Abstract
The polymerization of octamethylcyclotetrasiloxane (D4) catalyzed by Maghnite-H+, a nontoxic and green solid catalyst, is studied. The Maghnite-H+ is a montmorillonite type 2:1 dioctahedral phyllosilicates whose interlayer ions are exchanged by hydronium ions after activation with sulfuric acid which gives it its catalytic appearance. D4 was polymerized cationically by ring opening at 60 °C without solvent using Maghnite-H+ contents less than 5% by weight. The molecular structure of the polymer obtained was identified by IR, 1H NMR and 13C NMR. The DSC was used to study the thermal properties. The operating conditions were optimized so that we can achieve best performance for obtaining a linear polymer with high average molecular mass. The variation of the molecular mass distribution was verified by GPC. Finally, a reaction mechanism was proposed to show the role of the Maghnite-H+ during the different steps of the reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To preserve the environment and human health, it is recommended to reduce the use of solvents and homogeneous catalysts, which have a very negative impact, especially in industrial chemistry (Clark and Rhodes 2000). The heterogeneous catalysts present a very good alternative to those homogeneous and also to strong acids such as Brönsted acids and Lewis acids. The good feature of this new class of catalysts is not limited solely to the fact that they are environmentally friendly catalysts, but also to their: easy filtration of the reaction mixture, high specific surface and possible regeneration and reuse while achieving a satisfactory yields, moreover, they allow to work under mild conditions (Chen et al. 2007).
The siloxane polymerization technology has been taken for the first time by Friedel and Crafts (1877) and Crafts (1900), when they have synthesized the first polysiloxane (Scheme 1a), then several polymerizations have been carried out previously by achieving different efficiencies, depending on the catalyst used; about 95% by phosphazene bases (Molenberg and Möller 1995; Pibre et al. 2008), 90% by strong bases (Gee 2015; Sun et al. 2014), 77% by triflic acid (Conan et al. 2003), 85% by dodecylbenzenesulfonic acid (Jiang et al. 2010), 58% by trifluoromethanesulfonic acid (Wilczek et al. 1986) and 95% by tris(pentafluorophenyl)borane (Chojnowski et al. 2007), etc.
Currently, polydimethylsiloxanes (Scheme 1b) are the most common class in the polysiloxane industry, accounting for 70% of the worldwide silicon market, they are a mixture of fully methylated linear siloxane polymers containing repeating units of the formula (CH3)2SiO and stabilized at the end by trimethylsiloxy blocking units of the formula (CH3)3SiO or by hydroxyl groups. In accordance with the Codex Alimentarius, polydimethylsiloxanes are generally used as emulsifiers, anti-caking and anti-foaming agents as in cooking oils to avoid splashing. They can be added to a wide variety of products, including dietary supplements and spirits. They serve as a processing aid in the preparation of dyes and aromas, also in pharmaceutical and cosmetics sectors (Rodriquez 1989; DeGroot et al. 2004; Narins and Beer 2006; Dumitriu 2002).
The synthesis of the polydimethylsiloxanes can be done with three different methods: hydrolysis of chlorosilanes, anionic ring opening polymerization and cationic ring opening polymerization (Kendrick et al. 1991; Chojnowski and Cypryk 2000). The polydimethylsiloxane obtained from the reaction contains sometimes small amounts of cyclic polymers, which are resulting in side reactions such as the reticulation and backbiting phenomena. The percentage of cyclic polymers depends mainly on the operating conditions and also the type of catalyst used (Sigwalt 1987; Chojnowski et al. 2002). Chen et al. polymerized the D4 with a commercialized acidified bentonite, by introducing the tetramethyldihydrogensiloxane as a terminator, the reaction requires periods of time greater than 24 h. The results showed that the average molecular mass obtained for a maximum conversion (93%) is Mn ≈ 6000 (Chen et al. 2007).
The novelty of our work is based on the use of the Maghnite-H+; prepared Algerian clay, as a heterogeneous solid catalyst for the polymerization in bulk of D4 (Scheme 1c), making it possible to obtain a conversion of 93% with a high average molecular mass. The reaction time required is relatively short. The reaction can be terminated with a simple deactivation of Maghnite-H+ with water. The Maghnite is a natural clay of montmorillonite type, obtained from western Algeria (Maghnia), characterized by its high percentage of silica compared to alumina, this property gives it an excellent catalytic capacity, because the uptake of protons on the silica is more important than on the alumina, considering the high electronegativity of the silica (Kherroub et al. 2014a, b; Belbachir and Bensaoula 2001). Activation with sulfuric acid results in an increase in the interlayer distance; this can be clearly distinguished by the XRD analysis.
Various researches have been made on introducing the Maghnite-H+ as initiator for several polymerization reactions, like the polymerization of: N-vinyl-2-pyrrolidone (Meghabar et al. 2003) and hexachlorocyclotriphosphazene (Bouchama et al. 2015), it was also used as a support for the synthesis of nanocomposites (Kherroub et al. 2013, 2015a, b, c), but not yet for the synthesis of silicon polymers.
The polydimethylsiloxane obtained in the polymerization of D4 by the Maghnite-H+ was characterized by IR, 1H NMR and 13C NMR, showing the interchain bonds and the cyclic forms that appear over time. The DSC was introduced to confirm the production of the polydimethylsiloxane and also to check its purity. The influence of reaction time, temperature and catalyst content on the average molecular weight of the obtained polymer, and the monomer conversion was studied using the GPC. In each test, the degree of branching was controlled by the determination of the molecular mass distribution (MMD).
2 Experimental
2.1 Materials
Octamethylcyclotetrasiloxane (D4 98%) was used as purchased from Aldrich chemical, without further purification. Methanol was purified by vacuum distillation. All other products have been used as received. Maghnite was obtained from Algerian company of bentonite (BENTAL), without any pretreatment.
2.2 Preparation of Maghnite-H+
A mass of 30 g of raw Maghnite is combined with 120 ml of distilled water at room temperature; the suspension is left under stirring. After 30 min, 100 ml of a solution of sulfuric acid (0.23 M) is added, and the stirring is continued for 48 h. After filtration and subsequent washing, the activated Maghnite is dried in an oven for 24 h at a temperature of 105 °C. Finally, Maghnite-H+ was crushed, sieved and stored away from air and moisture.
2.3 Polymerization Procedure
0.15 g of Maghnite-H+ is heated before use under vacuum with mechanical stirring for 30 min. The polymerization was carried in bulk. The dried amount of Maghnite-H+ is added to a flask containing 5 g of D4, the flask is immersed in an oil bath and brought to a temperature of 60 °C under reflux at while being stirred. After 8 h, the reaction was stopped by deactivating the Maghnite-H+ by adding cold water to the reaction mixture. The Maghnite-H+ is recovered by filtration, and the filtrate is precipitated in methanol (non-solvent). The insoluble product was dried at 80 °C in vacuum for 6–8 h and weighed as polymer. Excess water was retrieved by evaporation at 105 °C, the amount of water necessary to stop the reaction would be then the difference between the initial amount and the recovered amount. It was assumed that the residual material is the remaining monomers and the oligomers formed during the reaction. Regarding the kinetic study, the same procedure described above was repeated by changing the temperature, time and the percentage of the catalyst.
2.4 Characterization Methods
2.4.1 X-Ray Diffraction
The XRD patterns of the samples were carried out at room temperature on a Bruker D8 Advance X-Ray diffractometer (40 kV, 30 mA) with a graphite monochromator, using CuKα radiation (λ = 0.154 nm) at the rate of 5 min−1 in the range of 2θ = 2°–80°.
2.4.2 Infrared Spectroscopy
Infrared analysis of the polymers obtained was done using a Bruker Alpha FT-IR spectrometer equipped with an ATR accessory.
2.4.3 Nuclear Magnetic Resonance
1H and 13C NMR spectrums were recorded under ambient temperature on Bruker Advance 300 NMR spectrometer, using tetramethylsilane as internal standard and deuterated chloroform as solvent.
2.4.4 Differential Scanning Calorimetry
The different thermal characteristic such as Tg, Tc and Tm of the synthesized polymer were measured by DSC from the corresponding thermal changes in the DSC thermogram using a Setaram 92 DSC apparatus.
2.4.5 Molecular Weight Measurements
Gel permeation chromatography (GPC) measurements of the samples was performed using a WISP Model 712, Waters Associates chromatograph, THF was used as a solvent and the apparatus was calibrated in an initial approximation with polymethyl methacrylate of known molecular weight.
3 Results and Discussion
3.1 X-Ray Diffraction (XRD)
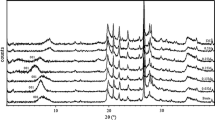
Figure 1 shows the characterization by XRD analysis of the raw Maghnite and Maghnite treated with sulfuric acid. It is obvious that the treatment led to the removal of minerals such as Calcite and Mica, this is confirmed by the decline of the intensity of their peaks compared to the strong peak corresponding to montmorillonite (2θ = 5.73°), this elimination is clearer for the quartz, as shown by the reduction of the two peaks at 2θ = 21.93° and 26.71°. Moreover, the acid treatment caused a shift of the peak of montmorillonite to small values of 2θ from 8.41° to 5.73°, corresponding to an increase of the interlayer distance of montmorillonite (d001) from 10.50 to 15.41 Å, this can be explained by the substitution of interlamellar cations of Maghnite by the hydrogen protons in the form of H3O+.
3.2 Infrared Spectroscopy (IR)
Figure 2 provides the infrared spectra of the monomer and the obtained products for 4, 6, 8, 10 and 12 h at 60 °C. The broad peak seen at 3465 cm−1 for the obtained products is attributed to the OH stretching of the Si–OH end groups in the PDMS chains, the appearance of this peak is due to the linkage between the released proton of Maghnite-H+ and the oxygen atom at the end after the D4 ring opening (Scheme 2), the decrease in its intensity with time of the reaction is clearly noticeable, which may be explained by the increase of polymerization degree leading to smaller number of OH chain ends. The two bands at 2971 and 2916 cm−1 are, respectively, due to C–H asymmetric/symmetric stretching of CH3. The signal at 1281 cm−1 is assigned to the CH3 symmetric deformation of Si–CH3. Peaks appearing at 1071 and 478 cm−1 are, respectively, attributed to the stretching vibrations and deformation vibrations of the linear Si–O–Si structures. The signal at 825 cm−1 is due to the Si–C stretching vibrations. The infrared spectrum of obtained PDMS using Maghnite-H+ as catalyst revealed no differences from those obtained by other researchers (Ya-Qing et al. 2010; Jian et al. 2015).
3.3 Proton Nuclear Magnetic Resonance (1H NMR)
To identify more precisely the structure of the polymer obtained by the polymerization of D4 using the Maghnite-H+ as catalyst, the product was analyzed before and after reaction by NMR analysis by comparing the two spectra: that of the monomer and that of polymer obtained at 60 °C for 8 h. The results are shown in Fig. 3 which shows the different chemical shifts. In both spectra, the dominant peak observed at about 0.15 ppm is attributed to the methyl groups, it is more intense in the spectrum of the polymer meaning the large number of methyl groups in the polymeric chain. The small peak appearing at 4.90 ppm is assigned to the OH groups at the ends of polymer chains during the reaction. Similar results were obtained by Narayana et al. (2011).
3.4 Carbon Nuclear Magnetic Resonance (13C NMR)
It was, therefore, necessary to analyze the products obtained by 13C NMR to provide a complement to the previous study. The results are shown in Fig. 4. The spectrum of the monomer is characterized by a single peak at approximately −0.40 ppm corresponding to the carbon of CH3 (curve above). The other two spectra correspond to 13C NMR spectrum of the polymer obtained after 8 h (curve in the middle) and the DEPT-135 spectrum of the polymer obtained after 10 h (curve below), Both spectra have a characteristic peak at −0.96 ppm, which corresponds to methyl groups in the polymer chain. Moreover, there is a creation of a down peak at 33.13 ppm for the polymer obtained after 10 h, that is attributed to the carbon of CH2, indicating the formation of ethylene bridges between linear polymer chains. These results show that beyond 8 h of reaction time, the polymer chains can be crosslinked to form organopolysiloxane elastomers.
3.5 Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was used as a thermal analysis, to identify and test at the same time the purity of the product obtained. Figure 5 shows the DSC thermogram of the polymer obtained after 8 h of reaction time. The thermogram presents a glass transition, exothermic peak and two endothermic peaks. The Tg is located at −120 °C. The exothermic peak (Tc) detected at −76 °C corresponds to the cold crystallization phenomenon, indicating the existence of amorphous regions. The multiple endothermic peaks appear around −46 and −40 °C corresponding to the melting temperature is related to the fact that there are different crystalline forms in the polymer. These results are consistent to a large extent with those found in previous studies (Dollase et al. 2002; Namrata et al. 2012).
3.6 Effect of Temperature
In an effort to understand and control more the polymerization reaction of D4 catalyzed by Maghnite-H+, we have examined the effect of the temperature of the medium on the reaction that takes place there. Table 1 gives measured values of the monomer conversion and number average molecular mass of the polymers obtained in a temperature range of 20–100 °C. The increase in temperature leads to a significant increase in conversion reaching 93% at 60 °C, beyond this temperature, this increase becomes negligible until the conversion stabilizes at its maximum at about 80 °C. On the other hand, the variation in average molecular mass shows two different behaviors, a gradual increase from 20 to 60 °C, followed by a reduction after just exceed its highest value at about 60 °C, we assume that it is the ceiling temperature, this decrease in average molecular mass can be explained by the fragmentation of the chains suggests the thermal decomposition of PDMS after the breaking of Si–O bonds when approaching the boiling point. The thermal degradation phenomenon reflects a wide divergence between the molecular mass values, resulting in the increase of the polydispersity index, which is found in Table 1.
3.7 Effect of Maghnite-H+/Monomer Weight Ratio
To study the catalytic action of Maghnite-H+ as a heterogeneous catalyst in the polymerization reaction of D4, we performed the reaction with a catalyst content ranging from 1 to 5% by weight, so that for each catalyst content, the reaction time varied from 1 to 12 h. The results of the influence of the Maghnite-H+ content on the monomer conversion and on the number average molecular mass are shown in Figs. 6 and 7, respectively. In all tests, the reaction was carried out in bulk and at a temperature of 60 °C. It is clearly noticeable that for all the different Maghnite-H+ contents, the reaction time has an effect proportionally positive on the monomer conversion before 10 h. After this duration, the effect of reaction time has become negative (Fig. 6). The reduction in the monomer conversion for large periods may be explained by the occurence of depolymerization phenomenon of polymer chains caused by the active cites of the Maghnite-H+ still remaining in the reaction medium. This result indicates that the Maghnite-H+ can play the opposite role after periods of time sufficiently large. Similar results were previously obtained by several authors (Kherroub et al. 2014a, b, 2015a, b, c). On the other hand, the average molecular mass increases with increasing reaction time, the maximum value for the different Maghnite-H+ contents is about 8 h, then it is almost stabilizes for 2 h, so that it begins to decrease. The reduction of the peak corresponding to OH groups over time, which exist only at the end of the polymer chains of PDMS synthesized by Maghnite-H+ showing by the infrared analysis (Fig. 2), indicates that the chains became longer, resulting in large molecular mass. The temporary stabilization between 8 and 10 h of the average molecular mass is due to the crosslinking phenomenon leading to branched structures because of the formation of ethylene bridges between the linear chains, this explanation is clearly supported by what has been obtained by 13C NMR analysis (Fig. 4). The decrease in the average molecular mass after 10 h can be explained by backbiting degradation in the growing polymer chains, which generates oligomers and cyclic polysiloxanes of varying sizes, thereby increasing the polydispersity index (Table 2).
3.8 Kinetics and Mechanism of the Polymerization
To study the chemical kinetics of the polymerization reaction of D4 catalyzed by Maghnite-H+, we followed the evolution of the concentration of monomer over time, we were interested just for t ≤ 10 h, where there was not the depolymerization phenomenon. The results clearly indicate that the reaction is first order with respect to monomer (Fig. 8). Scheme 3 shows the probable reaction mechanism.
4 Conclusion
In this work, a new method of synthesis of a polydimethylsiloxane polymer was studied; it consists of the application of the Maghnite-H+ to catalyze the polymerization reaction of the octamethylcyclotetrasiloxane by ring opening polymerization process.
The structure of the Maghnite is described by the X-ray diffraction showing that it is of the aluminosilicates montmorillonite type, it showed after an acid activation a significant increase in the distance interfoliaire. The polymerization reaction of octamethylcyclotetrasiloxane without solvent was carried out successfully using Maghnite-H+, the polymer was characterized by IR, 1H NMR, 13C NMR, DSC and GPC. The analysis of the structure of the polymer obtained allowed us to propose a reaction mechanism, this mechanism is based on electrophilic attack of H+ on cyclic oxygen resulting in the breaking of the bond Si–O with the formation of a silyl group capable to propagate the reaction, the termination of this reaction was carried out by adding water leading subsequently an OH group at the end of the polymer chain. The kinetic study has allowed knowing the evolution of the conversion and average molecular weight under different reaction conditions.
Maghnite-H+ has shown excellent catalytic capacity for the polymerization of octamethylcyclotetrasiloxane (about 94%), that is very similar to those obtained by strong acids and bases. The advantage of this technique lies in the fact that the polymerization of the octamethylcyclotetrasiloxane with Maghnite-H+ requires a fairly simple protocol; this catalyst greatly accelerates the reaction and thus allows it to occur in very mild conditions.
References
Narayana PL et al (2011) US Patent. 20110237740 A1
Belbachir M, Bensaoula A (2001) US Patent. No 6, 274,527B1
Bouchama A, Ferrahi MI, Belbachir M (2015) Copolymerization of ε-caprolactone with tetrahydrofuran by a solid acid, in the presence of acetic anhydride. J Mater Environ Sci 6:977–982
Chen B, Zhan X, Yi L, Chen F (2007) Cationic ring opening polymerization of octamethylcyclotetrasiloxane initiated by acid treated bentonite. Chin J Chem Eng 15:661–665
Chojnowski J, Cypryk M (2000) Silicon-containing polymers. Kluwer, Dordrecht
Chojnowski J, Cypryk M, Kazmierski K (2002) Cationic polymerization of a model cyclotrisiloxane with mixed siloxane units initiated by a protic acid. Mechanism of polymer chain formation. Macromolecules 36:9904–9912
Chojnowski J, Rubinsztajn S, Fortuniak W, Kurjata J (2007) Oligomer and polymer formation in hexamethylcyclotrisiloxane (D3)—hydrosilane systems under catalysis by tris(pentafluorophenyl)borane. J Inorg Orgamet Polym Mater 17:173–187
Clark JH, Rhodes CN (2000) Clean synthesis using porous inorganic solid catalysts and supported reagents. Royal Society of Chemistry, Cambridge
Conan JT, William PW, Guoping C (2003) Acid and base catalyzed ring-opening polymerization of 2,2,4,4,6,6-hexamethyl-8,8-diphenylcyclotetrasiloxane. Polymer 44:4149–4155
Crafts JM (1900) Friedel memorial lecture. J Chem Soc Trans 77:993–1000
DeGroot JV et al (2004) Highly transparent silicone materials. In: Norwood R, Eich M, Kuzyk M (eds) Linear and nonlinear optics of organic materials, 2nd edn. Proc SPIE IV, Midland, pp 116–123
Dollase T, Spiess HW, Gottlieb M, Yerushalmi-Rozen R (2002) Crystallization of PDMS: the effect of physical and chemical crosslinks. Europhys Lett 60:390–396
Dumitriu S (2002) Polymeric biomaterials. Marcel Dekker, New York
Friedel C, Crafts JM (1877) Comprehensive organic name reactions and reagents. Comptes Rendus 84:1392–1450
Gee RP (2015) Emulsion polymerization of dimethylcyclosiloxane in cationic emulsion: mechanism study utilizing two phase liquid–liquid reaction kinetics. Colloids Surf A Physicochem Eng Asp 481:297–306
Jian W, Xueming C, Panjin J, Qing H, Mingtao R (2015) Synthesis and characterization of the copolymers containing blocks of polydimethylsiloxane in low boiling point mixtures. Mater Chem Phys 149:216–223
Jiang S, Qiu T, Li X (2010) Kinetic study on the ring-opening polymerization of octamethylcyclotetrasiloxane (D4) in miniemulsion. Polymer 51:4087–4094
Kendrick TC, Parbhoo B, White JW (1991) The silicon–heteroatom bond. Wiley, Chichester
Kherroub DE, Belbachir M, Lamouri S, Bouhadjar L, Chikh K (2013) Synthesis of polyamide-6/montmorillonite nanocomposites by direct in situ polymerization catalysed by exchanged clay. Orient J Chem 29:1429–1436
Kherroub DE, Belbachir M, Lamouri S (2014a) Cationic ring opening polymerization of ε-caprolactam by a montmorillonite clay catalyst. BCREC 9:74–80
Kherroub DE, Belbachir M, Lamouri S (2014b) Preparation and characterization of organophilic montmorillonite (12-Maghnite) using Algerian clay. Orient J Chem 30:1647–1651
Kherroub DE, Belbachir M, Lamouri S (2015a) Nylon 6/clay nanocomposites prepared with Algerian modified clay (12-Maghnite). Res Chem Intermed 41:5217–5228
Kherroub DE, Belbachir M, Lamouri S (2015b) Study and optimization of the polymerization parameter of furfuryl alcohol by Algerian modified clay. Arab J Sci Eng 40:143–150
Kherroub DE, Belbachir M, Lamouri S (2015c) Synthesis of poly(furfuryl alcohol)/montmorillonite nanocomposites by direct in situ polymerization. Bull Mater Sci 38:57–63
Meghabar R, Megherbi A, Belbachir M (2003) Maghnite-H+, an ecocatalyst for cationic polymerization of N-vinyl-2-pyrrolidone. Polymer 44:4097–4100
Molenberg A, Möller M (1995) A fast catalyst system for the ring-opening polymerization of cyclosiloxanes. Macromol Rapid Commun 16:449–453
Namrata ST, Florence DJ, Lawrence F, Jacques L (2012) Oxidation, chain scission and cross-linking studies of polysiloxanes upon ageings. OJOPM 2:13–22
Narins RS, Beer K (2006) Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg 118:77–84
Pibre G, Chaumont P, Fleury E, Cassagnau P (2008) Ring-opening polymerization of decamethylcyclopentasiloxane initiated by a superbase: kinetics and rheology. Polymer 49:234–240
Rodriquez F (1989) Principles of polymer systems. Hemisphere Publishing Corp, New York
Sigwalt P (1987) New developments in cationic polymerization of cyclosiloxanes. Polym J 19:567–580
Sun CN, Shen MM, Deng LL, Mo JQ, Zhou BW (2014) Kinetics of ring-opening polymerization of octamethylcyclotetrasiloxane in microemulsion. Chin Chem Lett 25:621–626
Wilczek L, Rubinsztajn S, Chojnowski J (1986) Comparison of the cationic polymerization of octamethylcyclotetrasiloxane and hexamethylcyclotrisiloxane. Makromol Chem 187:39–51
Ya-Qing Z, Xiang K, Xiao-Li Z, Zheng-Hong L (2010) Particle kinetics and physical mechanism of microemulsion polymerization of octamethylcyclotetrasiloxane. Powder Technol 201:146–152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kherroub, D.E., Belbachir, M., Lamouri, S. et al. Cationic Ring Opening Polymerization of Octamethylcyclotetrasiloxane Using a Cost-Effective Solid Acid Catalyst (Maghnite-H+). Iran J Sci Technol Trans Sci 43, 75–83 (2019). https://doi.org/10.1007/s40995-017-0269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-017-0269-y