, and the deforestation of mangroves for timber product supply (Sofawi et al., 2017). Land use activities involve removal of surface vegetation which might reduce nutrient uptake and might also increase pools of labile P in the surface (Tuukkanen et al., 2017). Knowledge of the potential release of P from mangrove sediment is useful since it can serve as an indicator for the contribution of this element to the eutrophication of the water environment (Cheng et al., 2019).
Can Gio, a coastal district of Ho Chi Minh City, south of Vietnam, has a large area covered by a mangrove forest that was designated as a Biosphere Reserve by UNESCO (Tuan & Kuenzer, 2012). This is a dynamic transitional coastal ecosystem with natural and anthropogenic impacts such as tidal regime, nutrient accumulation through mangrove tree root system, organic matter decomposition by litter mineralization process in mangrove forest, aquaculture activities, and effluents from municipal sewage (Taillardat et al., 2019). Growing shrimp industry has led to wetland conversion into shrimp farms. This has raised the concern about adverse environmental effects of development activities due to high mangrove losses (Spalding et al., 2010). Statistics from Can Gio People’s Committee showed that aquaculture is the main component contributing to the whole economic value of this district (Ho Chi Minh City People’s Committee, 2016). More than 1500 operational shrimp farms located in the transitional buffer zones around the Biosphere Reserve officially were recorded in Can Gio (McDonough et al., 2014). Research about P in Can Gio mangrove sediments which takes into account the difference in land use patterns has received little attention, especially with regard to available pools of P that might be released into aquatic environments.
The goals of this study were to (1) evaluate the P enrichment in core sediments in the Can Gio area, as well as the factors controlling the distribution of P; (2) investigate different P fractions in the core sediments and determine whether human-impacted areas have significant differences in P concentrations and pools in the core sediments; and (3) assess the risk of P pollution from sediment to the environment. This study will contribute to a better understanding of the leaching behavior of P in sediment when external environmental conditions change, as well as the risk associated with the mobilization of P in sediments to the aquatic environment, particularly in a dynamic area where anthropogenic activities have changed rapidly and may impact the mangrove forest like Can Gio.
Materials and methods
Sampling and sample pretreatment
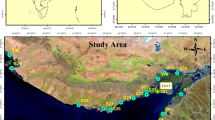
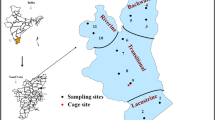
Sampling campaigns were carried out in May and June 2020. Three sediment cores (C1, C2, and C3, with a length of 105, 125, and 185 cm, respectively) were collected by piston coring. Cores C1 and C2 were located in human settlement and densely populated shrimp farming zones, while core C3 was in the protected zone of the mangrove biosphere reserve, where fewer human activities were expected (Fig. 1). One sample site for each land use pattern is indeed a limitation to distinguishing the differences. However, the topography and geomorphology properties of alluvial core sediment in a sub-land are similar due to the relative similarity in sedimentary formation conditions over time. Moreover, the sampling location was chosen to be a condensed version of a specific land use pattern (i.e., shrimp farming or human settlement). Therefore, we assumed that it is adequate for investigating relative changes in the chemical compositions of the sub-land. It is difficult to differentiate strictly the land use pattern across the area since shrimp farming existed in the human settlement area as well as human settlement being recognized in the aquaculture area. However, during the field survey and communication with local authorities, the dominant land use pattern in the area where C1 was collected was human settlement, and the dominant use pattern in the area where C2 was collected was shrimp farming. Hence, based on the dominant land use patterns, the areas were classified into 3 groups: C1 and C2 represent the human settlement and shrimp farming areas, respectively, while C3 represents the mangrove area.
The sediment cores were sectioned over 5–15-cm intervals. Core C1 and core C3 were sectioned over 5-cm intervals (from 0 to 5 cm) and 10-cm intervals (from 5 cm to the bottom of the cores). Due to an analytical mistake, core C2 was sectioned with a thickness of 5 cm (from 0 to 5 cm), 15 cm (from 5 to 20 cm), and 10 cm (from 20 cm to the bottom of the core). Consequently, cores C1, C2, and C3 were divided into 11, 13, and 19 subsamples in total, respectively. After sample collection, the samples were packaged immediately in polyethylene film and stored in a cold incubator in the dark during transport to the laboratory. The samples were air-dried, homogenized in a porcelain mortar, and sieved over a 2-mm mesh sieve. Although the vacuum freeze dryer is good to retain the in situ chemical composition, air-drying should be satisfactory for investigations into relative changes in sediment P (Pierzynski, 2000).
General sample characterization
Organic carbon (OC) was inferred from the organic matter determined by the Walkley and Black manual titration method (Nelson & Sommers, 1982). Grain size distribution was determined by sieving and hydrometer methods (Head, 1992). First, air-dried sediments were washed to eliminate salts and then were boiled for 2 h with NH4OH (25%) to increase pH to 8 or 9 and disperse the fraction with a grain size of > 63 µm. The fraction coarser than 63 µm was determined by sieving, and the fraction ≤ 63 µm was determined by the hydrometer method (Head, 1992). The pH of each sample was measured in water (1:5 ratio w/w). The total elemental content of the samples was determined after digestion by the aqua regia digestion method (3:1, v/v, HClconc to HNO3 conc) in a Teflon beaker on a hot plate. The major elements (Al, Ca, Fe, K, Mg) in the digested solutions were analyzed with ICP-OES (Perkin Elmer Optima 7300 DV), while P was determined by the molybdate blue method of Murphy and Riley (1962).
Phosphorus fraction determination
Since P can exist in several forms in sediments with different mobility performance, fractionation of P by sequential extractions to water-soluble, salt-extractable, pH-sensitive, organic, and residual forms is often used to evaluate the potential release of P to aquatic environment (Abu-Hmeidan et al., 2018; Cheng et al., 2019). However, sequential extractions are laborious and time-consuming. Single extractions with the reagents representing different environmental conditions that the sediments might encounter during resuspension could be fast, simple, and relatively cheap to assess the potential release of elements from sediments to water environment (Cappuyns, 2012).
The different fractions of P in the sediments were determined by three independent single extractions with different reagents and conditions. The most labile (or weakly loosely) P was determined by a single extraction with a mild salt (Pierzynski, 2000). Apatite phosphorus (AP) bound to Ca-Mg and non-apatite inorganic phosphorus (NAIP) bound to Al, Fe, and Mn (hydr)oxides (Cavalcante et al., 2018) were determined by a modified Standards Measurements and Testing Program (SMT protocol) (Ruban et al., 2001). Detailed experimental procedures and conditions were described as follows.
For the labile P (LP), about 1.0000 g of dried sediment was weighed into a centrifuge tube, and then, 25 mL of 0.01 M CaCl2 was added. The sediment was extracted by shaking for 1 h. Regarding apatite P (AP), another 0.2000 g portion of the dried sediment was weighed into a centrifuge tube; then, 20 mL of 1 M HCl was added and the solution was shaken for 16 h. For the non-apatite inorganic P, 20 mL of 1 M NaOH was added to 0.2000 g of sediment. After shaking for 16 h at room temperature and centrifuging for 10 min at 4000 rpm, 10 mL of clear supernatant was mixed with 4 mL of 3.5 M HCl. Let the mixture stand for 16 h at room temperature.
All the extractions were carried out at room temperature. After each extraction, the samples were centrifuged (4000 rpm for 10 min) and filtered through Whatman No. 42 filter paper, and the phosphorus in the extracts was determined accordingly by the molybdate blue method of Murphy and Riley (1962). All P forms were expressed as the amount of P in milligrams per kilogram of sediment (mg kg−1) taking into account the volume of extraction solvent for each extraction.
Quality assurance and quality control
All reagents were of analytical grade. For each sample, duplicate (total element concentrations and single extractions) or triplicate (pH and organic matter) analyses were performed to ensure analytical precision and reproducibility. Results are displayed as the averages of the replicates. Duplicate blanks were also inserted into each batch.
Ecological risk assessment
Ecological risk assessment of P from sediments was based on both the total concentration of P and the bioavailability of P from the extractions.
Ecological risk assessment of P from sediments is first evaluated by using sediment quality guidelines (SQGs). Comparison of measured concentrations of P within the sediments with SQGs values provides a basic indication of the degree of pollution and risk to ecology. Currently, there are no Vietnamese standards for the ecological risk assessment of P in sediments. We used SQGs values derived from SQGs of the Department of Environment and Energy of Ontario as a reference (Persaud et al., 1993). The guidelines consist of three levels. Level I: there are no toxic effects in aquatic organisms (600 mg/kg); level II: the sediment is contaminated, but most benthic organisms are viable (600–2000 mg/kg); and level III: the benthic community has been significantly harmed (2000 mg/kg).
One of the limitations of using the SQGs is that they do not take into account the differences in fractions of P in sediments. Phosphorus can exist in many forms in sediments. However, the SQGs are based on the total P concentration. This comes with the assumption that all the forms of P possess an equal risk with regard to the ecosystem. To overcome this limitation, the extractable fraction of P was used in risk assessment in sediments. Hence, the risk of P in sediments was further assessed based on the Risk Assessment Index (RI), which takes into account both the total concentration and the bioavailability of P. In this method, the ratio of bioavailable P to non-bioavailable P was defined as the bioavailable coefficient of P (Ye et al., 2019).
The Risk Assessment Index (RI) was calculated as:
where RI is the Risk Assessment Index, K is the biological efficiency coefficient, Cs is the standard concentration of P based on the Sediment Quality Guidelines (600 mg/kg) (Persaud et al., 1993), and BAP is concentration of bioavailable forms of phosphorus. This simple classification was drawn up based on the guidelines for environmental quality assessment developed by the Department of Environment and Energy of Ontario, Canada (1992), and has been used in several studies about the risk assessment of P in sediment (Peng et al., 2020; Ye et al., 2019; Zhang et al., 2015). The risk levels of RI is divided into four levels, ranging from level I to level IV based on the value of RI. According to this classification, if RI is < 0.5, the sediment is classified as clean or there is no risk of pollution, 0.5 ≤ RI < 1 reflects a slight risk, 1 ≤ RI < 1.5 indicates medium risk, and 1.5 ≤ RI indicates for a serious risk of pollution, respectively. Generally, the NAIP, which is the P fraction that is extracted by NaOH, is an indicator of algal available P (Zhou et al., 2001). Hence, we used the concentration of this fraction as the concentration of bioavailable forms of P (BAP).
Statistical analyses
R studio was used for all statistical analyses. Correlation analysis (Spearman Rank Order) was used to assess the relationship between parameters, and the Mann–Whitney U test was used to determine the difference between areas. The significance level was set at p < 0.05.
Results and discussion
Sediment characteristics
The average of the fine fraction (FF) (≤ 63 µm, silt and clay), OC, pH, and elemental concentrations in the sediment is presented in Table 1 and Fig. 2. Vertical distribution of pH in all the three cores indicated low average values, where C3 showed the highest value ranging from 4.86 to 6.72, followed by C1 with a value from 3.57 to 6.11. The pH of C2 was the lowest, with a value of 2.37 to 4.93. Changing the potential acid sulfate soil, common to many parts of Can Gio area, to actual acid sulfate soil due to disturbance and exposure to air through shrimp pond reclamation might decrease the pH in the C2 area. Acidification due to shrimp farming was also reported in Can Gio area by Bao and Ha (2018). The profiles of C1 (30–125 cm) and C2 (75–125 cm) show a decreasing trend of pH, which is coincident with the decreasing trend in concentrations of P (Fig. 2), while C3 does not indicate any clear trend. The Mann–Whitney U analysis showed that pH was significantly different across the three areas (p < 0.05).
Grain size analysis revealed the dominance of fine fraction (> 98%). The composition of clay and silt was recorded high in C1 and C3, while sand composition was the highest in C2, especially at 0–20 cm, ranging from 49 to 71%. We found that FF was significantly different between the three areas (p < 0.05). This is because the construction as well as digging activities to build houses and prepare shrimp ponds might disturb and affect the grain size composition in C1 and C2, which leads to the difference in FF of the three areas.
In general, sediments found in the mangrove biosphere reserve area (C3) had less variation of Al, Ca, Fe, and P with depth than sediments in the impacted areas (C1 and C2) (Fig. 2). Average concentrations of Al in the cores were in the following order: C1 > C3 > C2 (38,113 mg/kg, 22,348 mg/kg, and 35,851 mg/kg, respectively) and were rather constant with depth within C3, while it showed an enriched trend in the 0–60-cm layer of C1 and in the 0–55-cm layer of C2. Aluminum did not show any significant variation between areas.
The average Ca concentration in the cores ranged between 639 and 2279 mg/kg. Calcium concentrations in core sediments increased slightly in the 50–85-cm layer of the cores within cores C1 and C2 (Fig. 2), while they showed little variation from the surface to the bottom of C3. The concentration ranges of Mg were rather similar between the three cores (3559–5692 mg/kg) and did not change much with depth (Fig. 2), supporting the hypothesis that they are not influenced by human activities, which would be reflected by changes in concentrations in the uppermost, i.e., youngest sediments. Results indicated that average concentrations of Ca and Mg were significantly different between the areas (p < 0.05). Both concentrations of Ca and Mg were very low in these sediment cores, indicating that the sediments in the Can Gio area contain a comparatively low carbonate concentration.
From C1 to C3, average Fe concentrations decreased, with average values of 50,415 mg/kg, 45,224 mg/kg, and 32,083 mg/kg, respectively. The Mann–Whitney U test revealed that the Fe concentration differed significantly (p < 0.05) between C1 and C3 and between C2 and C3. However, we did not find any significant difference between C1 and C2 (p > 0.05). The construction activity might introduce the Fe material to the environment, which might be attributed to the high concentrations of Fe in C1 and C2. However, residential land was the main land use in C1; hence, the highest concentration of Fe was observed in this area. With regard to depth profile, Fe concentrations were the highest at 20 cm of C2 (60,995 mg/kg) or at 55 cm of C1 (79,625 mg/kg), while Fe concentration was the highest at 185 cm (41,166 mg/kg) in C3, a deeper layer compared to C1 and C2.
Total phosphorus concentrations varied over a very wide range—from 287 to 669 mg/kg, with significant low values observed in C3 (129 mg/kg) compared to C1 and C2 (p < 0.05). Concentrations of P are usually low in the mangrove environment because they are rapidly consumed by plants (Sofawi et al., 2017). The average concentration of P in C1 and C2 was quite similar (669 and 607 mg/kg), and there was no significant difference in the average concentration found in the two areas (p > 0.05). It can be inferred that it is difficult to differentiate the source of P from human settlement and aquaculture, and they both contributed to the P in the sediment of the studied area to a similar extent. On the whole depth profile, concentrations of P were rather constant in C3, but they showed little enrichment at 55–85 cm of C1 and at 85–115 cm of C2 (Fig. 2). Total phosphorus in the sediments was in the same range as in a previous study in the Can Gio area (320–668 mg/kg) (Dung et al., 2020) and another study in an Indian mangrove forest (360–550 mg/kg) (Ramanathan et al., 2010), but higher than in Malaysia (Sofawi et al., 2017) and China (44.58–125.34 mg/kg) (You et al., 2022).
The average amount of organic carbon (OC) in core sediments varied from 20,342 to 40,268 mg/kg, with the highest value being observed at 125 cm of C2 (68,849 mg/kg). This result indicated evidence that the organic matter from the aquaculture activities (i.e., uneaten feed, shrimp feces, decomposed phytoplankton) led to an increase in OC concentration in the sediment. The Mann–Whitney U test confirmed this statement since the average concentration of OC in C2 was significantly different from C1 and C3 (p < 0.05). Regarding the depth profiles of OC, C1 and C3 were rather similar with little variation, while it did not show any clear trend of OC in C2 (Fig. 2).
P pools of the sediments
Only the sediments from the top 45 cm (or 50 cm) of each core were selected to investigate the behavior of P in sediments since it was widely accepted that the interaction between sediments and the overlying water happens mainly in the sediment’s top active layer (Gunduz et al., 2011). Figure 3 shows the vertical distribution characteristics of the LP, NAIP, and AP in the sediments. Generally, the sum of the labile P, apatite P, and non-apatite P forms is considered the mobile form, which is the P that can be released when external environmental conditions change. As a result, the difference between TP and the sum of LP, AP, and NAIP represents the non-mobile sediment P pool or the residual P (RP) (Fig. 4).
In C1 and C2, the main form of P was AP, which varied from 35 to 248 mg/kg and thus comprised 6 to 40% of their total P pools, whereas in C3, it was significantly lower, ranging from 20 to 38 mg/kg (as 4–12% of TP). The results indicate that AP was slightly higher in the 35–40-cm (C1) and 10–20-cm (C2) intervals, although it was relatively stable with depth in C3. The average AP concentration in core sediment samples was slightly lower than in other studies conducted in Vietnam (134–381 mg/kg) (Dung et al., 2020) and other countries (Kapanen, 2008; Lukawska-Matuszewska et al., 2013). This may be due to the fact that the sediments in this study have relatively low pH values, which may limit the concentration of AP since AP is greatly affected by the pH value. Samples having relatively higher apatite P amounts are expected to have higher pH values because precipitation of P with CaCO3 compounds occurs at higher pH values (Oxmann & Schwendenmann, 2015). However, in our study, this trend was not observed in all cores. We observed that C2 has the lowest pH, while C1 has the highest pH, but the concentration of AP in C2 is higher than that in C1. Consequently, we suggest that 1 M HCl not only dissolved the carbonates but also a small amount of other detrital and authigenic phosphate compounds. These phosphate compounds can be igneous or biogenic (fish debris) (Anschutz et al., 2007) or shrimp debris in C2 in our case.
The ranges of NAIP concentrations in C1 and C2 were 168–201 and 63–115 mg/kg, respectively, with a proportion of 26–31% and 10–15% of the TP. In the sediment of C3, NIAP presented the highest fraction, ranging from 84 to 98 mg/kg and accounting for between 19 and 29% of the TP. Non-inorganic apatite P concentrations were lower than those measured in Norway (Lukawska-Matuszewska et al., 2013) but higher than those observed in Estonia (Kapanen, 2008). The NIAP concentrations were a little higher in the upper 25-cm profiles than in the rest of the sediment profiles in C1 and C2, which might reflect the impact of anthropogenic activities (i.e., shrimp farming and human settlement) in this area that accelerated the accumulation of P in sediments in recent years. However, the NIAP concentration in C3 exhibited a generally stable pattern, suggesting a rather constant environment in the protected area of the mangrove forest.
The lowest form of P was LP, which only contributed to 1–4% of the total P pool (ranging from 4 to 25 mg/kg). This form of P is considered as loosely bound, labile, or exchangeable P, which represents the “real mobility” of P because dilute salt as CaCl2 0.01 M is considered as similar to pore water composition (Sahuquillo et al., 2003). Generally, the LP is quite low in spite of different extractants (e.g., NH4Cl) in other studies as well (Dung et al., 2020; Kapanen, 2008). Within the three cores, the LP concentrations were similar and revealed little variation on the whole depth profiles (Fig. 3).
The relationship between potential P release and sediment properties
Previous research found that changes in sediment P concentrations were related to the elemental composition of sediments (Que Vo et al., 2014). Metal hydroxides of Fe, Al, and Ca in sediments are well-known for fixing phosphorus into sediments (Han et al., 2011). Hence, preliminary information on the possible release and origination of P in the sediments can be predicted based on the relationship between TP and other geochemical properties. The relations among P forms and the physicochemical properties of sediments were investigated using Spearman’s correlation analysis (Table 3).
According to Reddy and Delaune (2008), organic phosphorus mineralization and the OC:TP ratio are inversely connected. As a result, a lower OC:TP ratio leads to a higher possibility of P release from organic matter (Joseph et al., 2011). The average OC:TP ratios in the sediments were 33, 71, and 93 for C1, C2, and C3, respectively. The single extractions used in this work do not allow for the evaluation of P release from organic matter, but the OC:TP ratio indicates that P mobility from organic matter in the investigated sediments was in the following order: C1 > C2 > C3. The strong correlation between OC and OC:TP ratio (r = 0.66) (Table 2) indicated that organic carbon and P were controlled by the same factors or that organic matter is the primary source of P in the sediments. This finding is consistent with Wang et al. (2008), who found organic phosphorus to be an important phosphorus fraction buried in the core sediment of three different lakes in China. Strong negative correlations were found between the OC:TP ratio and different released forms of P such as LP, NAIP, and AP (r = − 0.62 to − 0.77, p < 0.05), indicating that the majority of the P released was inorganic form.
According to Jensen et al. (1992), the ratio of total iron to total phosphorus (Fe:TP ratio) in the sediment is an indicator for the release of P into the aquatic environment. Fe:TP ratios greater than 15 (by weight) release less P under anoxic circumstances, while Fe:TP ratios less than 10 appear to be unable to hold P (Jensen et al., 1992). This is owing to the fact that the development of Fe (II) and P minerals is favored when Fe is abundant in the sediment (Lukawska-Matuszewska et al., 2013). The average Fe:TP ratios in the sediments were 84, 78, and 125 for C1, C2, and C3, respectively. The highest Fe:TP ratio (Fe:TP = 249) was observed at the deepest of C3, located in the protected mangrove forest area, whereas the lowest Fe:TP ratio (Fe:TP = 19) was found in sediment from C1, where human settlement was dominant. Iron was found to have significant positive correlations with both NAIP and AP, both of which were designated as inorganic phosphorus, implying that Fe may play a significant role in P availability in the investigated sediment. This assumption was supported by the fact that all mobile forms of P, including LP, NAIP, and AP, demonstrated a moderate to strong positive correlation (r = 0.40–0.73, p < 0.05) (Table 3) with Fe.
Weak bound P extracted by CaCl2 presenting for the exchanged P and P in pore water had a moderate to strong positive correlation with Al, NAIP, and AP (r = 0.5 to 0.71, p < 0.05), but a strong negative correlation with the OC and OC:TP ratio (r = − 0.66 to − 0.69, p < 0.05) (Table 3). The pore water P played an important role in the matter exchange and cycle between the water phase and the sediments, and it was found to be significantly positively correlated with the concentrations of AP and TP (Wang et al., 2008). However, we found no correlation between LP and TP in this study (Table 3). Similarly, significant intercorrelations were found between released P forms such as LP vs AP and NAIP (r = 0.50–0.71, p < 0.05) and NAIP vs AP (r = 0.79, p < 0.05) (Table 3). This result is in line with Que Vo et al. (2014), who also found a relationship between different P fractions in the sediment of the Han River (Korea). Despite differences in land use patterns that vary by area, the correlations between P forms and sediment components suggest a high affinity between these sediment components.
The concentrations of LP, NAIP, and AP in sediments varied according to land use pattern. In general, the profiles of these released P forms were lower in the protected mangrove sediments (C3) than in the human settlement (C1) and aquaculture areas (C2) (Fig. 3). However, only AP concentrations in the human settlement (C1) and aquaculture areas (C2) were found to be statistically significantly higher in sediments than in sediments from the protected mangrove site (C3) (p < 0.05).
Ecological risk and potential release assessment of P
Based on total concentrations
Ecological risk assessment of sediments based on the average concentrations of TP in the investigated sediments can be classified as level I (C3) to level II (C1 and C2). This result suggested that sediments in the protected mangrove forest had no toxic effects on aquatic organisms, whereas sediments in the human settlement and aquaculture area were slightly contaminated, but most benthic organisms were still viable. This statement was supported by a significant increase in TP in the human settlement and aquaculture areas when compared to the protected area of the mangrove forest (p < 0.05).
Based on the single extractions
Although total P concentrations can provide information on current pollution levels, they cannot predict potential P release under changing environmental conditions or assess the ecological risk associated with P mobilization in sediments. Based on the results of the single extractions, preliminary risk assessment and potential release of P from the sediments to the aquatic environment can be inferred.
The Risk Assessment Index (RI) of P in sediments ranged from 0.1 to 0.5 in this study (Fig. 5), and the risk levels were mostly in level I, with the exception of one sample at a depth of 15 cm classified as level II, indicating a slight risk (RI = 0.5). In other words, the majority of the sediment samples collected in the protected mangrove forest and the human settlement as well as the aquaculture areas were at no risk of P pollution. These results are in line with Ye et al. (2019), who also reported a free risk of pollution of P based on bioavailability in river sediments, although it was classified from a slight to serious risk based on total concentration of P. The findings demonstrated that, in addition to total concentration, mobility of P assessed by a single extraction was useful in providing an indication of risk during sediment suspension or under changing environmental conditions. Furthermore, because not all forms of P are readily releasable, monitoring the health of aquatic ecosystems should not be based solely on total concentration.
To assess the potential release of P as an internal load from sediment to the water environment, TP is divided into inorganic P (IP) and organic P (OP). The IP was calculated by summing AP and NAIP, which is the amount of P that can be released when external environmental conditions change. This is due to the fact that the NAIP is sensitive to changing redox conditions, while the AP may be released when the external pH decreases (Tran et al. 2020). The difference between TP and IP represents the OP, which is considered as a stable fraction and is only mobilized under the effect of microorganisms (Han et al., 2011).
The average IP in the core sediment was 386 mg/kg for C1, 190 mg/kg for C2, and 123 mg/kg for C3. This finding implies that the land use pattern in the Can Gio area had a significant impact on the concentration of IP in core sediments. Although it is difficult to estimate the effect of land use on IP contribution, we found that IP increased in human settlement and aquaculture areas, while the decomposition of acid phosphatase from plants and reactive oxygen species to the OP probably occurred in the sediment–water interface. This finding is consistent with the findings of Ye et al. (2019), who also found that the accumulation of P in sediments is positively related to human activities. These results further illustrated that the sediment in the mangrove forest had a low potential for internal P loading from sediment; however, changes in land use practices may pose a risk of P release because human activities may increase the mobile forms of P in the sediments.
Conclusions
The main physicochemical characteristics of core sediments from three different land use types including human settlement, aquaculture, and protected mangrove forest areas in Can Gio, a coastal area of Ho Chi Minh City, southern Vietnam, were determined. On the basis of the results obtained in this work, sediments at Can Gio mangrove forest have a high concentration of fine fraction (63 ≤ µm), a high organic matter concentration, and an average level of Al and Fe. The carbonate component (as inferred by Ca and Mg concentration) and pH were both low. Phosphorus concentrations varied greatly, with significantly lower values observed in the mangrove biosphere reserve zone compared to the human settlement and aquaculture areas. In general, sediments found in the mangrove biosphere reserve zone varied less with depth in terms of Al, Ca, Fe, and P than those found in the sediments in the human settlement and aquaculture areas. The average Fe:TP ratios in the core sediments of the human settlement and aquaculture areas were lower than those found in the protected mangrove forest significantly (p < 0.05). Within the 45-cm (or 50-cm) sediment profiles, the rank orders of P fractions were AP > NAIP > LP in the human settlement and aquaculture areas and NAIP > AP > LP in the mangrove biosphere reserve area. The LP represented ≤ 4% of the sediment total P, while the NAIP ranged from 10 to 31%. The apatite P showed considerable variation in the sediment total P loads, ranging from 4 to 40%. Strong positive correlations were discovered between the OC:TP ratio and OC, but negative correlations were found between the OC:TP ratio and different released forms of P such as LP, NAIP, and AP (p < 0.05), indicating that organic matter is the primary source of P in the sediments, but the majority of the P released was inorganic. There were positive correlations between Fe and both NAIP and AP, as well as intercorrelations between P fractions (p < 0.05). The risk assessment revealed that there was low risk of P pollution or release from the core sediment in the Can Gio area. Anthropogenic activities, on the other hand, may increase the IP fractions, which are more released under anoxic or pH decrease conditions.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
References
Abu-Hmeidan, H., Williams, G., & Miller, A. (2018). Characterizing total phosphorus in current and geologic Utah Lake sediments: Implications for water quality management issues. Hydrology, 5(1), 8.
Alongi, D. M. (2002). Present state and future of the world’s mangrove forests. Environmental Conservation, 29, 331–349.
Alongi, D. M. (2018). Impact of global change on nutrient dynamics in mangrove forests. Forests, 9(1), 1–13.
Anschutz, P., Chaillou, G., & Lecroart, P. (2007). Phosphorus diagenesis in sediment of the Thau Lagoon. Estuarine, Coastal and Shelf Science, 72(3), 447–456.
Bao, T. T., & Ha, M. B. (2018). Effect of land-use changes resulting from shrimp farming on acid sulfate soils in the Can Gio coastal wetland area (Vietnam). Polish Journal of Soil Science, 51(2), 205. https://doi.org/10.17951/pjss.2018.51.2.205
Cappuyns, V. (2012). A critical evaluation of single extractions from the SMT Program to determine trace element mobility in sediments. Applied and Environmental Soil Science, 2012, 1–15.
Cavalcante, H., Araújo, F., Noyma, N. P., & Becker, V. (2018). Phosphorus fractionation in sediments of tropical semiarid reservoirs. Science of the Total Environment., 619(620), 1022–1029.
Cheng, L., Pei, S. L., Zhang, H. L., Ai, M. J., Xue, G. C., Chen, T. A., Shuangyan, H., Lin, Y., & Hong, W. Y. (2019). A 30-year record of sedimentary phosphorus species in a coastal salt marsh Southwest of Hangzhou Bay. China. Cogent Environmental Science, 5(1), 1–13.
Dung, T. T. T., Thoang, T., & N., Tuyen, N. N., Chau, T. B., Hoang, T. M. (2020). Assessment of phosphorus release from alluvial sediments using single extraction: A case study in Can Gio, Southern Vietnam. Vietnam Journal of Science, Technology and Engineering, 62(4), 60–64.
Gunduz, B., Firat, A., Isil, A., & Candan, H. (2011). Study of phosphorus distribution in coastal surface sediment by sequential extraction procedure (NE Mediterranean Sea, Antalya-Turkey). Microchemical Journal, 98(1), 72–76.
Han, L., Suiliang, H., Stanley, C. D., & Osborne, T. Z. (2011). Phosphorus fractionation in core sediments from Haihe river mainstream. China. Soil and Sediment Contamination, 20(1), 30–53.
Head, K. H. (1992). Manual of Soil Laboratory Testing Volume I - Soil Classification and Compaction Tests (2nd edition) (p. 388). Pentech Press.
Ho Chi Minh City People’s Committee. (2016). Approved project construction planning of networks services for the development of aquaculture and salt production in Can Gio period 2016 – 2020 (in Vietnamese).
Jensen, H. S., Kristensen, P., Jeppesen, E., & Skytthe, A. (1992). Iron: Phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia, 235, 731–743.
Joseph, M. M., Kumar, C. S. R., Renjith, K. R., Kumar, T. R. G., & Chandramohanakumar, N. (2011). Phosphorus fractions in the surface sediments of three mangrove systems of southwest coast of India. Environmental Earth Science., 62(6), 1209–1218.
Kapanen, G. (2008). Phosphorus fractionation in lake sediments. Estonian Journal of Ecology, 57(4), 244–255.
Kristensen, E., Andersen, F. Ø., Holmboe, N., Holmer, M., & Thongtham, N. (2000). Caron and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket. Thailand. Aquatic Microbial Ecology., 22, 199–213.
Lukawska-Matuszewska, K., Rolf, D. V., & Ruikai, X. (2013). Phosphorus pools and internal loading in a eutrophic lake with gradients in sediment geochemistry created by land use in the watershed. Hydrobiologia, 713(1), 183–197.
McDonough, S., Gallardo, W., Berg, H., Trai, N. V., & Yen, N. Q. (2014). Wetland ecosystem service values and shrimp aquaculture relationships in Can Gio. Vietnam. Ecological Indicators, 46, 201–213.
Murphy, J., & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36.
Nelson, D. W., & Sommers, L. E. (1982). Total carbon, organic carbon and organic matter, 539–579. https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.c29pdf/10.2134/agronmonogr9.2.2ed.c29
Oxmann, J. F., & Schwendenmann, L. (2015). Authigenic apatite and octacalcium phosphate formation due to adsorption-precipitation switching across estuarine salinity gradients. Biogeosciences, 2(3), 723–738.
Peng, C., Shen, Y., Wu, X., Yuan, P., Jiang, L., Chen, S., Ze, S., Wang, X., & Song, X. (2020). Heavy metals, nitrogen, and phosphorus in sediments from the first drinking water reservoir supplied by Yangtze River in Shanghai, China: Spatial distribution characteristics and pollution risk assessment. Water, Air, and Soil Pollution, 2020, 231–298.
Persaud, D., Jaagumagi, R., & Hayton, A. (1993). Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario. Ontario.
Pierzynski, G. M. (2000). Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters. Southern Cooperative Series Bulletin No. # 396: North Carolina State University.
Que Vo, N. X., Yoonhwan, J., & Hojeong, K. (2014). Distribution of inorganic phosphorus fractions in sediments of the South Han River over a rainy season. Environmental Engineering Research, 19(3), 229–240.
Ramanathan, A. L., Ranjan, R. K., Prasad, M. B. K., Chauhan, R., & Singh, G. (2010). Sediment - nutrient dynamics in selected Indian mangrove ecosystems - land use and climate change implications. International Association of Hydrological Sciences, 337, 84–92.
Reddy, K., & Delaune, R. (2008). Biogeochemistry of wetlands: Science and applications (p. 387). CRC Press.
Ruban, V., Pardo, P., Rauret, G., & Muntau, H. (2001). Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments – A synthesis of recent works. Fresenius Journal Analytical Chemistry, 370, 224–228.
Sahuquillo, A., Rigol, A., & Rauret, G. (2003). Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends in Analytical Chemistry, 22, 152–159.
Sofawi, A. B., Nazri, M. N., & Rozainah, M. Z. (2017). Nutrient variability in mangrove soil: Anthropogenic, seasonal and depth variation factors. Applied Ecology and Environmental Research, 15(4), 1983–1998.
Spalding, M., Kainuma, M., & Collins, L. (2010). World Atlas of Mangroves (version 3.0). A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. London (UK): Earthscan, London. 319 pp.
Taillardat, P., Alan, D. Z., Daniel, A. F., David, W., Frank, D., Nobuhito, O., Takashi, N., Jaivime, E., Nguyen, T. N., & Truong, V. V. (2019). Assessing nutrient dynamics in mangrove porewater and adjacent tidal creek using nitrate dual-stable isotopes: A new approach to challenge the outwelling hypothesis ? Marine Chemistry, 214, 103662.
Tuan, V. Q., & Kuenzer, C. (2012). Can Gio Mangrove Biosphere Reserve Evaluation 2012: Current Status, Dynamics, and Ecosystem Services (p. 109). IUCN.
Tuukkanen, T., Marttila, H., & Kløve, B. (2017). Predicting organic matter, nitrogen, and phosphorus concentrations in runoff from peat extraction sites using partial least squares regression. Water Resources Research, 53, 5860–5876.
Wang, C., Qian, J., Guo, Z. Y., Zhao, L., & Li, X. C. (2008). Vertical distributions of phosphorus fractions in sediments of three typical shallow urban lakes in P.R. China. Polish Journal of Environmental Studies, 17(1), 155–162.
Ye, H., Hao, Y. N. H., Changchun, H., Tao, H., Guoping, L., Xuyin, Y., & Hong, W. (2019). Risk assessment based on nitrogen and phosphorus forms in watershed sediments: A case study of the upper reaches of the Minjiang watershed. Sustainability, 11(20), 5565.
You, S., Peisun, L., Zilong, L., Haiyan, Q., Siriporn, P., Le, T. P. Q., Chantha, O., Che, A., Rahim, M., Choon, W. L., Xixi, L., & Gusti, Z. A. (2022). Geochemical behavior of sedimentary phosphorus species in northernmost artificial mangroves in China. Forests, 18(610), 1–15.
Zhang, Z., Lv, Y., Zhang, W., Zhang, Y., Sun, C., & Marhaba, T. (2015). Phosphorus, organic matter and nitrogen distribution characteristics of the surface sediments in Nansi Lake. China. Environmental Earth Sciences, 73(9), 5669–5675.
Zhou, Q., Gibson, C. E., & Zhu, Y. (2001). Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere, 42(2), 221–225.
Acknowledgements
We would like to thank Le Van Truong Phong, Nguyen Do Thang, and Tran Van Anh for their assistance during field work and sample analysis. The personnel at the Can Gio Mangrove Protection Forest Management Board are also acknowledged for accessing the Can Gio mangrove biosphere reserve and support during sampling campaigns.
Funding
This research is funded by the Vietnam National University, Ho Chi Minh City (VNU-HCM), under grant number C2020-18–16.
Author information
Authors and Affiliations
Contributions
T.T.T. Dung interpreted the results with significant contributions from N.N. Tuyen and T.B. Chau. L.H. Quan and H.T.V. Anh performed the analysis and prepared the map. T. T. T. Dung wrote the main manuscript text, and a significant contribution to writing was made by T. M. Hoang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dung, T.T.T., Quân, L.H., Van Anh, H.T. et al. Vertical distributions and risk assessment of phosphorus in core sediments from the Can Gio coastal district in southern Vietnam. Environ Monit Assess 194, 859 (2022). https://doi.org/10.1007/s10661-022-10523-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10523-9